Abstract
Epidemiological studies have correlated embryonic arsenic exposure with adverse developmental outcomes such as stillbirths, neonatal mortality, and low birth weight. Additionally, arsenic exposure reduces neuronal cell migration and maturation, and reduces skeletal muscle cell formation, alters muscle fiber subtype, and changes locomotor activity. This study used P19 mouse embryonic stem cells to examine whether arsenic exposure could alter their differentiation into skeletal muscles and neurons. When P19 cells were exposed to 0.1, 0.5, or 1.0μM sodium arsenite, embryoid body (EB) formation was not altered. However, arsenic suppressed their differentiation into muscles and neurons, as evidenced by morphological changes accompanied by a significant reduction in myosin heavy chain and Tuj1 expression. Real-time PCR, immunofluorescence, and immunoblotting were used to confirm that the altered differentiation was due to the repression of muscle- and neuron-specific transcription factors such as Pax3, Myf5, MyoD, myogenin, neurogenin 1, neurogenin 2, and NeuroD in the arsenite-exposed cells. The reductions in transcription factors expression appear to be caused by repressed Wnt/β-catenin signaling pathways in early embryogenesis, as evidenced by decreased β-catenin expression in the arsenic-exposed EBs on differentiation days 2 and 5. Interestingly, the expression of Nanog, a transcription factor that maintains the pluripotency of stem cells, was increased after arsenite exposure, indicating that arsenite inhibits their differentiation but not proliferation. This study demonstrates that arsenic can perturb the embryonic differentiation process by repressing the Wnt/β-catenin signaling pathway. More importantly, this study may provide insight into how arsenic exposure affects skeletal and neuronal differentiation during embryogenesis.
Key Words: arsenite, P19, stem cells, β-catenin, myogenesis, neurogenesis.
Exposure to arsenic in drinking water can result in developmental toxicity. In humans and mice, arsenic can pass through the placental barrier (Concha et al., 1998; Jin et al., 2006) and in utero exposure of arsenic via the mother’s drinking water increases the incidence of neonatal deaths and miscarriages, and reduces birth weight (Concha et al., 1998; Raqib et al., 2009; von Ehrenstein et al., 2006).
Additionally, arsenic can accumulate in the brain (Jin et al., 2006; Xi et al., 2010), which may provide a rationale for the correlations between embryonic arsenic exposure and neurological diseases, mental retardation, and lower intelligence quotient scores (Dakeishi et al., 2006; Tsai et al., 2003). Arsenic trioxide exposure (3μM) also inhibited neurite outgrowth in Neuro-2a (N2a) cells (Wang et al., 2010), induced degeneration of neuronal cells in the cerebrum and cerebellum of mice provided 1–2 ppm arsenic trioxide in their drinking water (Piao et al., 2005), and resulted in thinly myelinated axons in the peripheral sensory nerves of rats given 10mg arsenite/kg/day in their drinking water (García-Chávez et al., 2007).
Arsenic-mediated adverse effects on muscle differentiation have also been reported. For example, exposure of 20nM arsenite to mouse C2C12 myoblast cells resulted in delayed differentiation into myotubes due to reduced myogenin expression (Steffens et al., 2011). In rodents, 0.5 and 5 ppm arsenic trioxide given to rodents for 8 weeks suppresses the regeneration of injured muscles (Yen et al., 2010). Additionally, rats exposed to arsenite or arsenate in utero had reduced locomotor activity and reductions in limb movements (Chattopadhyay et al., 2002; Rodriguez et al., 2002). Collectively, these results suggest that arsenic acts as a developmental toxicant by affecting the development of the musculature and neurons. However, the molecular mechanisms responsible these multiple adverse outcomes remain largely unknown.
The mouse P19 cell line consists of pluripotent cells capable of differentiating into multiple cell lineages, such as muscles and neurons (McBurney, 1993) and appear to recapitulate gene expression patterns during early mouse embryogenesis through similar signaling pathways (Kultima et al., 2010). One such signaling pathway, the Wnt/β-catenin pathway, plays an important role in somite formation and neural crest development (Geetha-Loganathan et al., 2008; Schmidt et al., 2008). In stem cells, β-catenin regulates self-renewal and cell fate decisions such that β-catenin-deficient mouse stem cells self-renew rather than differentiate (Ling et al., 2009; Lyashenko et al., 2011), whereas overexpression of β-catenin alone triggers stem cells to differentiate into muscles and neurons (Otero et al., 2004; Petropoulos and Skerjanc, 2002). Thus, the use of stem cells is a promising in vitro model to assess cell fate determination (Marikawa et al., 2009).
Indeed, it has been shown that exposing human stem cells to 76nM arsenic downregulated genes indicative of all the three germ layers (Flora and Mehta, 2009). Results from mouse embryonic stem cells indicate that 10mM arsenic reduced embryoid body (EB) formation and inhibited cardiac cell differentiation (Stummann et al., 2008). However, the molecular mechanisms responsible for arsenic’s effects during embryogenesis are not well understood. Thus, the goal of this study was to determine if arsenic reduced myogenesis and neurogenesis due to altered Wnt/ β-catenin signaling using embryonic stem cells.
MATERIALS AND METHODS
P19 cell culture and differentiation. The mouse embryonal carcinoma P19 cell line (ATCC, Manassas, VA) was maintained in α-MEM supplemented with 7.5% bovine calf serum (Hyclone, Logan, UT), 2.5% fetal bovine serum (Mediatech, Manassas, VA), 1% L-glutamine, and 1% penicillin/streptomycin (designated as growth medium) at 37°C in a humidified incubator containing 5% CO2. Medium renewal was conducted every 48h. To induce differentiation, P19 cells were aggregated by the hanging drop method (Wang and Yang, 2008) with some modifications. Briefly, P19 cells were trypsinized and suspended in differentiation medium (growth medium containing 1% dimethyl sulfoxide) with 0, 0.1, 0.5, or 1.0μM sodium arsenite at a density of 500 cells/20 μl drop. These concentrations correspond to 7.5, 37.5, and 75 μg/l arsenic. Human epidemiological studies examining maternal-newborn pairs determined that cord blood arsenic ranged from 2.9 to 74.6 μg/l, with an average of 15.7 μg/l when mothers drank water averaging 90.5 ppb arsenic (Hall et al., 2007), whereas placental and cord blood arsenic levels from babies born in Argentina from an area with 200 ppb arsenic in drinking water were 9 μg/l in the cord blood and 34 μg/kg in the placenta (Concha et al., 1998). Thus, the arsenic levels used in our study are similar to those that a developing embryo might encounter.
Ninety-six drops of cell suspension were placed on the upturned inner surface of the lid of a 150-mm petri dish, which was inverted and placed on top of the dish containing 10ml of PBS, and incubated for 2 days (day 2). After 2 days, each individual drop was transferred to a 96-well ultralow attachment plate containing 70 μl of fresh differentiation medium with 0, 0.1, 0.5, or 1.0μM arsenite. After 3 days (day 5), the EBs were transferred to a 0.1% gelatin-coated 48-well plate containing 300 μl of fresh differentiation medium with 0, 0.1, 0.5, or 1.0μM sodium arsenite. The medium was renewed every 48h until cells were harvested.
qPCR. P19 cells were cultured with 0, 0.1, 0.5, or 1.0μM sodium arsenite as described above. When harvesting aggregates on day 5 culture, EBs from a 96-well plate were collected and combined as one replicate (n = 3 per group per day), whereas for day 9 collection, cells from all wells in a 48-well plate were trypsinized and combined as one replicate (n = 3 per group per day). Total RNA were extracted using TRI reagent (Sigma Aldrich, St Louis, MO). RNA (2 μg) was reverse transcribed into cDNA, and the expression of paired box 3 (Pax3), paired box 7 (Pax7), myogenic factor 5 (Myf5), MyoD, myogenin (Mgn), neurogenin 1, neurogenin 2, NeuroD, and GAPDH were quantified by qPCR using SYBR Green (SABiosciences, Frederick, MD) and the appropriate primers (Pax3 Forward: 5′-CCT CTG CCC AAC CAT ATC CG-3′, Reverse: 5′-GAA ATG ACG CAA GGC CGA ATG-3′; Pax7 Forward: 5′-TCT CCA AGA TTC TGT GCC GAT-3′, Reverse: 5′-CGG GGT TCT CTC TCT TAT ACT CC-3′; Myf5 Forward: 5′-TGC TGT TCT TTC GGG ACC AGA CAG G-3′, Reverse: 5′-GGA GAT CCT CAG GAA TGC CAT CCG C-3′; MyoD Forward: 5′-ATG CTG GAC AGG CAG TCG AGG C-3′, Reverse: 5′-GCT CTG ATG GCA TGA TGG ATT ACA-3′; Mgn Forward: 5′- CCA ACC CAG GAG ATC ATT TG-3′, Reverse: 5′- ACG ATG GAC GTA AGG GAG TG-3′; Neurogenin 1 Forward: 5′-CCA GCG ACA CTG AGT CCT G-3′, Reverse: 5′-CGG GCC ATA GGT GAA GTC TT-3′; Neurogenin 2 Forward: 5′-AAC TCC ACG TCC CCA TAC AG-3′, Reverse: 5′-GAG GCG CAT AAC GAT GCT TCT-3′). GAPDH was used as the housekeeping gene (GAPDH Forward: 5′-TGC GAC TTC AAC AGC AAC TC-3′, Reverse: 5′-ATG TAG GCC ATG AGG TCC AC-3′). Samples were run in triplicate. A standard curve was constructed for each gene to determine the number of molecules, which was normalized against GAPDH as a housekeeper. The experiments were replicated at least three times.
Immunohistochemistry and immunoblotting of differentiated cells. Because the qPCR analysis indicated that gene expression was consistently changed in the 0.5 and 1.0μM arsenite exposure groups, EBs exposed with or without 0.5μM sodium arsenite were prepared as described above for immunohistological analysis. On day 5, EBs were plated onto 10-cm tissue culture dishes containing 0.1% gelatin-coated coverslips. Medium renewal was conducted every 48h until the day for immunofluorescence (n = 5 per group per day). Cells were fixed with methanol at −20°C for 5min, blocked in 1% bovine serum albumin, 0.1% Triton-X100 in PBS, and incubated with the appropriate primary antibody for 1h (β-catenin: 1:100 dilution, Gene Tex no. GTX101254; Pax3: 1:200 dilution, Gene Tex no. GTX100663; MyoD: 1:100 dilution, Santa Cruz no. SC-304; Tuj1: 1:100 dilution, Millipore no. MAB1637; myosin heavy chain: 50 μl of antibody supernatant, DSHB no. MF20; and Nanog: 1:200 dilution, Gene Tex no. GTX100863). The secondary antibody (1 μg/ml) conjugated to Alexa Fluor 488 (Invitrogen, Carlsbad, CA) was incubated with the cells, which were counterstained with DAPI (Invitrogen). Cells were examined by conventional immunofluorescence on a Ti Eclipse Inverted Microscope (Nikon, Melville, NY).
Protein extractions and immunoblots were performed according to standard methods. When harvesting aggregates on day 5 culture, EBs from a 96-well plate were collected and combined as one replicate (n = 3 per group per day), whereas for day 12 collection, cells from all wells in a 48-well plate were trypsinized and combined as one replicate (n = 3 per group per day). The same primary antibodies were used as for the immunofluorescence (Tuji: 1:100 dilution; myosin heavy chain: 1:50 dilution). Anti-Tbp antibody (1:1000 dilution, Abcam no. 818) and anti-GAPDH antibody (1:1000 dilution, IMGENEX no. IMG-5019A-1) were used as loading controls.
Immunohistochemical analysis of EBs. EBs at day 2 and day 5 were exposed to 0 or 0.5μM arsenite as described above. For day 2 collection, 96 drops of cell suspension were harvested from a 150-mm petri dish combined as one replicate (n = 3 per group per day), whereas for EBs on day 5 culture, aggregates from a 96-well plate were collected and combined as one replicate (n = 3 per group per day). Once harvested, EBs were fixed for 24h in 10% neutral buffered formalin, dehydrated in graded ethanol, processed, and embedded in paraffin. Sections (5 μm) were placed on slides, deparaffinized with xylene, and rehydrated in graded ethanol solutions. β-Catenin, Pax3, and Nanog staining was carried out in a dark humid chamber for 1h, using anti-β-catenin antibody (1:100 dilution, Gene Tex no. GTX101254), anti-Pax3 antibody (1:200 dilution, Gene Tex no. GTX100663), and anti-Nanog antibody (1:200 dilution, Gene Tex no. GTX100863). The secondary antibody (1 μg/ml) conjugated to Alexa Fluor 488 (Invitrogen) was incubated with the slides, which were counterstained with DAPI (Invitrogen). Slides were examined by conventional immunofluorescence on a Ti Eclipse Inverted Microscope (Nikon).
RESULTS
Arsenic Represses Skeletal Muscle and Neuronal Differentiation in P19 Cells
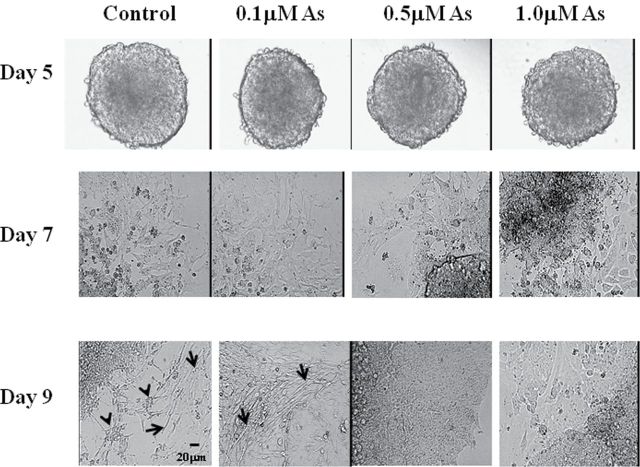
To examine the developmental effects of arsenic exposure on stem cell differentiation, EBs were formed from P19 cells exposed with 0, 0.1, 0.5, or 1.0μM sodium arsenite. There were no overt morphological differences in EB formation between the control and arsenic-exposed cells, and cellular outgrowth from the EBs could be seen on day 7 (Fig. 1). Myoblasts and myotubes, representing about 15% of the total cells, could be identified at day 9 (Fig. 1) in the control cells. These time frames and percentages are consistent with previous reports (McBurney, 1993). Interestingly, in our experiments, neurons were also formed at day 9 control cells (Fig. 1), representing 30–40% of the total cells by day 12 (data not shown). However, in the arsenic-exposed cells, the formation of myoblasts, myotubes, and neurons was significantly reduced. Although some cells differentiated in the 0.1μM exposure group, the majority of the outgrowing cells in the 0.5 and 1.0μM exposures retained an undifferentiated cell-like morphology (Fig. 1), whereas a small percentage of the total cell numbers in these two groups remained as EBs and did not show any signs of outward proliferation and/or differentiation (data not shown).
FIG. 1.
Arsenic represses skeletal muscle and neuronal differentiation in P19 cells. P19 cells were aggregated as hanging drop cultures with 0, 0.1, 0.5, or 1.0μM sodium arsenite for 5 days, and then transferred to gelatin-coated plates. On day 7, myoblast-like cells were observed around the EBs, whereas on day 9, myoblasts, myotubes, and neurons could be identified. Arrows show myotubes and arrowheads indicate the differentiated neurons.
Arsenic Represses β-Catenin Expression in the Differentiating EBs
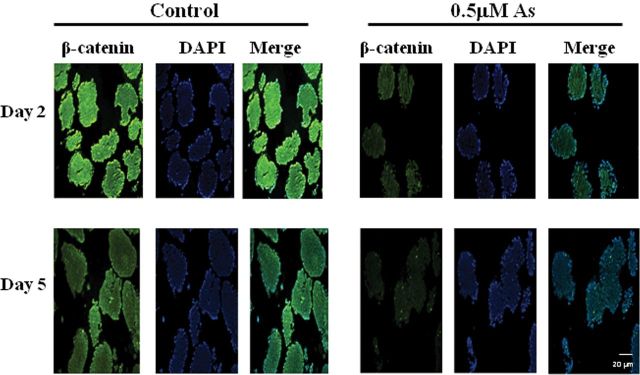
Because Wnt/β-catenin signaling plays an important role in the induction of early myogenesis and neurogenesis, the expression of β-catenin during EB formation was examined by immunofluorescence. In the control group of EBs, β-catenin was highly expressed on day 2 and expression levels were reduced on day 5 (Figs. 2A and 2C). However, in arsenic-treated EBs, the expression of β-catenin was much lower than controls at both day 2 and day 5 (Figs. 2B and 2D).
FIG. 2.
Arsenic represses β-catenin expression in the differentiating EBs. P19 cells were aggregated for 2 and 5 days with (A and C) or without (B and D) 0.5μM sodium arsenite. EBs were harvested and embedded with paraffin, and β-catenin expression was examined by immunofluorescence. Pictures are representative examples from 198 EBs per time point per group.
Arsenic Represses Pax3 Expression in the Exterior Regions of EBs
During embryogenesis, Pax3 is an essential transcription factor that regulates skeletal muscle and neuronal differentiation by targeting myogenic and neurogenic transcription factors (Ridgeway et al., 2000). Moreover, it has been shown that Wnt/β-catenin activates Pax3 expression in P19 cells (Marikawa et al., 2009; Petropoulos and Skerjanc, 2002). To this end, we examined whether repressed β-catenin expression in arsenic-exposed EBs lead to reduced Pax3 expression. In control cells, Pax3 was highly expressed in the EBs on day 2 and day 5 (Fig. 3A) and was also expressed in the cells differentiating out from EBs on day 9 (Fig. 3A). However, in arsenic-exposed group, Pax3 expression was repressed in the EBs on day 2 and day 5. And, Pax3 protein was almost absent in the day 9 cells proliferating out from the EBs in the arsenic-exposed group (Fig. 3A). qPCR corroborated the immunofluorescence, showing a significant reduction in Pax3 transcripts in the 0.1, 0.5, and 1.0μM arsenic-treated groups on day 5 (1.2-, 1.4-, and 1.7-fold reduction, respectively) and on day 9 (2.9-, 4.6-, and 4.2-fold reduction, respectively) (Fig. 3B).
FIG. 3.
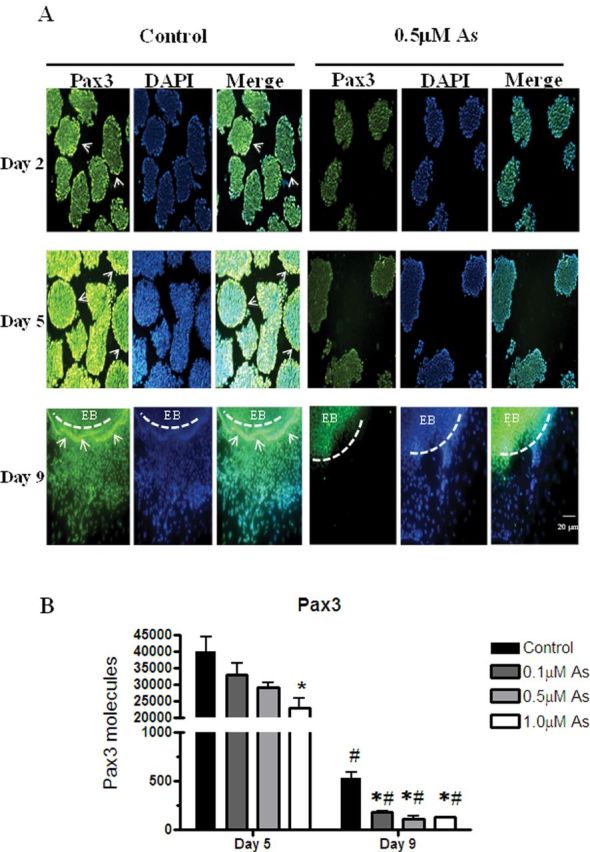
Arsenic represses Pax3 expression in the exterior regions of EBs. P19 cells were aggregated in the presence or absence of 0.5μM sodium arsenite for 5 days, then transferred to gelatin-coated plates for 4 days (day 9) with or without 0.5μM sodium arsenite to examine Pax3 expression by immunofluorescence (A). Arrows indicates the exterior regions of EBs with induced Pax3 expression. Dashed lines indicate the location of an EB. Pictures are representative examples from 198 independent EBs per time point per group. Pax3 mRNA expression on day 5 and day 9 from cells exposed to 0, 0.1, 0.5, or 1.0μM sodium arsenite was quantified by qPCR. Each sample was run in triplicate (n = 3), results were normalized to GAPDH, and are expressed as the average normalized number of molecules per 100ng cDNA. Statistical differences were determined by ANOVA followed by Tukey’s post hoc test (p < 0.05) to determine statistical differences between concentrations (*) and between days (#) (B).
Arsenic Represses Skeletal Muscle Differentiation by Reducing the Expression of Myogenic Regulatory Factors
The development of skeletal muscle is regulated by several myogenic transcription factors, including Myf5 and MyoD, which direct the differentiation of muscle progenitors to myoblasts, and then myogenin, which differentiates myoblasts into myotubes (recently reviewed in Yokoyama and Asahara [2011]). Thus, the expression Myf5, MyoD, and myogenin from P19 cells exposed with or without arsenic during differentiation was examined. In the control groups, Myf5 and MyoD mRNA transcripts were expressed on day 5 and then were diminished on day 9, with the Myf5 levels reduced to a much greater extent, as would be expected (Fig. 4A). The expression of myogenin was barely detectable on day 5, but it was induced by 11-fold on day 9 in the control cells (Fig. 4A). In the arsenic-exposed cells, the expression of Myf5 was not changed in the EBs at day 5, but was reduced by day 9 cells by ~4.5-fold in the 0.5 and 1.0μM groups. MyoD expression was reduced in a dose-responsive manner in response to arsenite exposure at both day 5 and day 9. Myogenin expression in the differentiating cells on day 9 was significantly repressed by 10.5-, 80-, and 135-fold in the cells exposed to 0.1, 0.5, and 1.0μM arsenite, respectively (Fig. 4A). To confirm these changes, MyoD protein levels were examined on day for the control and 0.5μM arsenite groups. These immunofluorescence studies indicated that MyoD expression was highly repressed in cells exposed to arsenic on day 9 (Fig. 4B). The reductions in these 3 myogenic transcription factors resulted in suppressed skeletal myotube formation, as indicated by reduced myosin heavy chain expression on day 12 in the cells exposed to 0.5μM arsenite, as seen by both immunofluorescence (Fig. 4C) and immunoblotting (Fig. 4D).
FIG. 4.
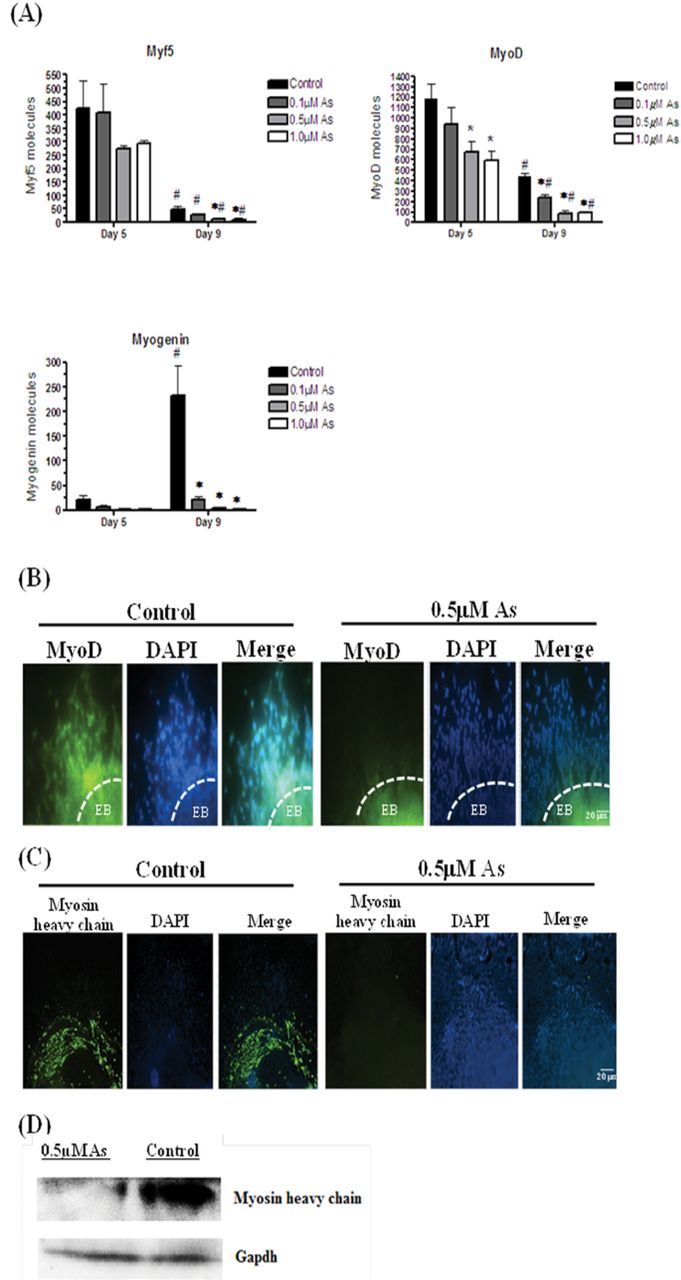
Arsenic represses skeletal muscle differentiation through the repressed myogenic regulatory factors. Myf5, MyoD, and myogenin mRNA expression on day 5 and day 9 of cells derived from EBs after exposure to 0, 0.1, 0.5, and 1.0μM arsenite were quantified by qPCR. Each sample was run in triplicate (n = 3), results were normalized to GAPDH, and are expressed as the number of molecules per 100ng cDNA. ANOVA followed by Tukey’s post hoc test (p < 0.05) was performed to determine statistical differences between concentrations (*) and between days (#) (A). Differentiated cells from EBs exposed with or without 0.5μM arsenite on day 9 were fixed and MyoD expression was examined by immunofluorescence (B). Cells grown out to day 12 were fixed and myosin heavy chain expression was examined by immunofluorescence (C). Dashed lines indicate the location of an EB. Pictures are representative examples from 198 independent EBs per time point per group. Myosin heavy chain protein expression was examined by immunoblotting, using EBs exposed with or without 0.5μM arsenic on day 12. GAPDH was used as a loading control (D).
Arsenic Represses Neuronal Differentiation by Reducing the Expression of Neurogenic Transcription Factors
Neurogenin 1 and neurogenin 2 are transcription factors that are expressed in neuronal precursor cells, whereas NeuroD is the transcription factor that promotes neuronal differentiation into sensory neurons (Howard, 2005). In control cells, the expression of neurogenin 1 and neurogenin 2 mRNA was at moderate levels in day 5 EBs, and was then increased in day 9 cells. In contrast, NeuroD expression was not detectable in day 5 EBs, but dramatically increased in the day 9 cells (Fig. 5A). Arsenite exposure reduced the expression of neurogenin 1 in a dose-responsive manner on both days 5 and 9. Neurogenin 2 expression was not changed in the day 5 cells, but again, arsenite reduced its expression by 2.2-, 3.1-, and 3.8-fold in the 0.1, 0.5, and 1.0μM arsenite groups, respectively. Finally, arsenic exposure reduced NeuroD expression by twofold in the 0.1μM arsenite-exposed cells, 37-fold in the 0.5μM exposed cells, and 125-fold in the 1.0μM exposed cells (Fig. 5A). The reductions in neurogenin 1, neurogenin 2, and NeuroD transcripts when cells are exposed to arsenite likely decreased the formation of sensory neurons, as shown in Figure 1. Indeed, the protein expression of the neuron-specific tubulin Tuj1 in day 12 cells is reduced in the 0.5μM arsenite-exposed cells, as seen by both immunofluorescence (Fig. 5B) and immunoblotting (Fig. 5C).
FIG. 5.
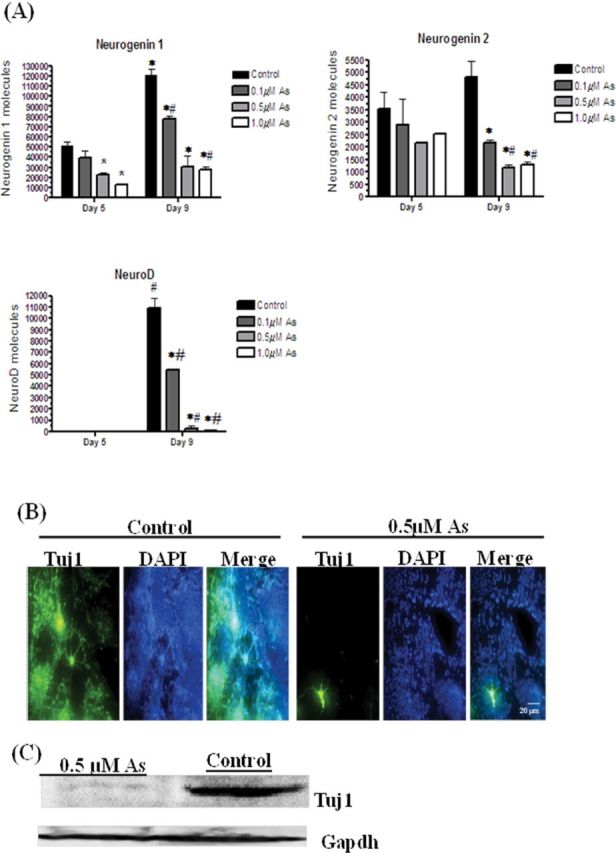
Arsenic inhibits neuronal differentiation by repressing neurogenic transcription factors. Neurogenin 1, neurogenin 2, and NeuroD mRNA expression on day 5 and day 9 of cells derived from EBs after exposure to 0, 0.1, 0.5, and 1.0μM arsenite were quantified by qPCR. Each sample was run in triplicate (n = 3), results were normalized to GAPDH, and are expressed as the number of molecules per 100ng cDNA. ANOVA followed by Tukey’s post hoc test (p < 0.05) was performed to determine statistical differences between concentrations (*) and between days (#) (A). Differentiated control cells and cells exposed to 0.5μM arsenite were fixed on day 12, and Tuj1 expression was examined by immunofluorescence (B). Pictures are representative examples from 198 independent EBs/time point/group. Tuj1 protein expression was examined by immunoblotting, using EBs exposed with or without 0.5μM arsenic on day 12, with GAPDH used as a loading control (C).
Arsenic Increases Nanog Expression
In mammals, Nanog is an essential transcription factor that maintains the pluripotency of stem cells (Mitsui et al., 2003). Many of the outgrowing cells in the arsenite exposures had a morphology similar to P19 cells (Fig. 1), and were not readily differentiating into myoblasts or neuronal cells. Thus, the effects of arsenite exposure on Nanog transcript levels and protein expression were examined. The qPCR study indicates that Nanog transcripts were increased by 1.4-fold in the 0.5 μΜ EBs and by 1.9-fold 1.0μM EBs (Fig. 6A). Immunofluorescence staining indicated that Nanog was expressed in both control and 0.5μM arsenic-exposed EBs at day 5, with its expression being somewhat higher in the arsenic-exposed group (Fig. 6B). However, by days 9 and 12, Nanog is highly expressed in the cells proliferating out from EBs in the arsenite exposure group, whereas little Nanog is present in the control cells (Fig. 6B).
FIG. 6.
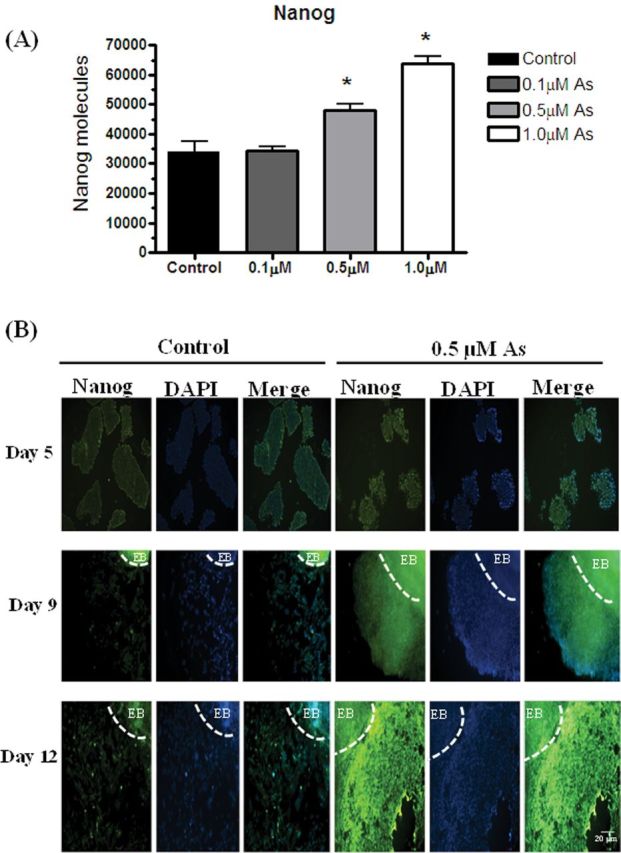
Arsenic increases Nanog expression during differentiation. Nanog mRNA expression in day 5 EBs after exposure to 0, 0.1, 0.5, and 1.0μM arsenite was quantified by qPCR. Each sample was run in triplicate (n = 3), results were normalized to GAPDH, and are expressed as the number of Nanog molecules per 100ng cDNA. ANOVA followed by Tukey’s post hoc test (p < 0.05) was performed to determine statistical differences (*) (A). P19 cells, cultured with or without 0.5μM sodium arsenite, were aggregated for 5 days, and then differentiated out to day 9 and 12. EBs were harvested on day 5 and embedded with paraffin, whereas differentiated cells were fixed on days 9 and 12. Nanog expression was examined by immunofluorescence. Dashed lines indicate the location of an EB. Pictures are representative examples (B).
DISCUSSION
The results from this study using P19 stem cells illustrate that sodium arsenite suppresses skeletal muscle and neuronal differentiation through repressed β-catenin expression. Lowered β-catenin levels leads to reductions in the specific transcription factors needed to convert stem cells into neurons and skeletal myotubes (Fig. 7). Arsenic is known to affect other signaling pathways. For example, arsenic exposure can cause apoptosis in neuronal cells via the nuclear factor-kappa B and mitogen-activated protein kinase (MAPK) pathways (Felix et al., 2005; Yen et al., 2011), can inhibit neuronal outgrowth via the LKB1-AMPK signaling pathway at 3μM arsenite (Wang et al., 2010), and inhibits myocyte differentiation and muscle regeneration via the AKT pathway (Yen et al., 2010). However, these studies were conducted in neuronal and muscle progenitor cells. In stem cells, it appears that one potential mechanism by which low levels of arsenic can repress stem cell differentiation is via the Wnt/β-catenin pathway.
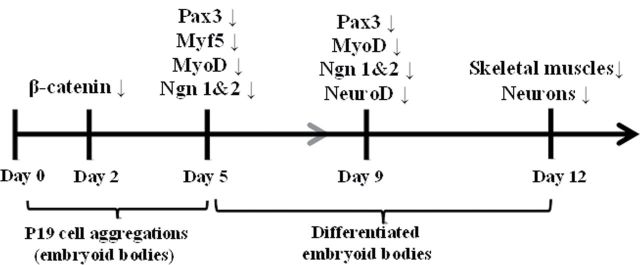
FIG. 7.
Model of the transcription factor cascade that inhibits skeletal myotube and sensory neuron formation after arsenite exposure. During P19 aggregation, β-catenin expression was repressed by arsenic exposure on day 2. The repressed β-catenin leads to the reduction of Pax3, which plays an important role in specification of muscle and neuronal precursors. Pax3 regulates Myf5, MyoD, neurogenin 1, and neurogenin 2, all of which were down-regulated by arsenite exposure expression on day 5 and day 9. The expression of myogenin and NeuroD, which regulate terminal skeletal and neuronal differentiation, was reduced by arsenic on day 9, which therefore leads to the suppressed formation of skeletal muscles and neurons on day 12.
To induce P19 differentiation, DMSO has long been used as a chemical reagent for the induction of mesoderm and endoderm lineages (McBurney, 1993). DMSO can induce neuronal differentiation in neuroblastoma cells (Kimhi et al., 1976), but this has not been reported in P19 cells. Neurogenin 1 overexpression in P19 cells appears to be sufficient for inducing neurons (Kim et al., 2004), even in the absence of retinoic acid. In this study, both neurogenin 1 and neurogenin 2 transcripts were seen during EB formation. Previous studies have demonstrated that coculturing DMSO-exposed P19 EBs with the primitive streak mesoderm-like germinal center B cells (GCLB) could induce neuronal cell differentiation in P19 cells, which was hypothesized to be due to the synergistic action of a Pax3 signal from the GCLB cells and the DMSO treatment (Pruitt, 1994). It is known that Pax3 promotes sensory neuron differentiation in mammals (Koblar et al., 1999) and Pax3 can be induced when P19 cells treated with DMSO (Petropoulos and Skerjanc, 2002). Therefore, it is reasonable to expect that DMSO-induced Pax3 can trigger neurogenin expression in P19 cells.
Immunofluorescence analysis of sectioned EBs indicates that β-catenin was expressed on day 2 in the control groups. On day 5, the β-catenin levels were greatly reduced after exposure to 0.5μM arsenite, but are not completely knocked down. Such temporal patterns of β-catenin expression are consistent with previous reports using P19 cells. For example, the level of Wnt/β-catenin was maximal on day 2 of P19 cell differentiation into mesodermal cells, and its expression started to decrease on day 3 (Marikawa et al., 2009). In mice, it has been shown that β-catenin is necessary for embryonic development but no longer required for fetal development, such as the differentiation of myoblasts (Hutcheson et al., 2009). Although it is not completely absent as in a knockout mouse model, the repressed β-catenin expression in our P19 cells exposed to arsenite indicates that Wnt signaling was likely repressed in the early stage of EB development. This may be the initial regulatory mechanism responsible for the reduced skeletal muscle and neuronal cell formation on day 9 after arsenic exposure. Indeed, it has been shown that β-catenin can directly activate Myf5, as two β-catenin responsive elements in Myf5 promoter are essential for mouse myotomal development (Borello et al., 2006), and can also induce the expression of neurogenin 2 (Hirsch et al., 2007).
Recently, altered Wnt/β-catenin signaling has been reported after arsenic exposure. For example, 2μM arsenic trioxide reduces cytoplasmic β-catenin accumulation and induces apoptosis in human primary myeloma cells (Zhou et al., 2008), whereas 2.5–10μM arsenic trichloride-induced reactive oxygen species (ROS) formation promotes cell transformation and tumorigenesis through the induction of Wnt/β-catenin signaling in human colorectal adenocarcinoma DLD1 cells (Zhang et al., 2011). Interestingly, ROS-mediated β-catenin reduction can be regulated by overexpressing nucleoredoxin, which is a thioredoxin family member, in HEK293 cells and in Xenopus (Funato and Miki, 2010). Because arsenic toxicity has been related to the induction of ROS (Piao et al., 2005), arsenic-induced ROS may play a role in the reduction of β-catenin expression in P19 cells.
During differentiation, β-catenin signaling triggers myogenesis and neurogenesis through Pax3 (Petropoulos and Skerjanc, 2002; Ridgeway et al., 2000). In the arsenite-exposed EBs, Pax3 transcript levels were lowered on day 5, and its protein expression was lowered on both day 2 and day 5, likely due to reduced β-catenin levels. It is interesting that the spatial expression of Pax3 differed between control and arsenite-exposed cells. In control cells, Pax3 is highly expressed at the edges of the EBs and is later expressed in the differentiating cells on day 9. In the arsenic-exposed cells, Pax3 is expressed in the interior of the EB, but its expression is extremely low at the edges of EBs. By day 9, Pax3 expression is almost absent in the differentiated regions from arsenic-exposed EBs. In contrast, on day 9, Pax3 is expressed in the differentiated and differentiating outgrowing cells where the skeletal myotubes and neurons are formed on day 9 and day 12. This reduced expression is also confirmed by qPCR data.
In the nervous system, Pax3 upregulates neurogenin 2 that plays a key role in the specification of neuronal subtypes, neural crest development, and sensory neurogenesis (Nakazaki et al., 2008). In skeletal muscle progenitors, Pax3 upregulates Myf5, MyoD, and myogenin (reviewed in Buckingham, 2007). As a result of the reductions in Pax3 expression, these key transcription factors for muscle and neuronal differentiation were also repressed by arsenic exposure in this study in a dose-responsive manner. Moreover, Pax7, which has also been suggested to play a key role in muscle and neuronal cell fate determination in mice (Jostes et al., 1990), is reduced by 3.2-, 8.2-, and 4.6-fold in the 0.1, 0.5, and 1.0μM arsenite-exposed cells on day 9 of EB differentiation (data not shown). In contrast, Nanog expression, which maintains the pluripotency of stem cells (Mitsui et al., 2003), is between 1.4- and 1.9-fold higher in the arsenic-exposed cells. These results indicate that, rather than undergoing skeletal and neuronal lineages, the majority of arsenic-exposed cells were maintained in the self-renewal status, thereby leading to the reduced formation of skeletal muscles and neurons.
In conclusion, our results indicate that 0.5μM sodium arsenite suppresses skeletal muscle and neuronal formation from P19 mouse embryonic stem cells likely due to repressed Wnt/β-catenin signaling, which leads to reductions in myogenic and neurogenic transcription factor expression. This study provides insights into how arsenic exposure affects skeletal and neuronal differentiation during embryogenesis, and highlights the utility of using embryonic stem cells to assess cell fate determination.
FUNDING
National Institutes of Health (ES016640 and ES016640-01S1 to L.J.B.).
Acknowledgments
We thank Terry Bruce and Nancy Korn for their help with the immunofluoresence assays.
References
- Borello U.,, Berarducci B.,, Murphy P.,, Bajard L.,, Buffa V.,, Piccolo S.,, Buckingham M.,, Cossu G. (2006).. The Wnt/beta-catenin pathway regulates Gli-mediated Myf5 expression during somitogenesis. Development 133, 3723––3732 [DOI] [PubMed] [Google Scholar]
- Buckingham M. (2007).. Skeletal muscle progenitor cells and the role of Pax genes. C. R. Biol. 330, 530––533 [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S.,, Bhaumik S.,, Purkayastha M.,, Basu S.,, Nag Chaudhuri A.,, Das Gupta S. (2002).. Apoptosis and necrosis in developing brain cells due to arsenic toxicity and protection with antioxidants. Toxicol. Lett. 136, 65––76 [DOI] [PubMed] [Google Scholar]
- Concha G.,, Vogler G.,, Lezcano D.,, Nermell B.,, Vahter M. (1998).. Exposure to inorganic arsenic metabolites during early human development. Toxicol. Sci. 44, 185––190 [DOI] [PubMed] [Google Scholar]
- Dakeishi M.,, Murata K.,, Grandjean P. (2006).. Long-term consequences of arsenic poisoning during infancy due to contaminated milk powder. Environ. Health 5, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix K.,, Manna S. K.,, Wise K.,, Barr J.,, Ramesh G. T. (2005).. Low levels of arsenite activates nuclear factor-kappaB and activator protein-1 in immortalized mesencephalic cells. J. Biochem. Mol. Toxicol. 19, 67––77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora S. J., Mehta A. (2009).. Monoisoamyl dimercaptosuccinic acid abrogates arsenic-induced developmental toxicity in human embryonic stem cell-derived embryoid bodies: Comparison with in vivo studies. Biochem. Pharmacol. 78, 1340––1349 [DOI] [PubMed] [Google Scholar]
- Funato Y., Miki H. (2010).. Redox regulation of Wnt signalling via nucleoredoxin. Free Radic. Res. 44, 379––388 [DOI] [PubMed] [Google Scholar]
- García-Chávez E.,, Segura B.,, Merchant H.,, Jiménez I.,, Del Razo L. M. (2007).. Functional and morphological effects of repeated sodium arsenite exposure on rat peripheral sensory nerves. J. Neurol. Sci. 258, 104––110 [DOI] [PubMed] [Google Scholar]
- Geetha-Loganathan P.,, Nimmagadda S.,, Scaal M.,, Huang R.,, Christ B. (2008).. Wnt signaling in somite development. Ann. Anat. 190, 208––222 [DOI] [PubMed] [Google Scholar]
- Hall M.,, Gamble M.,, Slavkovich V.,, Liu X.,, Levy D.,, Cheng Z.,, van Geen A.,, Yunus M.,, Rahman M.,, Pilsner J. R.,, et al. (2007).. Determinants of arsenic metabolism: Blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environ. Health Perspect. 115, 1503––1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch C.,, Campano L. M.,, Wöhrle S.,, Hecht A. (2007).. Canonical Wnt signaling transiently stimulates proliferation and enhances neurogenesis in neonatal neural progenitor cultures. Exp. Cell Res. 313, 572––587 [DOI] [PubMed] [Google Scholar]
- Howard M. J. (2005).. Mechanisms and perspectives on differentiation of autonomic neurons. Dev. Biol. 277, 271––286 [DOI] [PubMed] [Google Scholar]
- Hutcheson D. A.,, Zhao J.,, Merrell A.,, Haldar M.,, Kardon G. (2009).. Embryonic and fetal limb myogenic cells are derived from developmentally distinct progenitors and have different requirements for beta-catenin. Genes Dev. 23, 997––1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.,, Xi S.,, Li X.,, Lu C.,, Li G.,, Xu Y.,, Qu C.,, Niu Y.,, Sun G. (2006).. Arsenic speciation transported through the placenta from mother mice to their newborn pups. Environ. Res. 101, 349––355 [DOI] [PubMed] [Google Scholar]
- Jostes B.,, Walther C.,, Gruss P. (1990).. The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech. Dev. 33, 27––37 [DOI] [PubMed] [Google Scholar]
- Kim S.,, Yoon Y. S.,, Kim J. W.,, Jung M.,, Kim S. U.,, Lee Y. D.,, Suh-Kim H. (2004).. Neurogenin1 is sufficient to induce neuronal differentiation of embryonal carcinoma P19 cells in the absence of retinoic acid. Cell Mol. Neurobiol. 24, 343––356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhi Y.,, Palfrey C.,, Spector I.,, Barak Y.,, Littauer U. Z. (1976).. Maturation of neuroblastoma cells in the presence of dimethylsulfoxide. Proc. Natl. Acad. Sci. U.S.A. 73, 462––466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblar S. A.,, Murphy M.,, Barrett G. L.,, Underhill A.,, Gros P.,, Bartlett P. F. (1999).. Pax-3 regulates neurogenesis in neural crest-derived precursor cells. J. Neurosci. Res. 56, 518––530 [DOI] [PubMed] [Google Scholar]
- Kultima K.,, Jergil M.,, Salter H.,, Gustafson A. L.,, Dencker L.,, Stigson M. (2010).. Early transcriptional responses in mouse embryos as a basis for selection of molecular markers predictive of valproic acid teratogenicity. Reprod. Toxicol. 30, 457––468 [DOI] [PubMed] [Google Scholar]
- Ling L.,, Nurcombe V.,, Cool S. M. (2009).. Wnt signaling controls the fate of mesenchymal stem cells. Gene 433, 1––7 [DOI] [PubMed] [Google Scholar]
- Lyashenko N.,, Winter M.,, Migliorini D.,, Biechele T.,, Moon R. T.,, Hartmann C. (2011).. Differential requirement for the dual functions of ß-catenin in embryonic stem cell self-renewal and germ layer formation. Nat. Cell Biol. 13, 753––761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikawa Y.,, Tamashiro D. A.,, Fujita T. C.,, Alarcón V. B. (2009).. Aggregated P19 mouse embryonal carcinoma cells as a simple in vitro model to study the molecular regulations of mesoderm formation and axial elongation morphogenesis. Genesis 47, 93––106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney M. W. (1993).. P19 embryonal carcinoma cells. Int. J. Dev. Biol. 37, 135––140 [PubMed] [Google Scholar]
- Mitsui K.,, Tokuzawa Y.,, Itoh H.,, Segawa K.,, Murakami M.,, Takahashi K.,, Maruyama M.,, Maeda M.,, Yamanaka S. (2003).. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113, 631––642 [DOI] [PubMed] [Google Scholar]
- Nakazaki H.,, Reddy A. C.,, Mania-Farnell B. L.,, Shen Y. W.,, Ichi S.,, McCabe C.,, George D.,, McLone D. G.,, Tomita T.,, Mayanil C. S. (2008).. Key basic helix-loop-helix transcription factor genes Hes1 and Ngn2 are regulated by Pax3 during mouse embryonic development. Dev. Biol. 316, 510––523 [DOI] [PubMed] [Google Scholar]
- Otero J. J.,, Fu W.,, Kan L.,, Cuadra A. E.,, Kessler J. A. (2004).. Beta-catenin signaling is required for neural differentiation of embryonic stem cells. Development 131, 3545––3557 [DOI] [PubMed] [Google Scholar]
- Petropoulos H., Skerjanc I. S. (2002).. Beta-catenin is essential and sufficient for skeletal myogenesis in P19 cells. J. Biol. Chem. 277, 15393––15399 [DOI] [PubMed] [Google Scholar]
- Piao F.,, Ma N.,, Hiraku Y.,, Murata M.,, Oikawa S.,, Cheng F.,, Zhong L.,, Yamauchi T.,, Kawanishi S.,, Yokoyama K. (2005).. Oxidative DNA damage in relation to neurotoxicity in the brain of mice exposed to arsenic at environmentally relevant levels. J. Occup. Health 47, 445––449 [DOI] [PubMed] [Google Scholar]
- Pruitt S. C. (1994).. Discrete endogenous signals mediate neural competence and induction in P19 embryonal carcinoma stem cells. Development 120, 3301––3312 [DOI] [PubMed] [Google Scholar]
- Raqib R.,, Ahmed S.,, Sultana R.,, Wagatsuma Y.,, Mondal D.,, Hoque A. M.,, Nermell B.,, Yunus M.,, Roy S.,, Persson L. A.,, et al. (2009).. Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh. Toxicol. Lett. 185, 197––202 [DOI] [PubMed] [Google Scholar]
- Ridgeway A. G.,, Petropoulos H.,, Wilton S.,, Skerjanc I. S. (2000).. Wnt signaling regulates the function of MyoD and myogenin. J. Biol. Chem. 275, 32398––32405 [DOI] [PubMed] [Google Scholar]
- Rodríguez V. M.,, Carrizales L.,, Mendoza M. S.,, Fajardo O. R.,, Giordano M. (2002).. Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol. Teratol. 24, 743––750 [DOI] [PubMed] [Google Scholar]
- Schmidt C.,, McGonnell I.,, Allen S.,, Patel K. (2008).. The role of Wnt signalling in the development of somites and neural crest. Adv. Anat. Embryol. Cell Biol. 195, 1––64 [DOI] [PubMed] [Google Scholar]
- Steffens A. A.,, Hong G. M.,, Bain L. J. (2011).. Sodium arsenite delays the differentiation of C2C12 mouse myoblast cells and alters methylation patterns on the transcription factor myogenin. Toxicol. Appl. Pharmacol. 250, 154––161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummann T. C.,, Hareng L.,, Bremer S. (2008).. Embryotoxicity hazard assessment of cadmium and arsenic compounds using embryonic stem cells. Toxicology 252, 118––122 [DOI] [PubMed] [Google Scholar]
- Tsai S. Y.,, Chou H. Y.,, The H. W.,, Chen C. M.,, Chen C. J. (2003).. The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology 24, 747––753 [DOI] [PubMed] [Google Scholar]
- von Ehrenstein O. S.,, Guha Mazumder D. N.,, Hira-Smith M.,, Ghosh N.,, Yuan Y.,, Windham G.,, Ghosh A.,, Haque R.,, Lahiri S.,, Kalman D.,, et al. (2006).. Pregnancy outcomes, infant mortality, and arsenic in drinking water in West Bengal, India. Am. J. Epidemiol. 163, 662––669 [DOI] [PubMed] [Google Scholar]
- Wang X.,, Meng D.,, Chang Q.,, Pan J.,, Zhang Z.,, Chen G.,, Ke Z.,, Luo J.,, Shi X. (2010).. Arsenic inhibits neurite outgrowth by inhibiting the LKB1-AMPK signaling pathway. Environ. Health Perspect. 118, 627––634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Yang P. (2008).. In vitro differentiation of mouse embryonic stem (mES) cells using the hanging drop method. J. Vis. Exp. 17, e825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi S.,, Guo L.,, Qi R.,, Sun W.,, Jin Y.,, Sun G. (2010).. Prenatal and early life arsenic exposure induced oxidative damage and altered activities and mRNA expressions of neurotransmitter metabolic enzymes in offspring rat brain. J. Biochem. Mol. Toxicol. 24, 368––378 [DOI] [PubMed] [Google Scholar]
- Yen C. C.,, Ho T. J.,, Wu C. C.,, Chang C. F.,, Su C. C.,, Chen Y. W.,, Jinn T. R.,, Lu T. H.,, Cheng P. W.,, Su Y. C.,, et al. (2011).. Inorganic arsenic causes cell apoptosis in mouse cerebrum through an oxidative stress-regulated signaling pathway. Arch. Toxicol. 85, 565––575 [DOI] [PubMed] [Google Scholar]
- Yen Y. P.,, Tsai K. S.,, Chen Y. W.,, Huang C. F.,, Yang R. S.,, Liu S. H. (2010).. Arsenic inhibits myogenic differentiation and muscle regeneration. Environ. Health Perspect. 118, 949––956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Asahara H. (2011).. The myogenic transcriptional network. Cell Mol. Life Sci. 68, 1843––1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.,, Wang X.,, Cheng S.,, Sun L.,, Son Y. O.,, Yao H.,, Li W.,, Budhraja A.,, Li L.,, Shelton B. J.,, et al. (2011).. Reactive oxygen species mediate arsenic induced cell transformation and tumorigenesis through Wnt/ß-catenin pathway in human colorectal adenocarcinoma DLD1 cells. Toxicol. Appl. Pharmacol. 256, 114––121 [DOI] [PubMed] [Google Scholar]
- Zhou L.,, Hou J.,, Fu W.,, Wang D.,, Yuan Z.,, Jiang H. (2008).. Arsenic trioxide and 2-methoxyestradiol reduce beta-catenin accumulation after proteasome inhibition and enhance the sensitivity of myeloma cells to Bortezomib. Leuk. Res. 32, 1674––1683 [DOI] [PubMed] [Google Scholar]