Abstract
The presence of toxic amounts of transition metals in the environment may originate from a range of human activities and natural processes. One method for the removal of toxic levels of metals is through chelation by small molecules. However, chelation is not synonymous with detoxification and may not affect the bioavailability of the metal. To test the bioavailability of chelated metals in vivo, the effects of several metal/chelator combinations were tested in the environmentally relevant organism Caenorhabditis elegans. The effect of metal exposure on nematode growth was used to determine the toxicity of cadmium, copper, nickel, and zinc. The restoration of growth to levels observed in nonexposed nematodes was used to determine the protective effects of the polydentate chelators: acetohydroxamic acid (AHA), cyclam, cysteine, calcium EDTA, desferrioxamine B, 1,2-dimethyl,3-hydroxy,4-pyridinone, and histidine. Cadmium toxicity was removed only by EDTA; copper toxicity was removed by all of the chelators except AHA; nickel toxicity was removed by cyclam, EDTA, and histidine; and zinc toxicity was removed by only EDTA. These results demonstrate the utility of polydentate chelators in the remediation of metal-contaminated systems. They also demonstrate that although the application of a chelator to metal contaminants may be effective, binding alone cannot be used to predict the level of remediation. Remediation depends on a number of factors, including metal complex speciation in the environment.
Key Words: C. elegans, cadmium, copper, zinc, nickel, chelator, remediation
A number of transition metal ions are essential to biological processes. A deficiency of a metal will result in the inability of the organism to perform important biological processes ranging from small molecule transport (e.g., iron in hemoglobin and copper in hemocyanin) to epigenetic regulation of gene expression (e.g., zinc in histone deacetylase) (Silva and Williams, 2001). Exposure of organisms to an excess of metals from environmental sources can result in toxicity due to undesirable reactions including oxidative damage to biomolecules, blocking of ion channels, and cross-linking of DNA. Organisms have developed stress responses to protect against metal toxicity or to remove metals from the system. However, when those stress responses are overwhelmed, the organism will suffer from metal toxicity. It is important to understand the toxicity of metals due to the prevalence of transition metals in a variety of products and industrial processes.
Exposure of organisms to toxic metals from environmental sources is a major topic of concern. The release of toxic metals including arsenic, cadmium, and chromium into the environment from residential and industrial sources can result in contamination that leads to adverse health effects in humans, due to both direct exposure and bioaccumulation in lower organisms. Remediation of metal-contaminated sites is necessary to protect humans from exposure and can be undertaken by a number of methods depending on the type of contamination. Most remediation techniques for metals involve mechanically or chemically immobilizing the contaminant. However, most current techniques are expensive and time consuming. The development of novel, more effective remediation techniques can improve efficiency and protection and decrease the cost of remediation efforts.
One potential route of remediation of toxic metal contamination in the environment is by immobilization or chelation of the metal. The effectiveness of remediation techniques involving chelation can be related to a number of factors involving the interaction between the target metal and the chelating agent. First, the chelating agent must bind the metal more strongly than any competing chelator in the environmental system. The strength of binding is expressed through the metal complex stability equilibrium constant, log β (Martell and Hancock, 1996). Complexes with higher log β values form more stable complexes in aqueous solutions. The log β value is also indicative of the ability of chelators to interact with metals that have been adsorbed to mineral surfaces, such as would be observed in the case of environmental contamination by toxic metals. In systems where multiple chelators are competing to bind a metal, the concentration of both chelators will be central to determining which complex will be predominant. An alternative expression of complex stability is the pM value (Harris et al., 1979). This value represents the free uncomplexed metal ion concentration in the presence of a chelator at an arbitrary set of conditions, including concentration and pH.
One advantage of using pM values to measure a chelator’s affinity for a specific metal is that it provides a measure of the free uncomplexed metal ion, which is generally the most bioavailable form. For a remediation technique to be effective, the chelated form of the metal cannot be bioavailable to the organism. If the complex is bioavailable, then the metal may still be taken up by organisms and be potentially toxic (Wenger et al., 2005).
Chelating agents that have been developed for treatment of metal-based diseases in humans are potential candidates for such remediation strategies. For example, the iron-specific chelating agents Desferal, or desferrioxamine B (Supplementary figure S1), and Deferiprone, or 3-hydroxy-1,2-dimethylpyridin-4-one, are used in the treatment of iron overload in β-thalassemia patients (Faa and Crisponi, 1999; Galanello, 2007). Calcium EDTA (Supplementary figure S1) is currently in clinical use for treatment of lead and cadmium toxicity (Brown et al., 2006).
Caenorhabditis elegans is a small nematode whose anatomy and genetics have been extensively studied (Brenner, 1974; Riddle, 1997; The C. elegans Sequencing Consortium, 1998). It is viewed as a model for higher order organisms at the biomolecular level, expressing many similar responses to those observed in vertebrate systems (Freedman et al., 1993; Heschl and Baillie, 1990; Leung et al., 2008). It is also useful as a model system for observing toxic responses in biological organisms due to its relatively short life cycle, its fully characterized biology, and the ease of observing cells within the living organism by light microscopy. In addition to these practical benefits, C. elegans is a soil-based organism, so its response to remediation strategies can serve as an indicator of how soil remediation techniques would affect toxic metal bioavailability in the environment (Boyd and Williams, 2003).
A number of criteria can be used to investigate the toxicity of chemicals to C. elegans, including growth, reproductive rate, feeding, and locomotion. Medium- and high-throughput methods using the COPAS Biosort flow sorting system have been developed, allowing for the rapid evaluation of potential toxicants over a range of concentrations (Boyd et al., 2010). Based on Biosort data, mathematical models have been developed that describe the growth of C. elegans (Smith et al., 2009). This model has been used to determine the effect of the organophosphate pesticide chlorpyrifos on the organism’s larval development (Boyd et al., 2009).
In this study, the growth assay and mathematical model were used to investigate the effects of transition metals, cadmium, copper, nickel, and zinc, on C. elegans. The protective effects of the chelating agents; desferrioxamine B, 3-hydroxy-1,2-dimethylpyridin-4-one (DMHP), and acetohydroxamic acid (AHA), iron-specific chelators; cyclam, cysteine, and histidine, zinc and copper chelators; and calcium EDTA (CaEDTA), a stable chelator for a number of transition metal ions, were determined (Supplementary figure S1) (Chaube and Murphy, 1966; Chen et al., 2009; Faa and Crisponi, 1999). In addition, the toxicity of the chelators alone was assessed, as they may have undesirable toxic effects, possibly due to chelation of essential metals from the organism. The results of these studies provided information on which chelating agent was most appropriate for protecting against toxic effects associated with exposure to each type of metal.
MATERIALS AND METHODS
Nematode culture. The Bristol N2 (wild-type) strain of C. elegans was obtained from the Caenorhabditis Genetic Center (Minneapolis, MN) and maintained at 20°C on K-agar plates (2% bacto-agar, 0.25% bacto-peptone, 51mM sodium chloride, 32mM potassium chloride, 13µM cholesterol) seeded with E. coli OP50 (Brenner, 1974; Williams and Dusenbery, 1988). Age-synchronized adult nematodes were prepared as previously described (Khanna et al., 1997).
Materials. All solutions were prepared in K medium (Williams and Dusenbery, 1990) (51mM sodium chloride, 32mM potassium chloride). Zinc chloride (97%), anhydrous nickel chloride (98%), anhydrous cadmium chloride (99.99+%), and cupric chloride dihydrate (99.999+%) were obtained from Sigma Aldrich and used as received to prepare metal solutions.
Desferrioxamine B mesylate salt (95%), deferiprone (DMHP [98%]), L-histidine (98%), AHA (98%), 1,4,7,11-tetrazacyclotetradecane (cyclam, 98%), and CaEDTA were all obtained from Sigma Aldrich and used as received to prepare chelator solutions. The pH was adjusted to between 5 and 8 for use in exposure growth assays using 0.10M NaOH or concentrated HCl.
Metal-chelator assay plate design. To test the ability of the chelators to protect C. elegans from the toxic effects of the metals, 96-well plates were prepared containing chelator and untreated nematodes and metal-chelator mixtures. A diagram of plate design is included in the Supplementary figure S2. To provide a better comparison between the chelator treatment and the metal-chelator treatment, the same concentration of chelator was used for both conditions. For each metal, at least three replicate plates were prepared to ensure reproducibility between experiments.
Concentrations of metal ions used were chosen to ensure reproducible toxic responses in treated nematodes and were higher than the previously determined LC50 values (Williams and Dusenbery, 1990). The concentrations of metal ions used are accurate within two significant figures, as no standardization was performed upon addition of the metal solutions to the K medium. Speciation models were calculated for the complexes formed between the metal ions and the chelators to determine the concentration of chelator required for complete complex formation (see Supplemental Information). Speciation models were calculated using the program HySS 2006 and the literature values of the stability constants of the metal-chelator complexes taken from the IUPAC Stability Constant Database (Alderighi et al., 1999; Powell). If a complex was not predicted to form or if partial complex formation was predicted based on the thermodynamic simulations, the highest concentration that produced no toxic effect in C. elegans was used in exposure wells.
Growth and activity assays. For the growth and activity experiments, metal and chelator solutions were prepared in complete K medium (K medium plus, 3mM calcium chloride, 3mM magnesium sulfate, 13µM cholesterol (Williams and Dusenbery, 1990)). Nematode populations were measured using the COPAS Biosort. (Union Biometrica Inc., Somerville, MA) (Pulak, 2006). Growth assays were modified from Boyd et al. (2009). Briefly, 50 L1 nematodes were dispensed into each well of a 96-well exposure plate containing complete K medium, varying concentrations of the chelating agents and metals, and OP50 E. coli as a food source. After exposure, samples were aspirated from exposure plates using the COPAS Biosort ReFlx, and measurements of individual nematodes were recorded by the Biosort.
To determine the toxicological effects of the metals and/or chelators, range-finding experiments were initially performed. Nematodes were exposed to concentrations of chelators ranging from 0 to 42.5mM. After a 48-h exposure period, the effects of the chelators on growth and movement were observed visually and the concentration response to the chelator was classified based on the yield and relative size of the nematodes, compared with controls. The Biosort was also used to measure two size characteristics: time of flight (TOF) and extinction (EXT). EXT is a measure of the optical density of the body of the nematode, and TOF is a measure of the length of the nematode. The values for both parameters increase as the nematode develops.
Data analysis. At the end of the exposure period, visual inspections of the nematodes by light microscopy were performed. In addition, growth of C. elegans was classified based on the size of the nematodes as a group. Toxic responses based on visual inspection were graded as one of three levels of response: “nontoxic” for C. elegans that did not differ significantly in size or level of activity from the control nematodes (< 15% diminishment of growth or activity compared with control nematodes), “slightly toxic” for nematodes that exhibited approximately 70–85% of growth or levels of activity (head-thrashing) than the controls, and “toxic” for greatly diminished growth (approximately 40–70% of the length of control nematodes) and activity compared with the control nematodes. An EC50 value was defined for each population, which represented the concentration of treatment that decreases nematode growth by 50% compared with nonexposed controls, based on visual observations by light microscopy.
Following visual inspection by light microscopy, log(EXT) and log(TOF) measurements were analyzed using the Markov growth model, as previously described (Smith et al., 2009). Analysis was performed using the means of the log(EXT) measurements per well or the number of nematodes recovered alive per well with a two-way ANOVA (Matlab, MathWorks, Natick, MA) with metal and chelator exposure as the two factors. Interaction effects were used to characterize protection by chelators. The p values produced from statistical analysis are representative of the probability that the observed result of treatment by chelator could have arisen in the C. elegans population by chance. Lower p values (p ≤ 0.10) are more suggestive of a protective effect on the specimens due to treatment with chelators, whereas larger p values suggest the probability that the result arose due to chance.
RESULTS
Chelator and Metal Toxicity Assays
Initially, range-finding experiments were performed for the chelators and metals alone to determine the appropriate maximum concentrations of both to be used in the metal-chelator assays. AHA was not toxic to nematodes up to a concentration of 1.7mM, above which a “slightly toxic” response was obtained through 11mM, with some loss of growth and decrease in nematode movement. A “toxic” response was observed, with severe growth inhibition and death at the highest AHA concentration tested. Cysteine produced no toxic response up to 2.7mM, above which a precipitate was observed in the solution that was accompanied by nematode death. DMHP was not toxic up to 0.67mM and was “slightly toxic” through 2.7mM. Cyclam was not toxic through 17mM and “slightly toxic” at higher levels. CaEDTA was “slightly toxic” through 4.25mM and “toxic” at higher concentrations. Desferrioxamine B and histidine were not toxic at any of the concentrations tested.
Metals were toxic at much lower concentrations than the chelators. Cadmium produced a “toxic” response at concentrations above 0.20mM. Copper produced a “toxic” response at concentrations above 0.32mM. Nickel produced a “toxic” response at 0.225mM, and zinc produced a “toxic” response at 0.50mM. The determined EC50 values of both chelator-only and metal-only toxicity studies are summarized in Table 1.
TABLE 1 .
Concentration of Ligands and Metals That Produce Toxicity in C. elegans
| Chemical | Effective concentration50 (mM)a | |
|---|---|---|
| Chelators | ||
| AHA | 6.7 | |
| Cysteine | 4.25 | |
| Cyclam | 42.5 | |
| Desferrioxamine B | > 42.5 | |
| DMHP | 2.7 | |
| Ca EDTA | 2.7 | |
| Histidine | > 42.5 | |
| Metals | ||
| Cadmium | 0.35 | |
| Copper | 0.32 | |
| Nickel | 0.32 | |
| Zinc | 0.60 | |
aThe concentration that decreases nematode growth by 50% compared with nonexposed controls, based on visual inspection by light microscopy.
Metal Chelation Protection Assays
To test the ability of chelators to ameliorate the toxic effects of these metals, nematodes were exposed to metals in the presence of slightly toxic or nontoxic concentrations of chelators. The results of the visual inspection of the metal-chelator protection assays by light microscopy are shown in Table 2. Protection of C. elegans from metal toxicity by chelator treatments was analyzed twice statistically by examining the interaction effects between metal exposure and chelators: once using the numbers of recovered nematodes and again using the size as indicated by log(EXT) (Table 3). The number of recovered nematodes was used as an approximation of the lethality of the treatment to the nematodes. The corresponding p values are shown in Table 3. Concentrations of metal and chelator used in each assay are listed along with the protective effect of each assay. For each metal, the concentration used was above the minimum concentration that produced a toxic effect (defined as > 30% growth reduction). This ensured that reproducible toxic effects were obtained for the metal treatment. Some chelators required higher concentrations than necessary for full complex formation (> 90% of metal chelated in some form), as lower concentrations provided little or no protection.
TABLE 2 .
Ability of Chelators to Protect C. elegans Against Metal Toxicitya
| Chelator | Metal | |||||||
| Cadmium (0.35mM) | Copper (0.32mM) | Nickel (0.32mM) | Zinc (0.60mM) | |||||
| AHA | 0(4.25) | 0(1.1) | 0(1.7) | 0(4.25) | ||||
| Cyclam | 0(11) | +(1.7) | ++(0.425) | +(0.67) | ||||
| Cysteine | 0(2.7) | +(1.1) | 0(1.7) | 0(1.7) | ||||
| DMHP | 0(1.7) | ++(1.7) | 0(1.1) | 0(1.7) | ||||
| Desferrioxamine B | 0(29.7) | ++(1.7) | 0(27.2) | 0(2.7) | ||||
| CaEDTA | ++(0.425) | ++(0.425) | ++(0.425) | ++(0.67) | ||||
| Histidine | +(29.7) | ++(2.7) | ++(1.1) | 0(6.7) | ||||
aConcentrations of metal (above the minimum concentration that produced a toxic effect, defined as > 30% growth reduction) are listed at the top of the column, whereas the millimolar concentration of chelator used for each metal is listed in parentheses below each result. Chelators that provided protection against specific metals are highlighted in gray; 0, no protection; +, slight protection or up to 50% restoration of growth compared with untreated nematodes; ++, full protection or 50–100% restoration of growth compared with untreated nematodes.
TABLE 3 .
Statistical Analysis of the Results of Metal-Chelator Protection Assaysa
| Chelator | Metal | |||||||
|---|---|---|---|---|---|---|---|---|
| Cadmiumb | Copperc | Nickelc | Zincc | |||||
| AHA | 0.7513 | 0.839 | 0.375 | 0.709 | ||||
| 0.106 | 0.911 | 0.944 | ||||||
| Cysteine | 0.6251 | 0.561 | 0.168 | 0.448 | ||||
| 0.767 | 0.710 | 0.951 | ||||||
| Cyclam | 0.9177 | 0.814 | 0.046 | 0.215 | ||||
| 0.453 | 0.223 | 0.482 | ||||||
| DMHP | 0.3658 | 0.916 | 0.461 | 0.276 | ||||
| 0.093 | 0.822 | 0.999 | ||||||
| Desferrioxamine B | 0.9516 | 0.214 | 0.625 | 0.627 | ||||
| 0.052 | 0.480 | 0.577 | ||||||
| EDTA | 0.0005 | 0.166 | 0.002 | 0.005 | ||||
| 0.013 | 0.072 | 0.004 | ||||||
| Histidine | 0.0640 | 0.255 | 0.001 | 0.280 | ||||
| 0.003 | 0.312 | 0.158 | ||||||
aConcentrations of metal and chelator used are the same as those listed in Table 1. Values listed are p values; representative of the probability that the observed result of treatment by chelator could have arisen in the C. elegans population by chance: lower p values are more suggestive of a protective effect on the specimens due to treatment with chelator.
bp values for cadmium were calculated using the number of specimens recovered alive, as treatment with the metal resulted in death in the absence of the chelator.
cp values for copper, nickel, and zinc were calculated both by comparing the number of specimens recovered alive in the presence and absence of chelator (top value) and by comparing the log EXT values of metal-treated specimens with log EXT values of metal-and-chelator-treated specimens (bottom value).
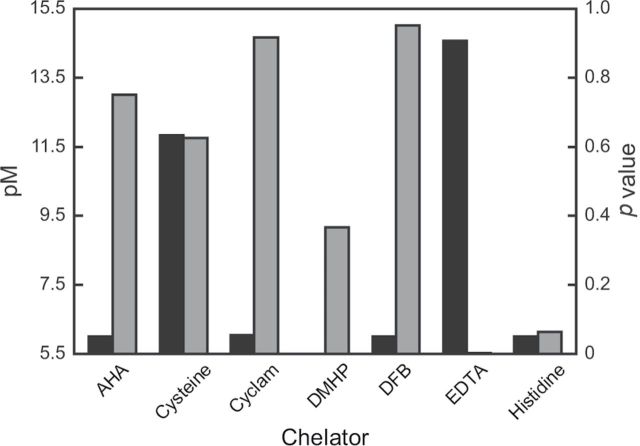
In the absence of chelators, 0.35 mM cadmium resulted in the death of > 90% of nematodes. A toxic effect was observed after cadmium exposure in the presence of all chelators except CaEDTA and histidine. CaEDTA has an extremely low p value, which strongly suggests protection of the nematodes from metal toxicity by the ligand (Table 3). This was also true for histidine, which exhibited a low p value (0.064), suggestive of some protective effect.
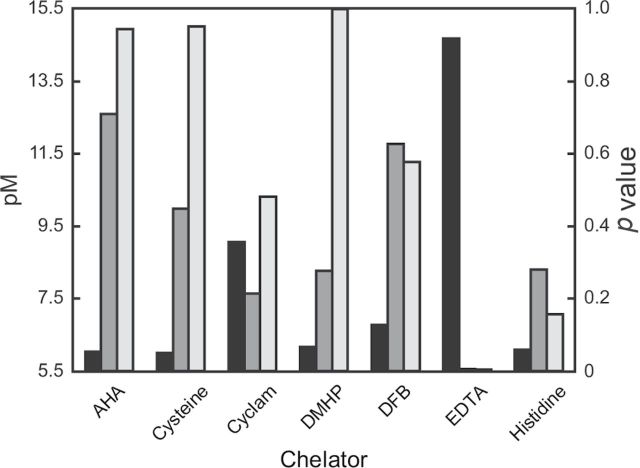
The toxicity associated with exposure to copper was not observed in the presence of desferrioxamine B, DMHP, CaEDTA, and histidine based on the low calculated p values for nematode size, as shown in Table 3. The Biosort data suggested little effect due to exposure to cysteine (p = 0.766) and cyclam (p = 0.453). Calculated p values for AHA treatment suggested that it provided no protection from copper toxicity (p = 0.106; Tables 2 and 3).
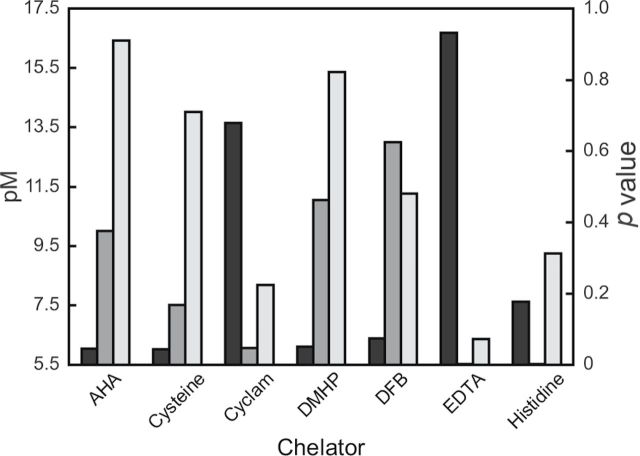
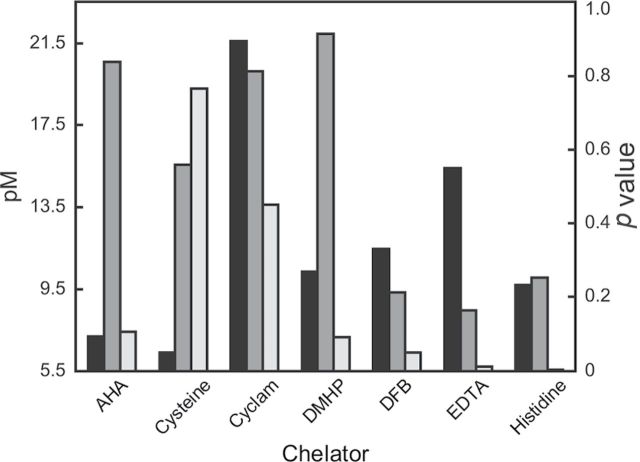
Based on calculated p values using the numbers of recovered nematodes, nickel toxicity was alleviated by treatment with cyclam (p = 0.046), CaEDTA (p = 0.002), and histidine (p = 0.0005) (Table 3). A highly significant decrease in zinc toxicity was observed after treatment of the nematodes with CaEDTA (p = 0.005) (Table 3). Although histidine alone did not produce a toxic effect, histidine in the presence of zinc killed the nematodes consistently during the L3 phase of growth (> 90% of nematodes dead after 48h).
DISCUSSION
A variety of anthropogenic and nonanthropogenic sources contribute toxic metals to the environment that pose a significant health threat. One method of remediation is to use stable chelators that may bind metals in nonbioavailable forms, thus reducing their toxicity. Because the characteristics of a chelator that can provide effective detoxification are unclear, it is necessary to utilize in vivo assays to characterize chelator-based metal remediation strategies. C. elegans has been used to test metal toxicity (Tatara et al., 1998) and in this study was used to monitor the effectiveness of chelator-based remediation. One important characteristic of a chelator-based remediation strategy is that the treatment should not produce its own toxic response. Therefore, in addition to testing the effectiveness of different chelators on reducing metal toxicity, the toxicity of the chelators alone was determined.
None of the chelators were highly toxic at the lowest concentrations tested although some produced a slight decrease in nematode growth at higher concentrations. AHA, a chelator with a strong affinity for high–charge density metal ions, was toxic at 4.25mM. This is not surprising as AHA is also toxic in humans and may be teratogenic (Holmes, 1996). Cysteine precipitated from solution at concentrations above 4.25mM and was toxic to C. elegans. Cysteine oxidizes in aqueous solution, forming reactive oxygen species that could have harmed the nematode (Gerwe, 1932). Cyclam is a stable molecule with high hydrophilicity and low bioavailability, which may contribute to its low toxicity (Schrap and Opperhuizen, 1990; Semple et al., 2003). Desferrioxamine B, as a tris hydroxamate siderophore, chelates iron(III) and other high–charge density metal cations. It has low bioavailability, low serum t1/2, and was not toxic to C. elegans (Lee et al., 1993). DMHP is also an effective iron(III) chelator, but there are some concerns about its toxicity in humans (Olivieri et al., 1998). This concern is reflected by the observed toxicity of DMHP at low concentrations to C. elegans. Ca EDTA had a slight toxic effect at low concentrations in C. elegans, which may be due to the chelation of essential metals. Histidine exhibited low toxicity likely because it is least reactive of the chelators tested and an essential amino acid.
The toxicity of metals is related to their bioavailability. The bioavailability of any metal complex species in a system is influenced by its size, shape, and charge. These characteristics allow a species to interact with any number of uptake pathways in the biological system (Campbell, 1995). Complexation of a metal changes many of these factors. For example, a change in the inner coordination sphere can result in an increase in the effective size and shape of the metal complex compared with the metal ion. As most of the chelators used in the current study feature ionizable sites, complexation of the metal will result in a more negative charge for the complex, compared with the aquated metal ion. Chelation does not necessarily have to be complete or in the highest order of complexation to be seen as effective. Partial chelation (i.e., partial coordination saturation of the metal) can reduce the effective bioavailable metal concentration.
Supplementary table S1 provides calculated pM values for the metal-chelator systems. Speciation diagrams for the various metal-ligand systems studied here are also provided in supplementary information. These plots show the relative percent metal complexed at the conditions of our experiments and relative degrees of formation of ML and ML2 complexes. Figures 1–4 show histograms that correlate the calculated pM values to the observed p values for metal-ligand systems. A low pM value denotes a relatively weak affinity of the chelator for sequestering the metal. A low p value indicates a lower toxicity in the presence of the chelator compared with the nonchelated metal. For example, a combination of high pM and low p value for a particular metal/chelator pair suggests low metal toxicity brought about by strong metal sequestration. The case of CaEDTA is particularly illustrative of this principle. All of the metals exhibited high pM values with this chelator (pM > 14), and all exhibited p values below 0.1 upon treatment with the ligand. This suggests that the addition of a strongly chelating ligand can be linked to a high probability of protection of the nematodes from metal toxicity.
FIG. 1.
Histogram correlating pM values (black bars) of individual chelators with cadmium to calculated p values (dark gray bars) after the treatment of C. elegans with metal and chelator. pM and p values are taken from Supplemental Table S1 and Table 3, respectively. DFB, desferrioxamine B.
FIG. 4.
Histogram correlating pM values (black bars) of individual chelators with zinc to calculated p values for survival (dark gray bars) or log EXT (light gray bars) after the treatment of C. elegans with metal and chelators. pM and p values are taken from Supplemental Table S1 and Table 3, respectively. DFB, desferrioxamine B.
AHA was ineffective at protecting C. elegans from any of the metals tested, possibly due to a relatively low degree of chelation. At the experimental concentrations of chelator used, between 2% (cadmium) and 80% (nickel) of the total metal was chelated in some form by the ligand (see speciation model diagrams in supporting information). However, the forms of the AHA found in solution were generally lower order complexes, suggesting that chelation of a degree lower than full coordination is insufficient for protection.
Cysteine formed stable complexes with copper and cadmium, but only provided partial protection from copper toxicity (Figs. 1 and 2). It also did not protect against zinc and nickel toxicity. This demonstrates little correlation between protective effect and pM value for the experimental conditions, as both nickel and zinc exhibit relatively high pM values, 13.6 and 9.0 (Figs. 3 and 4), respectively. However, there is also the possibility of the catalysis of side reactions due to the presence of metals (Gilmour and McAuley, 1970). Cyclam was predicted to form stable complexes with copper, zinc, and nickel. It also exhibited some protective effects from these metals although it only exhibited full protection against nickel toxicity (Fig. 3).
FIG. 3.
Histogram correlating pM values (black bars) of individual chelators with nickel to calculated p values for survival (dark gray bars) or log EXT (light gray bars) after the treatment of C. elegans with metal and chelators. pM and p values are taken from Supplemental Table S1 and Table 3, respectively. DFB, desferrioxamine B.
Desferrioxamine B strongly chelates copper (Supplementary table S1) and was able to reduce copper toxicity but was not effective against the other metals studied. This further suggests a link between the relative strength of chelation and protection against metal toxicity in vivo. DMHP chelates copper relatively strongly and provides partial chelation of nickel and zinc. This low degree of complex stability is likely related to the inability of DMHP to protect C. elegans from nickel and zinc toxicity. The stability of the cadmium-DMHP complex has yet to be characterized although it is likely that the complex stability is relatively low, as DMHP features hard donor atoms and cadmium is a moderate Lewis acid on the Pearson Hard-Soft Acid/Base scale (Pearson, 1997).
CaEDTA was the only chelator that provided protection from all four metals. EDTA is known as a stable chelator of many metals, resulting in its widespread application. The use of CaEDTA is preferable to Na2H2EDTA, as it is less likely to coordinate and remove essential metal ions such as calcium and zinc. The use of Na2H2EDTA has been associated with hypocalcemia and other metal-deficient conditions in humans (Brown et al., 2006). By using the calcium coordinated form of the chelator, the removal of calcium from the biological system is avoided. In addition, using approximately the same concentration of chelator as the target metal present in the system prevents a large excess of chelator that would sequester other essential metals.
Interestingly, histidine was found to have a protective effect against most of the metals, despite its relatively low degree of chelation of cadmium and nickel. One study suggested that excess histidine abrogates nickel toxicity through mechanisms other than chelation of the metal, possibly involving the storage of excess histidine in vacuoles in the cell (Pearce and Sherman, 1999).
The results obtained in our study demonstrate that, although in most cases strong thermodynamics of complex formation (i.e., high pM value) correlates with protection from metal toxicity, this factor alone is not sufficient for the development of effective chelating agents. One possible explanation for this is the variety of environments that the metal complex encounters in vivo. Although the complexes may encounter approximately neutral environments in the test solution, the environment in the nematode intestine may be more acidic than the external environment (the lumenal pH of C. elegans can be inferred to be slightly acidic from the pH of optimal activity of the intestinal enzymes of C. elegans) (Beh et al., 1991; Sarkis et al., 1988). Thus, complexes that are stable at pH 6.5 could dissociate at the lower pH, making the metal bioavailable. It is also unclear what mechanism promotes bioavailability of the aquated metal in vivo. Although some metals may remain partially chelated in vivo, these complexes may still be bioavailable upon ingestion. These findings are consistent with previous reports by van Leeuwen et al. who showed that a predictive model for bioavailability of metals and metal complexes must include numerous factors beyond simple equilibrium models, including kinetics of complex formation, kinetics of transport across cell membranes, and partition coefficients (van Leeuwen, 1999; van Leeuwen et al., 2005).
Although a number of soil remediation techniques are already in use, the primary purpose in the development of chelation-based remediation techniques is to decrease metal bioavailability. Our results demonstrate that although a compound may bind metals with a high affinity, it does not guarantee that it will alleviate metal toxicity. In the environment, there are numerous other factors related to physical and chemical properties of soils, including cation-exchange capacity, mode of adsorption, soil pH, environmental redox properties, and natural organic matter content, that will also affect the bioavailability of metals in the environment that are not taken into account in this model assay. However, the use of the organism C. elegans as a model organism for soil-based organisms to assess bioavailability of the metal is an effective, rapid technique for gauging the effectiveness of potential remediation techniques. In addition, the results of our experiments support the use of EDTA in the remediation of metal-contaminated sites (Boyd and Williams, 2003).
SUPPLEMENTARY DATA
Supplementary data are available online at http:// toxsci.oxfordjournals.org/.
FIG. 2.
Histogram correlating pM values (black bars) of individual chelators with copper to calculated p values for survival (dark gray bars) or log EXT (light gray bars) after the treatment of C. elegans with metal and chelators. pM and p values are taken from Supplemental Table S1 and Table 3, respectively. DFB, desferrioxamine B.
Supplementary Material
Funding
A.L.C. thanks the National Science Foundation (CHE CHE0809466) for partial financial support. Nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources. This work was supported (in part) by the Intramural Research Program of the NIH, NIEHS, and the NTP (Z01ES102045, Z01ES102046). Work by M.V.S. was supported by NIEHS contract HHSN273201000086U. A.L.C. and J.M.H. thank the Duke University Center for Biomolecular and Tissue Engineering for partial financial support.
References
- Alderighi L., Gans P., Ienco A., Peters D., Sabatini A., Vacca A. (1999). Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 184, 311–318 [Google Scholar]
- Beh C. T., Ferrari D. C., Chung M. A., McGhee J. D. (1991). An acid phosphatase as a biochemical marker for intestinal development in the nematode Caenorhabditis elegans. Dev. Biol. 147, 133–143 [DOI] [PubMed] [Google Scholar]
- Boyd W. A., Williams P. L. (2003). Availability of metals to the nematode Caenorhabditis elegans: Toxicity based on total concentrations in soil and extracted fractions. Environ. Toxicol. Chem. 22, 1100–1106 [PubMed] [Google Scholar]
- Boyd W. A., Smith M. V., Kissling G. E., Freedman J. H. (2010). Medium- and high-throughput screening of neurotoxicants using C. elegans. Neurotoxicol. Teratol. 32, 68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd W. A., Smith M. V., Kissling G. E., Rice J. R., Snyder D. W., Portier C. J., Freedman J. H. (2009). Application of a mathematical model to describe the effects of chlorpyrifos on Caenorhabditis elegans development. PLoS One 4, e7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. J., Willis T., Omalu B., Leiker R. (2006). Deaths resulting from hypocalcemia after administration of edetate disodium: 2003-2005. Pediatrics 118, e534–e536 [DOI] [PubMed] [Google Scholar]
- Campbell P. G. C. (1995). Interactions between trace metals and organisms: Critique of the free-ion activity model. In Metal Speciation and Bioavailability in Aquatic Systems (Tessier A., Turner D., Eds.), pp. 45–102 Wiley, Chichester, U.K [Google Scholar]
- Chaube S., Murphy M. L. (1966). The effects of hydroxyurea and related compounds on the rat fetus. Cancer Res. 26, 1448–1457 [PubMed] [Google Scholar]
- Chen T., Wang X., He Y., Zhang C., Wu Z., Liao K., Wang J., Guo Z. (2009). Effects of cyclen and cyclam on zinc(II)- and copper(II)-induced amyloid beta-peptide aggregation and neurotoxicity. Inorg. Chem. 48, 5801–5809 [DOI] [PubMed] [Google Scholar]
- Faa G., Crisponi G. (1999). Iron chelating agents in clinical practice. Coord. Chem. Rev. 184, 291–310 [Google Scholar]
- Freedman J. H., Slice L. W., Dixon D., Fire A., Rubin C. S. (1993). The novel metallothionein genes of Caenorhabditis elegans. Structural organization and inducible, cell-specific expression. J. Biol. Chem. 268, 2554–2564 [PubMed] [Google Scholar]
- Galanello R. (2007). Deferiprone in the treatment of transfusion-dependent thalassemia: A review and perspective. Ther. Clin. Risk Manag. 3, 795–805 [PMC free article] [PubMed] [Google Scholar]
- Gerwe E. G. (1932). The spontaneous oxidation of cysteine. Science 76, 100–101 [DOI] [PubMed] [Google Scholar]
- Gilmour A. D., McAuley A. (1970). Kinetics of the reaction of molecular oxygen with iron(II)-cysteine complexes. J. Chem. Soc. A 1970, 1006–1008 [Google Scholar]
- Harris W. R., Carrano C. J., Raymond K. N. (1979). Coordination chemistry of microbial iron transport compounds.16. Isolation, characterization, and formation-constants of ferric aerobactin. J. Am. Chem. Soc. 101, 2722–2727 [Google Scholar]
- Heschl M. F., Baillie D. L. (1990). The HSP70 multigene family of Caenorhabditis elegans. Comp. Biochem. Physiol. B 96, 633–637 [DOI] [PubMed] [Google Scholar]
- Holmes L. B. (1996). Hydroxamic acid: A potential human teratogen that could be recommended to treat ureaplasma. Teratology 53, 227–229 [DOI] [PubMed] [Google Scholar]
- Khanna N., Cressman C. P.,, 3rd, Tatara C. P., Williams P. L. (1997). Tolerance of the nematode Caenorhabditis elegans to pH, salinity, and hardness in aquatic media. Arch. Environ. Contam. Toxicol. 32, 110–114 [DOI] [PubMed] [Google Scholar]
- Lee P., Mohammed N., Marshall L., Abeysinghe R. D., Hider R. C., Porter J. B., Singh S. (1993). Intravenous infusion pharmacokinetics of desferrioxamine in thalassaemic patients. Drug Metab. Dispos. 21, 640–644 [PubMed] [Google Scholar]
- Leung M. C., Williams P. L., Benedetto A., Au C., Helmcke K. J., Aschner M., Meyer J. N. (2008). Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol. Sci. 106, 5–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell A. E., Hancock R. D. (1996). Metal Complexes in Aqueous Solutions. Plenum Press, New York, NY [Google Scholar]
- Olivieri N. F., Brittenham G. M., McLaren C. E., Templeton D. M., Cameron R. G., McClelland R. A., Burt A. D., Fleming K. A. (1998). Long-term safety and effectiveness of iron-chelation therapy with deferiprone for thalassemia major. N. Engl. J. Med. 339, 417–423 [DOI] [PubMed] [Google Scholar]
- Pearce D. A., Sherman F. (1999). Toxicity of copper, cobalt, and nickel salts is dependent on histidine metabolism in the yeast Saccharomyces cerevisiae. J. Bacteriol. 181, 4774–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. G. (1997). Chemical Hardness. Wiley-VCH, Weinheim, Germany; New York, NY [Google Scholar]
- Powell K. J. (2005). IUPAC SC-Database Academic Software. Timble, Otley, Yorks, UK: . [Google Scholar]
- Pulak R. (2006). Techniques for analysis, sorting, and dispensing of C. elegans on the COPAS flow-sorting system. Methods Mol. Biol. 351, 275–286 [DOI] [PubMed] [Google Scholar]
- Riddle D. L. (1997). C. elegans II. Cold Spring Harbor Laboratory Press, Plainview, NY: [PubMed] [Google Scholar]
- Sarkis G. J., Kurpiewski M. R., Ashcom J. D., Jen-Jacobson L., Jacobson L. A. (1988). Proteases of the nematode Caenorhabditis elegans. Arch. Biochem. Biophys. 261, 80–90 [DOI] [PubMed] [Google Scholar]
- Schrap S. M., Opperhuizen A. (1990). Relationship between bioavailability and hydrophobicity—Reduction of the uptake of organic-chemicals by fish due to the sorption of particles. Environ. Toxicol. Chem. 9, 715–724 [Google Scholar]
- Semple K. T., Morriss A. W. J., Paton G. I. (2003). Bioavailability of hydrophobic organic contaminants in soils: Fundamental concepts and techniques for analysis. Eur. J. Soil Sci. 54, 809–818 [Google Scholar]
- da Silva J. J. R. F., Williams R. J. P. (2001). The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, 2nd ed Oxford University Press, New York, NY [Google Scholar]
- Smith M. V., Boyd W. A., Kissling G. E., Rice J. R., Snyder D. W., Portier C. J., Freedman J. H. (2009). A discrete time model for the analysis of medium-throughput C. elegans growth data. PLoS One 4, e7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatara C. P.,, Newman M. C.,, McCloskey J. T., Williams P. L. (1998). Use of ion characteristics to predict relative toxicity of mono-, di- and trivalent metal ions: Caenorhabditis elegans LC50. Aquat. Toxicol. 42, 255–269 [Google Scholar]
- The C. elegans Sequencing Consortium (1998). Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 282, 2012–2028 [DOI] [PubMed] [Google Scholar]
- van Leeuwen H. P. (1999). Metal speciation dynamics and bioavailability: Inert and labile complexes. Environ. Sci. Technol. 33, 3743–3748 [Google Scholar]
- van Leeuwen H. P., Town R. M., Buffle J., Cleven R. F., Davison W., Puy J., van Riemsdijk W. H., Sigg L. (2005). Dynamic speciation analysis and bioavailability of metals in aquatic systems. Environ. Sci. Technol. 39, 8545–8556 [DOI] [PubMed] [Google Scholar]
- Wenger K., Tandy S., Nowack B. (2005). Effects of chelating agents on trace metal speciation and bioavailability. In Biogeochemistry of Chelating Agents (Nowack B., VanBriesen J. M., Eds.), pp. 204–225 American Chemical Society, Washington, DC [Google Scholar]
- Williams P. L., Dusenbery D. B. (1988). Using the nematode Caenorhabditis elegans to predict mammalian acute lethality to metallic salts. Toxicol. Ind. Health 4, 469–478 [DOI] [PubMed] [Google Scholar]
- Williams P. L., Dusenbery D. B. (1990). Aquatic toxicity testing using the nematode, Caenorhabditis elegans . Environ. Toxicol. Chem. 9, 1285–1290 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.