Editor's Highlight: Melamine and cyanuric acid were added to pet food in the United States and baby formula in China to boost apparent protein content, resulting in widespread poisoning that was characterized by renal toxicity. The research conducted by the Goering group in this issue demonstrated that renal toxicity could be monitored by the analysis of urinary RPA-1 (distal tubule and collecting duct injury biomarker). This represents a novel and valuable biomarker for the noninvasive monitoring of obstructive nephropathy associated with melamine-cyanuric acid exposure.
Key Words: kidney injury, obstructive nephropathy, nephrotoxicity biomarker, melamine, cyanuric acid
Abstract
Oral coexposure of rats to melamine (MEL) and cyanuric acid (CYA) results in a dose-dependent increase in the formation of MEL-CYA crystals in the kidney. The aim of this study was to determine if urinary biomarkers of acute kidney injury could be used to noninvasively detect renal damage associated with crystal formation in the kidneys of MEL- and CYA-exposed rats. Urine was obtained on days 0 (predose), 2, 4, 14, and 28 from male and female Fischer 344 rats fed a diet supplemented with 0, 120, 180, or 240 ppm each of MEL and CYA. A number of urinary protein biomarkers (kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, osteopontin, albumin, alpha-GST, GST-Yb1, renal papillary antigen 1 [RPA-1], and clusterin) were measured using a multiplex assay system. The results showed that RPA-1 (distal tubule and collecting duct injury biomarker) was elevated on day 28 at the 120 ppm dose and higher in male rats and at the 180 ppm dose and higher in female rats; however, other urinary protein biomarkers were significantly elevated only at the 240 ppm dose. Significant elevation in blood urea nitrogen and serum creatinine levels, and severe renal damage evidenced by histopathology, were observed after 28 days of exposure to the highest dose, despite the fact that MEL-CYA crystals were observable at the 120 and 180 ppm doses. These data indicate that RPA-1 may serve as a noninvasive urinary biomarker for the detection and monitoring of obstructive nephropathy associated with MEL-CYA exposure.
In the spring of 2007, there was an outbreak of acute renal failure associated with food contamination in cats and dogs across North America, which led to the largest pet food recall in U.S. history. The contaminated pet foods were investigated and were found to have been adulterated intentionally with melamine (MEL) and MEL-related compounds, including cyanuric acid (CYA) (Brown et al., 2007; Dobson et al., 2008; Filigenzi et al., 2008; Puschner and Reimschuessel, 2011). The following year in China, the adulteration of baby formula with MEL resulted in the illness of approximately 300,000 infants, among which more than 50,000 underwent hospitalization and six deaths were reported (WHO, 2008).
MEL (2,4,6-triamino-1,3,5-triazine) is a commonly used compound in industry for manufacturing laminates, plastics, coating, commercial filters, glues, and some dishes and kitchenware. Given its high nitrogen content (ca. 66% by weight), MEL has been marketed as a fertilizer (Lattupalli et al., 2008; Shelton et al., 1997). Because commonly used methods for food protein analysis are based on total nitrogen content, they cannot distinguish between nitrogen from protein and from non-protein sources. MEL was deliberately added to food to artificially boost its apparent protein content. CYA (1,3,5-triazine-2,4,6-triol), a deaminated derivative of MEL, is commercially used in preparation of herbicides, dyes, resins, and antimicrobial agents, and as a stabilizer and disinfectant in outdoor swimming pools (Downes et al., 1984). As a by-product of MEL synthesis, it was also present in the “scrap MEL” that was used in the adulteration of the pet food. Studies in animal models have shown that MEL or CYA individually have low toxicity. MEL has low direct cytotoxicity in vitro when tested in renal cell lines (Choi et al., 2010). In animal studies, MEL exhibited low toxicity when administered to rats, cats, and dogs and did not have an effect on renal function based on blood urea nitrogen (BUN) or creatinine (Cr) concentrations (Choi et al., 2010; Jacob et al., 2011; Puschner et al., 2007). CYA has also been evaluated for acute and chronic toxicity in rats, mice, and dogs. Similar to MEL, CYA has a low toxicity with an oral LD50 in rats of > 10g/kg. In a subchronic toxicity study, rats fed a high dose of CYA alone developed bladder calculi, but there was no indication of renal toxicity (Hammond et al., 1986; Puschner et al., 2007). In contrast, the administration of a combination of MEL and CYA is extremely nephrotoxic and has been recognized as a potent nephrotoxicant in animals (Dalal and Goldfarb, 2011). Combined exposure of MEL and CYA to rats resulted in a 100-fold potentiation of toxicity compared with exposure to each chemical individually (Choi et al., 2010). Recently, Jacob et al. (2011) showed that MEL or CYA individually did not induce any significant kidney toxicity after 7 days of exposure in rats. In contrast, coexposure to MEL and CYA mixtures caused severe kidney injury and a significant elevation of serum Cr and BUN. The combination of MEL and CYA can lead to the formation of MEL-CYA crystals in kidney tubules. The crystals physically block the renal tubules that results in the development of obstructive nephropathy and can lead to acute kidney failure (Dobson et al., 2008; Filigenzi et al., 2008; Puschner et al., 2007; Reimschuessel et al., 2010).
Nephrotoxicity has been traditionally evaluated by histopathology and elevated clinical chemistry markers such as BUN and serum Cr; however, these routinely used nephrotoxicity biomarkers often do not indicate that renal injury has occurred until a significant degree of renal function is lost (Schnellmann, 2008). Thus, there is an urgent need for the use of new and sensitive biomarkers to detect renal injury at earlier stages. The Food and Drug Administration’s Critical Path Initiative calls for the development of improved methods for a “better product safety toolkit,” including more sensitive and predictive biomarkers of toxicity (U.S. Food and Drug Administration [U.S. FDA], 2009). Seven urinary renal safety biomarkers (kidney injury molecule-1 [Kim-1], albumin, clusterin, trefoil factor 3, total protein, cystatin C, and β2-microglobulin) submitted by the Predictive Safety Testing Consortium’s Nephrotoxicity Working Group have been qualified by U.S. FDA and European Medicines Agency for monitoring nephrotoxicity in preclinical testing (Dieterle et al., 2010). Early and sensitive biomarkers of nephrotoxicity are valuable in drug discovery, preclinical safety evaluation of drugs and medical device materials, clinical trials, and evaluation of toxic responses following exposure to environmental toxicants. In our previous studies, we showed that a number of novel biomarkers (Kim-1, neutrophil gelatinase-associated lipocalin (NGAL), alpha-GST, GST-Yb1, renal papillary antigen 1 [RPA-1]) can detect acute kidney injury (AKI) at earlier stages following exposure of rats to nephrotoxicants, such as gentamicin, mercury, and chromium, than the traditionally used markers, BUN and Cr (Zhou et al., 2008). The objective of this study was to determine if these urinary biomarkers of AKI can be used to detect nephrotoxicity associated with crystal formation and obstructive nephropathy in rat kidneys following dietary coexposure to MEL and CYA.
MATERIALS AND METHODS
Rat urine samples were obtained from animals evaluated in the 28-day study at NCTR-FDA reported by Gamboa da Costa et al. (forthcoming), where a detailed description of the animal treatment, histopathological, and clinical chemistry procedures is provided. Briefly, F344 rats (12 males and 12 females per dose group, 10 weeks old) were fed ad libitum for 28 days with NIH-41 irradiated meal containing 0, 120, 180, or 240 ppm each of MEL and CYA. On days −1, 1, 3, 13, and 27, the rats were transferred to individual metabolic cages and a 24-h urine sample was collected on days 0 (pre-exposure sample), 2, 4, 14, and 28. The urine was collected on ice in 50-ml polypropylene tubes containing 1ml of 1% sodium azide. The volume of the urine was measured and the urine was stored at −80°C until analysis. Urinary Cr levels were determined using an Alfa Wassermann ALERA analyzer (West Caldwell, NJ). At the end of the 28-day exposure period, the animals were euthanized by carbon dioxide inhalation. Blood was collected by cardiac puncture for biochemical analyses and the kidneys were fixed in formalin for histopathological analysis. The results of these analyses are reported in detail in Gamboa da Costa et al. (forthcoming).
Urinary biomarkers analyses for renal injury. For assessment of nephrotoxicity biomarkers in urine, a subset of 8 male and 8 female rats from each treatment group were selected at random. Urine Cr was used as an internal control to normalize urinary biomarker concentrations. Urinary protein biomarkers of Kim-1, albumin, osteopontin, alpha-GST, GST-Yb1, NGAL, RPA-1, and clusterin were measured using commercially available rat multiplex assay kits on the SECTOR Imager 2400 electrochemiluminescence detection platform (Meso Scale Discovery, Gaithersburg, MD).
Statistical analyses. Data analysis was conducted using GraphPad Prism 4.0 statistical software. A one-way analysis of variance followed by Bonferroni’s post hoc test was used to compare the samples obtained from different days to those of day 0 within the same dose group. Data are expressed as mean ± SD (n = 8). A p value < 0.05 was considered statistically significant.
RESULTS
As reported in Gamboa da Costa et al. (forthcoming), acute kidney toxicity was observed in the 240 ppm dose group and only 9 rats were maintained until the end of the scheduled treatment (28 days). Therefore, in order to exclude rats with advanced renal damage from this study, urinary biomarkers were analyzed only on days 0, 2, 4, and 14 at the 240 ppm dose group.
Renal Histopathological Changes
The histopathological findings are reported in detail in Gamboa da Costa et al. (forthcoming). The incidence and severity of kidney lesions in rats used for urinary biomarker analysis are summarized in Table 1. The renal lesion severity for individual animals is shown in Supplementary table 2. Renal histopathological changes were prominent in the 240 ppm MEL and CYA treatment groups and, to a lesser extent, in the 180 ppm treatment group. The 120 ppm group did not reveal significant histopathological changes but did have minimal crystal deposits in > 50% of the animals. Renal lesions affected both the cortex and medulla with involvement of both proximal and distal tubules along with collecting ducts. Intratubular yellow-brown crystals (presumably MEL cyanurate) were evident in the distal and proximal areas of the kidney, resulting in renal tubular dilation accompanied by epithelial cell degeneration and/or necrosis. Significant tubular cell regeneration involving the proximal and distal convoluted tubules was also present. Intratubular and peritubular inflammatory cell infiltrates along with interstitial fibrosis were noted in many animals, but the severity was minimal to mild. Renal lesions occurred in both sexes, but the histopathological changes were more severe in male rats at doses of 120 and 180 ppm compared with the females.
Table 1.
Summary of Incidence and Severity of Rat Kidney Lesions after 28-day Exposure to Diet Containing 0 (control group), 120, 180, or 240 ppm of MEL and CYAa
| Dose group (ppm of MEL and CYA in feed) | 0 | 120 | 180 | 240 | ||||
| Sex | M | F | M | F | M | F | M | F |
| Crystals | 0b | 0 | 87 | 37 | 75 | 75 | 100 | 100 |
| Dilatation, renal tubule | 0 | 0 | 0 | 0 | 75 (2.4) | 25 (2.0) | 100 (4.0) | 100 (3.7) |
| Fibrosis | 0 | 0 | 0 | 0 | 12 (2.0) | 0 | 50 (3.2) | 50 (2.5) |
| Regeneration, renal tubule | 87 (1.0) | 37 (1.0) | 87 (1.1) | 50 (1.0) | 100 (2.5) | 100 (1.9) | 100 (4.0) | 100 (3.7) |
| Necrosis, renal tubule, epithelium | 0 | 0 | 0 | 0 | 25 (1.0) | 0 | 100 (2.2) | 100 (1.8) |
| Infiltration cellular, lymphocyte | 0 | 0 | 0 | 0 | 37 (1.0) | 25 (1.0) | 37 (2.0) | 37 (1.6) |
| Hyperplasia, pelvis, transitional epithelium | 0 | 0 | 12 (2) | 0 | 12 (3) | 12 (2) | 87 (2.0) | 100 (1.6) |
aData are from Gamboa da Costa et al. (forthcoming) and represent 8 rats per dose group used for urinary biomarker analysis. Kidney lesion severity values for individual rats are provided in Supplementary table 2.
b% incidence; severity value in parentheses: 0, not observed; 1, minimal; 2, mild; 3, moderate; 4, marked.
Blood Chemistry Findings
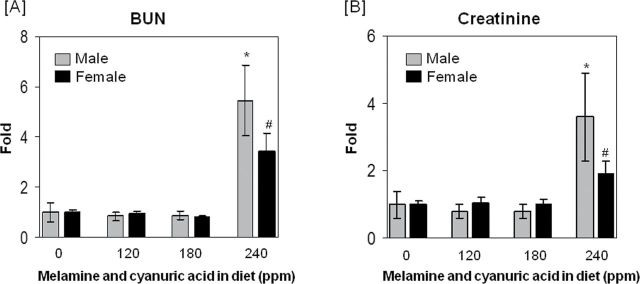
Figure 1 depicts the BUN and serum Cr levels for the subset of 8 male and 8 female rats from the Gamboa da Costa et al. (forthcoming) study that were evaluated for urinary protein biomarkers of nephrotoxicity in this study. Treatment of the rats with feed containing MEL and CYA caused a significant increase of BUN (Fig. 1A) and Cr (Fig. 1B) levels in the highest dose group (240 ppm) in both male and female animals (see numerical summary data in Supplementary table 1 and data for individual rats in Supplementary table 2). Elevations of BUN and serum Cr were higher in male rats compared with female rats; however, there were no significant differences observed between responses of male or female rats in the 120 and 180 ppm groups compared with their matching controls.
FIG. 1.
BUN and Cr changes in F344 rats after 28-day exposure to diet containing 0 (control group), 120, 180, or 240 ppm of MEL and CYA. At the end of study, blood was collected for BUN (panel A) and Cr (panel B) analysis. The data are expressed as means ± SD for each dose group (n = 8). BUN (control) = 17.1±6.3mg/dl (male), 16.4±1.92mg/dl (female); serum Cr (control) = 0.5±0.2mg/dl (male), 0.44±0.05mg/dl (female). Symbol * and # indicate the statistically significant difference with p < 0.05 between control and treatment group in male and female rats, respectively.
Analysis of Urinary Protein Biomarkers
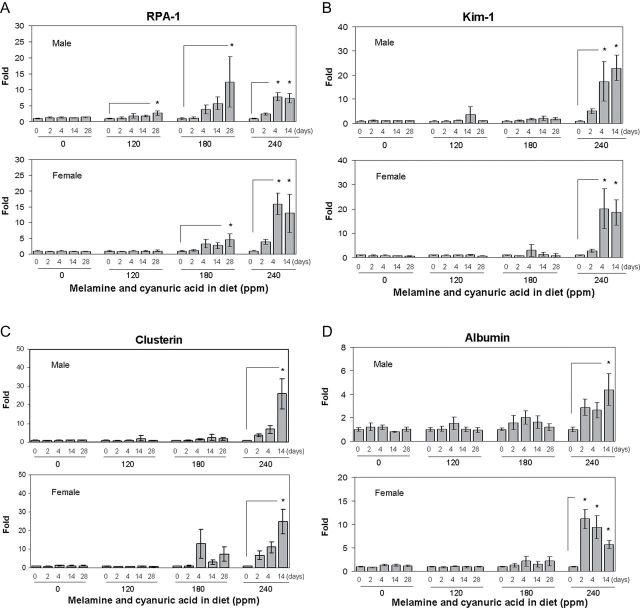
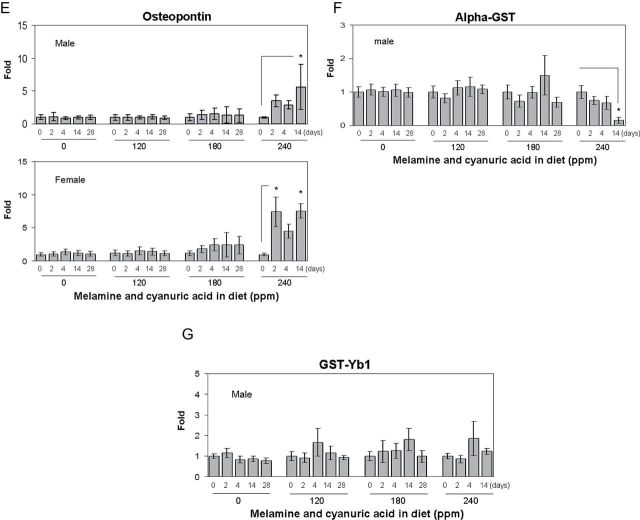
Twenty-four–hour urine samples of male and female F344 rats fed a diet containing 0, 120, 180 or 240 ppm of MEL and CYA were collected on days 0 (pre-exposure) 2, 4, 14, and 28 to allow monitoring of urinary levels of a number of biomarkers of AKI using a multiplex immunoassay system. Urinary biomarker data with means, SDs, and coefficients of variation are presented in Supplementary table 1. Among these biomarkers, a dose-dependent elevation of RPA-1 levels, starting with the lower doses, was observed. As shown in Figure 2A, in the males, there was a 2.7- and 12.4-fold increase of urinary RPA-1 on day 28 in the two lower dose groups (120 and 180 ppm) compared with day 0 values within each dose group (1.50±0.37 and 2.17±1.27 µg/mg Cr), respectively. In the highest dose group (240 ppm), changes in RPA-1 were significantly increased by 7.7- and 7.3-fold compared with day 0 values in the same dose group (1.51±0.53 µg/mg Cr) on day 4 and 14, respectively. In male rats of the low-dose groups (120 and 180 ppm), there was a trend toward time-dependent increases of RPA-1 levels from day 2 to 14; however, there was no statistically significant difference compared with day 0 values within each dose group. In female rats, there was a 4.4-fold increase observed in RPA-1 on day 28 at the 180 ppm dose group compared with the same dose group of day 0 control values (1.71±0.39 µg/mg Cr). In the highest dose group (240 ppm), RPA-1 was increased by 15.9- and 12.9-fold on day 4 and 14 compared with day 0 values within the dose group (1.2±0.28 µg/mg Cr), respectively. In contrast, other biomarkers (Kim-1, osteopontin, clusterin, and albumin) were observed to significantly increase only in the highest dose group (240 ppm), corresponding to the dose that produced severe histopathological damage in the kidney and high blood levels of BUN and Cr (Figs. 2B–E). For alpha-GST and GST-Yb1, the levels were an order of magnitude lower in females of both control and treated groups compared with male rats and also highly variable (data not shown). In males, treated rats exhibited no increases in alpha-GST and GST-Yb1 despite evidence of pathological injury at higher doses (Figs. 2F and 2G). Surprisingly, there was a time-dependent decrease of alpha-GST levels at the highest dose group (240 ppm) in male rats compared with the day 0 values within the dose group. Urinary levels of NGAL were generally undetectable across all groups and time points in both male and female rats (data not shown). Collectively, among the biomarkers tested, the results indicated that RPA-1 appears to be a relatively sensitive urinary protein biomarker to detect AKI following a dietary coexposure to MEL and CYA in rats.
FIG. 2.
Urinary biomarkers analysis in F344 rats following a 28-day exposure to diet containing 0 (control group), 120, 180, or 240 ppm of MEL and CYA. (A) RPA-1, (B) Kim-1, (C) clusterin, (D) albumin, (E) osteopontin, (F) alpha-GST, and (G) GST-Yb1. All the above urinary biomarkers are normalized by urinary Cr concentration. Data are expressed as mean ± SD for each group (n = 8). RPA-1 (control) = 1.45±0.36 µg/mg Cr (male), 1.75±0.31ng/mg Cr (female); Kim-1 (control) = 0.82±0.15ng/mg Cr (male), 1.08±0.24ng/mg Cr (female); clusterin (control) = 7.56±2.48ng/mg Cr (male), 4.72±0.71ng/mg Cr (female); albumin (control) = 37.3±12.7 µg/mg Cr (male), 10.4±2.7 µg/mg Cr (female); osteopontin (control) = 2.01±0.79ng/mg Cr (male), 2.75±1.44ng/mg Cr (female); alpha-GST (control) = 99.6±31.8ng/mg Cr (male); GST-Yb1 (control) = 20.8±5.06ng/mg Cr (male). Urine for day 28 from rats at high dose group (240 ppm) is not analyzed due to advanced renal damage. Asterisks represent statistically significant difference compared with samples obtained on day 0 within each dose group (p < 0.05).
DISCUSSION
Renal function is routinely monitored by serum Cr and BUN concentrations; however, these biochemical indicators often only show elevation when a significant amount of kidney function is lost. Early detection and diagnosis of kidney injury could prevent progression of the disease to more severe kidney damage and renal failure. As a result, new biomarkers are needed to aid in the early diagnosis and prediction of kidney injury. In recent years, a number of urinary proteins have been proposed as sensitive biomarkers of renal injury and as indicators of the localization of the kidney lesions. Included among these new biomarkers are Kim-1, NGAL, albumin, clusterin, glutathione-s-transferase (alpha-GST and GST-Yb1), and RPA-1 (Cruz et al., 2011; Dieterle et al., 2010; Khan et al., 2010). The goal of this study was to determine if these new biomarkers are able to detect kidney injury at early stages of obstructive nephropathy in rats following a dietary coexposure to MEL and CYA.
In this study, a suite of novel urinary nephrotoxicity biomarkers was investigated using a multiplex assay system. As shown in Figure 2A, RPA-1 was significantly increased starting at the 120 ppm group in male rats and 180 ppm group in female rats; however, other biomarkers were observed to increase only in the highest dose group (240 ppm), a dose group where severe histopathological damage in the kidney and high blood levels of BUN and Cr were observed. Renal histopathological changes were prominent in the 240 ppm group and to a lesser extent in the 180 ppm group, although variable amounts of crystals were detectable by wet-mount analysis in all rats in the 120 ppm group (Gamboa da Costa et al., forthcoming). RPA-1 was observed to increase in urine at this lowest dose level, suggesting that the biomarker was able to detect early crystal-induced kidney damage.
RPA-1 is a urinary biomarker used to detect injury of distal tubules and collecting ducts resulting from nephrotoxicity (Betton et al., 2012; Falkenberg et al., 1996; Hildebrand et al., 1999). Studies have shown that RPA-1 is an early preclinical marker of renal papillary collecting duct injury in Sprague Dawley and Wistar rats following exposure to toxicants such as 2-bromoethanamine and N-phenylanthranilic acid (Price et al., 2010). In addition, immunohistochemical studies demonstrated that RPA-1 was localized in the collecting duct and loop of Henle epithelia in Sprague Dawley rats exposed to gentamicin, mercury, or chromium (Zhang et al., 2008). In this study, detection of elevated RPA-1 levels occurred at lower exposure levels compared with other markers, which indicates that renal injury could initially localize in distal tubules and collecting ducts. This finding is in agreement with the detection of intratubular crystals in the proximal and distal areas of the kidneys of the rats in this study, and with other studies demonstrating the localization and distribution of crystals in renal tubules after coexposures to MEL and CYA in rats, cats, and dogs (Dobson et al., 2008; Kobayashi et al., 2010; Puschner et al., 2007). In our study, at the end of 28 days, renal histopathological changes showed that renal lesions occurred in both the cortex and medulla with involvement of both proximal and distal tubules along with collecting ducts. Intratubular yellow-brown crystals were evident in the distal and proximal areas of the kidney. Puschner et al. (2007) found that crystals were primarily present within the lumens of collecting ducts and distal tubules in cats fed a MEL-CYA mixture for 48h. Moreover, Kobayashi et al. (2010) reported that crystal formation was observed in the renal distal tubular lumens and the collecting ducts of the renal papilla after 2 weeks of exposure to an intermediate dose (12mg/kg/day of MEL and CYA) in rats. At a dose of 120mg/kg/day, crystallization was found not only in the collecting duct but also in proximal tubules of the renal cortex. In addition, histopathologic specimens from affected cats and dogs during the 2004 and 2007 outbreaks showed necrosis of distal tubular cells and dilated distal tubules containing crystals (Brown et al., 2007). In infants drinking MEL-contaminated milk, several studies using ultrasonographic imaging have localized stones to the renal collecting system (Lam et al., 2008, 2009). Taken together, the RPA-1 elevation suggests that the site of initial renal injury is the distal tubules and collecting ducts, a finding consistent with the site of injury identified by histopathological changes reported in cats and dogs and by ultrasonography in humans. It is not clear why MEL and CYA form crystals only after localizing in the papillary collecting ducts. A possible explanation is that water absorption generally occurs in this area, which increases the chemical saturation through the gradient of osmotic pressures in the tubules, facilitating the formation of MEL and CYA to insoluble precipitates in the kidney. The crystals physically block the renal tubules and cause acute kidney failure.
Of the other biomarkers examined, urine levels of Kim-1 (proximal tubular injury), clusterin (generalized renal injury), osteopontin, and albumin were found significantly increased only at the highest dose group (240 ppm). The finding in this dose group is consistent with the histopathological observations, i.e., evidence of overt AKI (Table 1). In both control and treated female rats, alpha-GST (proximal tubular injury) and GST-Yb1 (distal tubular injury) levels in urine were an order of magnitude lower than those in male rats and highly variable (data not shown). Recently, NGAL was reported to be a useful noninvasive biomarker of obstructive nephropathy in children (Wasilewska et al., 2011). In our study, NGAL was not detectable in the urine of control or treatment groups in male or female rats (data not shown). This result is consistent with other findings in which NGAL protein was also shown to be undetectable in the urine of untreated male F344 rats compared with urine of male Wistar and Sprague Dawley rats (Rached et al., 2008). The appearance of NGAL may be a strain-specific characteristic. Recently, kidney gene expression changes of these nephrotoxicity urinary biomarkers were evaluated using quantitative real-time reverse transcription PCR in MEL and CYA coexposed rats (Camacho et al., 2011). The F344 rats were exposed to 0, 7, 23, 69, 229, or 694 ppm MEL and CYA for 7 days; these concentrations resulted in a daily MEL and CYA exposure of ca. 0, 0.9, 2.8, 8.6, 17.6, or 29.8mg/kg bw/day, respectively. The results showed significantly increased expression changes of the genes encoding Kim-1, clusterin, osteopontin, and NGAL in both male and female rats at the higher doses of 229 or 694 ppm MEL-CYA mixtures. The study also reported extremely low basal expression of the gene encoding NGAL in this strain of rats. We reached the same conclusion in the detection of the urine protein NGAL in our current study. In addition, we also observed a sex-specific response in the levels of RPA-1 in the urine, with the male rats presenting a statistically significant elevation of the protein levels at a lower dose group (120 ppm). These observations are consistent with the more pronounced histopathological changes observed in the male rats (see Table 1 with discussion in detail in Gamboa da Costa et al., forthcoming, and Supplementary table 2 with data for individual rats used to analyze renal biomarkers).
In conclusion, a panel of new urinary biomarkers has been used to detect early kidney damage following dietary coexposure to MEL and CYA in F344 rats. We demonstrated that RPA-1 (distal tubule and collecting duct injury biomarker) was elevated at doses lower than those at which severe kidney histopathological changes occurred or commonly used biomarkers of nephrotoxicity (BUN and Cr) were elevated. The results suggest that RPA-1 may serve as a promising noninvasive urinary biomarker for earlier detection and monitoring of AKI associated with crystal obstruction following coexposure to MEL and CYA and possibly other forms of obstructive nephropathy as well.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
U.S. Food and Drug Administration and the National Toxicology Program at the National Institute of Environmental Health Sciences [FDA IAG 224-07-0007, NIH Y1ES1027].
Supplementary Material
References
- Betton G. R., Ennulat D., Hoffman D., Gautier J. C., Harpur E., Pettit S. (2012). Biomarkers of collecting duct injury in Han-Wistar and Sprague Dawley rats treated with N-phenylanthranilic acid. Toxicol. Pathol. 40, 682–694. [DOI] [PubMed] [Google Scholar]
- Brown C. A., Jeong K. S., Poppenga R. H., Puschner B., Miller D. M., Ellis A. E., Kang K. I., Sum S., Cistola A. M., Brown S. A. (2007). Outbreaks of renal failure associated with melamine and cyanuric acid in dogs and cats in 2004 and 2007. J. Vet. Diagn. Invest. 19, 525––531 [DOI] [PubMed] [Google Scholar]
- Camacho L., Kelly K. P., Beland F. A., Gamboa da Costa G. (2011). Gene expression of biomarkers of nephrotoxicity in F344 rats co-exposed to melamine and cyanuric acid for seven days. Toxicol. Lett. 206, 166––171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi L., Kwak M. Y., Kwak E. H., Kim D. H., Han E. Y., Roh T., Bae J. Y., Ahn I. Y., Jung J. Y., Kwon M. J., et al. (2010). Comparative nephrotoxicity induced by melamine, cyanuric acid, or a mixture of both chemicals in either Sprague Dawley rats or renal cell lines. J. Toxicol. Environ. Health A 73, 1407––1419 [DOI] [PubMed] [Google Scholar]
- Cruz D. N., de Geus H. R., Bagshaw S. M. (2011). Biomarker strategies to predict need for renal replacement therapy in acute kidney injury. Semin. Dial. 24, 124––131 [DOI] [PubMed] [Google Scholar]
- Dalal R. P., Goldfarb D. S. (2011). Melamine-related kidney stones and renal toxicity. Nat. Rev. Nephrol. 7, 267––274 [DOI] [PubMed] [Google Scholar]
- Dieterle F., Sistare F., Goodsaid F., Papaluca M., Ozer J. S., Webb C. P., Baer W., Senagore A., Schipper M. J., Vonderscher J., et al. (2010). Renal biomarker qualification submission: A dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat. Biotechnol. 28, 455––462 [DOI] [PubMed] [Google Scholar]
- Dobson R. L., Motlagh S., Quijano M., Cambron R. T., Baker T. R., Pullen A. M., Regg B. T., Bigalow-Kern A. S., Vennard T., Fix A., et al. (2008). Identification and characterization of toxicity of contaminants in pet food leading to an outbreak of renal toxicity in cats and dogs. Toxicol. Sci. 106, 251––262 [DOI] [PubMed] [Google Scholar]
- Downes C. J., Mitchell J. W., Viotto E. S., Eggers N. J. (1984). Determination of cyanuric acid levels in swimming pool waters by UV absorbance, HPLC and melamine cyanurate precipitation. Water Res. 18, 277––280 [Google Scholar]
- Falkenberg F. W., Hildebrand H., Lutte L., Schwengberg S., Henke B., Greshake D., Schmidt B., Friederich A., Rinke M., Schluter G., et al. (1996). Urinary antigens as markers of papillary toxicity. 1. Identification and characterization of rat kidney papillary antigens with monoclonal antibodies. Arch. Toxicol. 71, 80––92 [DOI] [PubMed] [Google Scholar]
- Filigenzi M. S., Puschner B., Aston L. S., Poppenga R. H. (2008). Diagnostic determination of melamine and related compounds in kidney tissue by liquid chromatography/tandem mass spectrometry. J. Agric. Food Chem. 56, 7593––7599 [DOI] [PubMed] [Google Scholar]
- Gamboa da Costa G., Jacob C. C., Von Tungeln L. S., Hasbrouck N. R., Olson G. R., Hattan D. G., Reimschuessel R., Beland F. A. (Forthcoming). Dose-response assessment of nephrotoxicity from a twenty-eight-day combined exposure to melamine and cyanuric acid in F344 rats. Toxicol. Appl. Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond B. G., Barbee S. J., Inoue T., Ishida N., Levinskas G. J., Stevens M. W., Wheeler A. G., Cascieri T. (1986). A review of toxicology studies on cyanurate and its chlorinated derivatives. Environ. Health Perspect. 69, 287––292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand H., Rinke M., Schluter G., Bomhard E., Falkenberg F. W. (1999). Urinary antigens as markers of papillary toxicity. II: Application of monoclonal antibodies for the determination of papillary antigens in rat urine. Arch. Toxicol. 73, 233––245 [DOI] [PubMed] [Google Scholar]
- Jacob C. C., Reimschuessel R., Von Tungeln L. S., Olson G. R., Warbritton A. R., Hattan D. G., Beland F. A., Gamboa da Costa G. (2011). Dose-response assessment of nephrotoxicity from a 7-day combined exposure to melamine and cyanuric acid in F344 rats. Toxicol. Sci. 119, 391––397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan E., Batuman V., Lertora J. J. (2010). Emergence of biomarkers in nephropharmacology. Biomark. Med. 4, 805––814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Okada A., Fujii Y., Niimi K., Hamamoto S., Yasui T., Tozawa K., Kohri K. (2010). The mechanism of renal stone formation and renal failure induced by administration of melamine and cyanuric acid. Urol. Res. 38, 117––125 [DOI] [PubMed] [Google Scholar]
- Lam C. W., Lan L., Che X., Tam S., Wong S. S., Chen Y., Jin J., Tao S. H., Tang X. M., Yuen K. Y., et al. (2009). Diagnosis and spectrum of melamine-related renal disease: Plausible mechanism of stone formation in humans. Clin. Chim. Acta 402, 150––155 [DOI] [PubMed] [Google Scholar]
- Lam H. S., Ng P. C., Chu W. C., Wong W., Chan D. F., Ho S. S., Wong K. T., Ahuja A. T., Li C. K. (2008). Renal screening in children after exposure to low dose melamine in Hong Kong: Cross sectional study. BMJ 337, a2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattupalli R., Yee J., Kolluru A. (2008). Nephrotoxicity of mala fide melamine: Modern era milk scandal. Sci. World J. 8, 949––950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S. A., Davies D., Rowlinson R., Copley C. G., Roche A., Falkenberg F. W., Riccardi D., Betton G. R. (2010). Characterization of renal papillary antigen 1 (RPA-1), a biomarker of renal papillary necrosis. Toxicol. Pathol. 38, 346––358 [DOI] [PubMed] [Google Scholar]
- Puschner B., Poppenga R. H., Lowenstine L. J., Filigenzi M. S., Pesavento P. A. (2007). Assessment of melamine and cyanuric acid toxicity in cats. J. Vet. Diagn. Invest. 19, 616––624 [DOI] [PubMed] [Google Scholar]
- Puschner B., Reimschuessel R. (2011). Toxicosis caused by melamine and cyanuric acid in dogs and cats: Uncovering the mystery and subsequent global implications. Clin. Lab. Med. 31 , 181––199 [DOI] [PubMed] [Google Scholar]
- Rached E., Hoffmann D., Blumbach K., Weber K., Dekant W., Mally A. (2008). Evaluation of putative biomarkers of nephrotoxicity after exposure to ochratoxin A in vivo and in vitro. Toxicol. Sci. 103, 371––381 [DOI] [PubMed] [Google Scholar]
- Reimschuessel R., Evans E. R., Stine C. B., Hasbrouck N., Mayer T. D., Nochetto C.,, Gieseker C. M. (2010). Renal crystal formation after combined or sequential oral administration of melamine and cyanuric acid. Food Chem. Toxicol. 48, 2898––2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnellmann, R. G. (2008). Toxic responses of the kidney. In Casarett and Doull’s Toxicology - The Basic Science of Poisons (C. D. Klaassen, Ed.), 7th ed., pp. 583––608 McGraw-Hill, New York, NY [Google Scholar]
- Shelton D. R., Karns J. S., McCarty G. W., Durham D. R. (1997). Metabolism of melamine by Klebsiella terragena. Appl. Environ. Microbiol. 63, 2832––2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilewska A., Taranta-Janusz K., Debek W., Zoch-Zwierz W., Kuroczycka-Saniutycz E. (2011). KIM-1 and NGAL: New markers of obstructive nephropathy. Pediatr. Nephrol. 26, 579––586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Brown R. P., Shaw M., Vaidya V. S., Zhou Y., Espandiari P., Sadrieh N., Stratmeyer M., Keenan J., Kilty C. G., et al. (2008). Immunolocalization of Kim-1, RPA-1, and RPA-2 in kidney of gentamicin-, mercury-, or chromium-treated rats: Relationship to renal distributions of iNOS and nitrotyrosine. Toxicol. Pathol. 36, 397––409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Vaidya V. S., Brown R. P., Zhang J., Rosenzweig B. A., Thompson K. L., Miller T. J., Bonventre J. V., Goering P. L. (2008). Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol. Sci. 101, 159––170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration (USFDA). (2009). The Critical Path Initiative Available at: http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/CriticalPathInitiative/UCM221651.pdf Accessed June 7, 2012.
- World Health Organization (WHO). (2008). Toxicological and Health Aspects of Melamine and Cyanuric Acid. Report of a WHO Expert Meeting in Collaboration with FAO Supported by Health Canada Ottawa, Canada, 1–4 December 2008. World Health Organization, Geneva: Available at: http://whqlibdoc.who.int/ publications/2009/9789241597951_eng.pdf . Accessed May 1, 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.