Abstract
Through the ectopic expression of four transcription factors, Oct4, Klf4, Sox2 and cMyc, human somatic cells can be converted to a pluripotent state, generating so-called induced pluripotent stem cells (iPSCs)1-4. Patient-specific iPSCs lack the ethical concerns that surround embryonic stem cells (ESCs) and would bypass possible immune rejection. Thus, iPSCs have attracted considerable attention for disease modeling studies, the screening of pharmacological compounds, and regenerative therapies5.
We have shown the generation of transgene-free human iPSCs from patients with different lung diseases using a single excisable polycistronic lentiviral Stem Cell Cassette (STEMCCA) encoding the Yamanaka factors6. These iPSC lines were generated from skin fibroblasts, the most common cell type used for reprogramming. Normally, obtaining fibroblasts requires a skin punch biopsy followed by expansion of the cells in culture for a few passages. Importantly, a number of groups have reported the reprogramming of human peripheral blood cells into iPSCs7-9. In one study, a Tet inducible version of the STEMCCA vector was employed9, which required the blood cells to be simultaneously infected with a constitutively active lentivirus encoding the reverse tetracycline transactivator. In contrast to fibroblasts, peripheral blood cells can be collected via minimally invasive procedures, greatly reducing the discomfort and distress of the patient. A simple and effective protocol for reprogramming blood cells using a constitutive single excisable vector may accelerate the application of iPSC technology by making it accessible to a broader research community. Furthermore, reprogramming of peripheral blood cells allows for the generation of iPSCs from individuals in which skin biopsies should be avoided (i.e. aberrant scarring) or due to pre-existing disease conditions preventing access to punch biopsies.
Here we demonstrate a protocol for the generation of human iPSCs from peripheral blood mononuclear cells (PBMCs) using a single floxed-excisable lentiviral vector constitutively expressing the 4 factors. Freshly collected or thawed PBMCs are expanded for 9 days as described10,11 in medium containing ascorbic acid, SCF, IGF-1, IL-3 and EPO before being transduced with the STEMCCA lentivirus. Cells are then plated onto MEFs and ESC-like colonies can be visualized two weeks after infection. Finally, selected clones are expanded and tested for the expression of the pluripotency markers SSEA-4, Tra-1-60 and Tra-1-81. This protocol is simple, robust and highly consistent, providing a reliable methodology for the generation of human iPSCs from readily accessible 4 ml of blood.
Keywords: Stem Cell Biology, Issue 68, Induced pluripotent stem cells (iPSCs), peripheral blood mononuclear cells (PBMCs), reprogramming, single excisable lentiviral vector, STEMCCA
Protocol
1. Isolation and Expansion of Peripheral Blood Mononuclear Cells (PBMCs)
DAY 0
Draw 4 ml of peripheral blood into a BD Vacutainer CPT Cell Preparation Tube with sodium citrate. Invert the tube 8 to 10 times and centrifuge at 1,800 x g for 30 min at room temperature. Ideally, this step should be done within 2 hr of collection.
Collect the mononuclear cells (MCs) by pipetting the buffy coat (cell layer between gel barrier and plasma) into a sterile 15 ml conical centrifuge tube. Bring total volume to 10 ml with sterile phosphate-buffered saline (PBS), invert several times and centrifuge at 300 x g for 15 min.
Resuspend the cells in 10 ml of sterile PBS and perform cell count. Transfer 1 to 2x106 cells into a sterile 15 ml conical centrifuge tube and centrifuge at 300 x g for 10 min.
Resuspend the cells in 2 ml of expansion medium (EM) (QBSF-60 Stem Cell Medium containing 50 μg/ml Ascorbic Acid, 50 ng/ml SCF, 10 ng/ml IL-3, 2 U/ml EPO, 40 ng/ml IGF-1, 1 μM Dexamethasone and 100 μg/ml primocin or 1% Pen/Strep) and transfer to one well of a 12-well plate. Incubate the cells in a 37 °C, 5% CO2 incubator.
Centrifuge remaining cells at 300 xg for 10 min and freeze ~2x106 cells/vial in FBS containing 10% DMSO.
To start the protocol using frozen PBMCs, thaw 1 vial of cells into 10 ml of QBSF medium and centrifuge at 300 x g for 10 min. Resuspend the cells in 2 ml of EM and transfer to one well of a 12-well plate. Incubate the cells in a 37 °C, 5% CO2 incubator.
DAY 3 and DAY 6
Transfer the cells to a sterile 15 ml conical tube and wash the well once with 1 ml of QBSF-60 Stem Cell Medium to collect adherent cells.
Centrifuge the cells at 300 x g for 10 min.
Resuspend the cells in 2 ml of EM and transfer to one well of a 12-well plate. Incubate the cells in a 37 °C, 5% CO2 incubator.
2. Transduction of PBMCs with STEMCCA Lentivirus
DAY 9
Transfer the cells to a sterile 15 ml conical tube and wash the well once with 1 ml of QBSF-60 Stem Cell Medium to collect adherent cells.
Centrifuge the cells at 300 x g for 10 min.
Resuspend the cells in 1 ml of fresh EM containing 5 μg/ml of polybrene and STEMCCA lentivirus (MOI=1 to 10) and transfer to one well of a 12-well plate. (The protocol describing production of lentiviral particles can be found at http://www.bumc.bu.edu/stemcells/protocols/)
Spin the plate at 2,250 rpm at 25 °C for 90 min.
After spin, add an additional 1 ml of fresh EMcontaining 5 μg/ml of polybrene for a total of 2 ml of medium and incubate the plate in a 37 °C, 5% CO2 incubator.
DAY 10
Transfer the cells to a sterile 15 ml conical tube and wash the well once with 1 ml of QBSF-60 Stem Cell Medium to collect adherent cells.
Centrifuge the cells at 300 x g for 10 min.
Resuspend the cells in 2 ml of EM and transfer to 1 well of a 12-well plate. Incubate the cells in a 37 °C, 5% CO2 incubator.
3. Plating Transduced Cells onto MEFs
DAY 11
Coat the wells of a 6-well plate with 0.1% gelatin and plate inactivated mouse embryonic fibroblasts (MEFs) at 2x105 cells/well in MEF medium (IMDM containing 10% FBS, 1% Non-Essential Amino Acids, 100 μM β-mercaptoethanol, 2 mM L-Glutamine, 100 μg/ml primocin or 1% Pen/Strep). Prepare 3 wells per infection.
DAY 12
Transfer the cells to a sterile 15 ml conical tube and wash the well once with 1 ml of QBSF-60 Stem Cell Medium to collect adherent cells.
Resuspend the cells in 3 ml of MEF medium containing 10 ng/ml bFGF, and Ascorbic Acid and growth factors at the same concentrations as used in EM medium (50 μg/ml Ascorbic Acid, 50 ng/ml SCF, 10 ng/ml IL-3, 2 U/ml EPO, 40 ng/ml IGF-1, 1 μM Dexamethasone).
Plate 1 ml of cells per well of a 6-well plate containing MEFs. Add 1.5 ml of MEF media with bFGF, Ascorbic Acid, and growth factors for a total of 2.5 ml of media/well.
Spin the plate at 500 rpm at 25 °C for 30 min. Incubate the plate in a 37 °C, 5% CO2 incubator.
DAY 14
Feed cells every other day with 2.5 ml of MEF media containing 10 ng/ml bFGF and 50 μg/ml Ascorbic Acid (no growth factors). Aspirate and discard floating cells with each feed.
Add additional MEFs as needed (usually once a week).
~ DAY 20
Once small colonies appear, feed cells daily with 2 ml of human embryonic stem cell (hESC) medium (DMEM/F12 containing 20% Knockout Serum Replacement, 1% Non-Essential Amino Acids, 100 μM β-mercaptoethanol, 2 mM L-Glutamine, 100 μg/ml primocin or 1% Pen/Strep and 10 ng/ml bFGF).
4. Picking and Expansion of iPSC Clones
~ DAY 30 - 40
Pick each colony in individual wells of a 12-well plate pre-seeded with inactivated MEFs containing 1 ml/well of hESC medium plus 10 μM of Stemolecule Y27632 (ROCK inhibitor).
Feed cells daily thereafter with 1 ml of hESC medium only (no ROCK inhibitor).
5. Immunofluorescence Staining for Pluripotency Markers
Staining was performed using the kit "ES Cell Marker Sample Kit" from Millipore and following the manufacturer's protocol.
6. Excision of Integrated STEMCCA Vector
Excision of reprogramming cassette is achieved utilizing a Cre-IRES-Puro expressing vector following transfection and brief Puromycin selection as described6.
Representative Results
We demonstrate a simple and effective protocol for the generation of human iPSCs from PBMCs using a single lentiviral vector. Figure 1A shows a schematic representation of the protocol. The blood is collected into a BD Vacutainer CPT Cell Preparation Tube with sodium citrate, and after centrifugation, mononuclear cells can be collected from the interface between the polyester gel and the plasma (buffy coat) (Figure 1B). The isolated PBMCs are then expanded in culture for 9 days. Figure 2 compares PBMCs at day 0 and day 9, showing that the cells are noticeably dividing. Approximately 10-15 days post-transduction with the STEMCCA lentivirus, appearance of small bright colonies are observed, with still undefined colony morphology. Following the addition of hESC medium, hESC-like colonies can be easily identified (Figure 2D). Some of these colonies are mechanically picked, expanded and characterized for the expression of pluripotency markers such as SSEA-4, Tra-1-60 and Tra-1-81 (Figure 3). Using a brief Puromycin selection approach6, we are able to consistently obtain transgene-free iPSC clones as depicted in Figure 4.
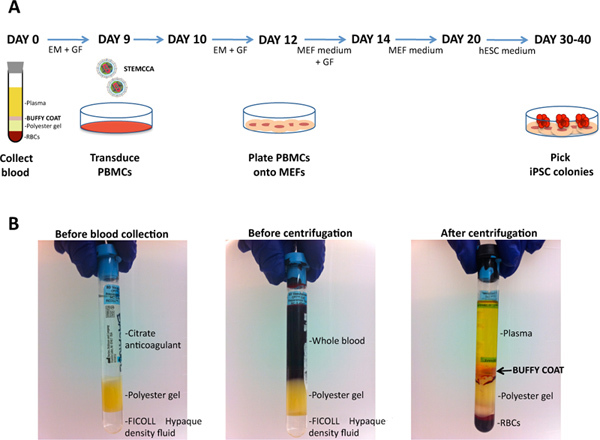 Figure 1. Generation of human iPSCs from peripheral blood. A) Schematic representation of the protocol used to generate human iPSCs from peripheral blood mononuclear cells (PBMCs) using the STEMCCA vector. EM: Expansion Medium; GF: Growth Factors; RBC: Red Blood Cells; MEF: Mouse Embryonic Fibroblast; hESC: human Embryonic Stem Cell. B) BD Vacutainer CPT Cell Preparation Tube with sodium citrate used for the collection of whole blood and the separation of mononuclear cells. The separation occurs during centrifugation when the gel barrier separates the mononuclear cells and plasma from the denser blood components. After centrifugation, mononuclear cells and platelets can be isolated from a whitish layer (buffy coat) just under the plasma layer. Click here to view larger figure.
Figure 1. Generation of human iPSCs from peripheral blood. A) Schematic representation of the protocol used to generate human iPSCs from peripheral blood mononuclear cells (PBMCs) using the STEMCCA vector. EM: Expansion Medium; GF: Growth Factors; RBC: Red Blood Cells; MEF: Mouse Embryonic Fibroblast; hESC: human Embryonic Stem Cell. B) BD Vacutainer CPT Cell Preparation Tube with sodium citrate used for the collection of whole blood and the separation of mononuclear cells. The separation occurs during centrifugation when the gel barrier separates the mononuclear cells and plasma from the denser blood components. After centrifugation, mononuclear cells and platelets can be isolated from a whitish layer (buffy coat) just under the plasma layer. Click here to view larger figure.
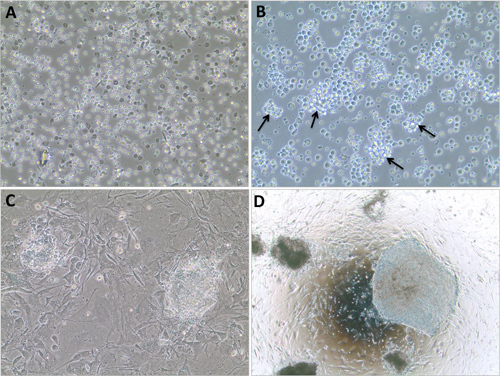 Figure 2. Representative images of morphological changes of the cells throughout the protocol. A) DAY 0: mononuclear cells are isolated from peripheral blood and cultured in expansion medium. 20x magnification. B) DAY 9: after expansion in culture for 9 days, 'bunch of grapes-like' clusters of cells are observed (arrows) suggesting that the cells are healthy and proliferating before being transduced. 20x magnification. C) DAY 20: colony formation is observed one week after the cells are plated onto MEFs. 10x magnification. D) DAY 30: hESC-like iPSC colonies show typical morphology and are ready for picking and expansion. 4x magnification.
Figure 2. Representative images of morphological changes of the cells throughout the protocol. A) DAY 0: mononuclear cells are isolated from peripheral blood and cultured in expansion medium. 20x magnification. B) DAY 9: after expansion in culture for 9 days, 'bunch of grapes-like' clusters of cells are observed (arrows) suggesting that the cells are healthy and proliferating before being transduced. 20x magnification. C) DAY 20: colony formation is observed one week after the cells are plated onto MEFs. 10x magnification. D) DAY 30: hESC-like iPSC colonies show typical morphology and are ready for picking and expansion. 4x magnification.
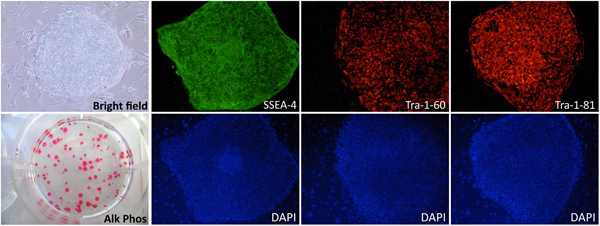 Figure 3. Characterization of human iPSCs generated from blood. Immunofluorescence analysis of iPSCs generated from PBMCs showing the expression of the pluripotency markers SSEA-4, Tra-1-60 and Tra-1-81. The iPSCs also stain positive for Alkaline Phosphatase (Alk Phos).
Figure 3. Characterization of human iPSCs generated from blood. Immunofluorescence analysis of iPSCs generated from PBMCs showing the expression of the pluripotency markers SSEA-4, Tra-1-60 and Tra-1-81. The iPSCs also stain positive for Alkaline Phosphatase (Alk Phos).
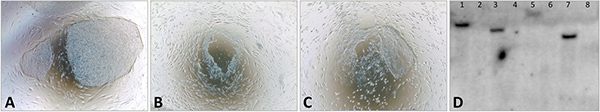 Figure 4. Generation of transgene-free iPSCs. iPSCs are transfected with a plasmid coexpressing Cre recombinase and a puromycin-resistance gene, as described in Somers et al, 2010. A) iPSCs before transfection. B) Cell death is observed after two days of puromycin selection C) New colonies emerge from resistant cells around 10 days after transfection. D) Some colonies are picked and expanded, and Southern Blot is performed to confirm the excision of the transgene. 1, 3, 5 and 7: iPSC clones before excision; 2, 4, 6 and 8: iPSC clones after excision. All phase contrast images at 4x magnification.
Figure 4. Generation of transgene-free iPSCs. iPSCs are transfected with a plasmid coexpressing Cre recombinase and a puromycin-resistance gene, as described in Somers et al, 2010. A) iPSCs before transfection. B) Cell death is observed after two days of puromycin selection C) New colonies emerge from resistant cells around 10 days after transfection. D) Some colonies are picked and expanded, and Southern Blot is performed to confirm the excision of the transgene. 1, 3, 5 and 7: iPSC clones before excision; 2, 4, 6 and 8: iPSC clones after excision. All phase contrast images at 4x magnification.
Discussion
We herein describe the use of the STEMCCA lentiviral vector to generate human iPSCs from mononuclear cells isolated from a few milliliters of freshly collected peripheral blood. The protocol can also be used to reprogram frozen PBMCs (obtained directly from the buffy coat), a detail of significant practical implications when utilizing donor cells acquired from a distant location. Before the induction of reprogramming, isolated PBMCs must undergo a critical expansion step that renders a healthy proliferating population of cells more amenable to nuclear reprogramming. This is easily observed by the presence of 'bunch of grapes-like' clusters of cells upon expansion in hematopoietic culture conditions. When less than 5x105 cells are transduced, cells may be plated onto one or two wells of a 6-well plate containing MEFs. We have experienced some variability in the time necessary to observe hESC-like colonies, in some cases up to 25 days post-transduction. The use of ROCK inhibitor is critical to enhance the survival and cloning efficiency upon picking of emerging iPSC colonies. Remarkably and in contrast to what we observed when picking colonies from reprogrammed fibroblasts, virtually 100% of picked colonies from reprogrammed PBMCs establish stable iPSC clones that can be expanded and cryopreserved. Finally, we believe that the simplicity of the sample collection combined with the highly efficient and consistent reprogramming approach described herein, represent a bona fide platform for applications in which large numbers of iPSC clones need to be generated.
Disclosures
No conflicts of interest declared.
Acknowledgments
These studies were funded in part by NIH UO1HL107443-01 Award to GJM and GM.
References
- Lowry WE, Richter L, Yachechko R. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24:2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers A, Jean JC, Sommer CA. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells. 2010;28:1728–1740. doi: 10.1002/stem.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YH, Hartung O, Li H. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7:15–19. doi: 10.1016/j.stem.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Yuasa S, Oda M. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Staerk J, Dawlaty MM, Gao Q. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7:20–24. doi: 10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou BK, Mali P, Huang X. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanden Akker E, Satchwell TJ, Pellegrin S, Daniels G, Toye AM. The majority of the in vitro erythroid expansion potential resides in CD34(-) cells, outweighing the contribution of CD34(+) cells and significantly increasing the erythroblast yield from peripheral blood samples. Haematologica. 2010;95:1594–1598. doi: 10.3324/haematol.2009.019828. [DOI] [PMC free article] [PubMed] [Google Scholar]


