Abstract
Background
Metformin has been proffered as a therapy for adolescent obesity, although long-term controlled studies have not been reported.
Objective
To test the hypothesis that 48 weeks of daily metformin hydrochloride extended release (XR) therapy will reduce body mass index (BMI) in obese adolescents, as compared with placebo.
Design
Multicenter, randomized, double-blind, placebo-controlled clinical trial.
Setting
The 6 centers of the Glaser Pediatric Research Network from October 2003 to August 2007.
Participants
Obese (BMI≥95th percentile) adolescents (aged 13–18 years) were randomly assigned to the intervention (n = 39) or placebo groups.
Intervention
Following a 1-month run-in period, subjects following a lifestyle intervention program were randomized 1:1 to 48 weeks’ treatment with metformin hydrochloride XR, 2000 mg once daily, or an identical placebo. Subjects were monitored for an additional 48 weeks.
Main Outcome Measure
Change in BMI, adjusted for site, sex, race, ethnicity, and age and metformin vs placebo.
Results
After 48 weeks, mean (SE) adjusted BMI increased 0.2 (0.5) in the placebo group and decreased 0.9 (0.5) in the metformin XR group (P = .03). This difference persisted for 12 to 24 weeks after cessation of treatment. No significant effects of metformin on body composition, abdominal fat, or insulin indices were observed.
Conclusion
Metformin XR caused a small but statistically significant decrease in BMI when added to a lifestyle intervention program.
Childhood obesity rates in the United States have more than tripled over the past 50 years, with recent reports indicating that 31.9% of all children are overweight or obese.1 Obesity in childhood, particularly during adolescence, is associated with significant morbidity, including type 2 diabetes mellitus and hypertension, and a high risk for adult obesity and associated risks for diabetes mellitus and cardiovascular disease.2 It is imperative that effective prevention and treatment modalities be identified to address the epidemic of childhood and adolescent obesity.
Current standard treatment of childhood obesity is lifestyle modification, including diet and exercise.3 However, short-term prospective trials using various lifestyle modification programs have shown that effectiveness is often related to the intensity of the program, shows high intersubject variability, and has limited longevity.4
Metformin hydrochloride is commonly used as a primary or adjunctive treatment in obese, nondiabetic adolescents. However, there are limited short-term data to support this therapy, and it is unclear whether any observed effects of metformin on body mass index (BMI) are associated with changes in body composition or insulin sensitivity. Therefore, we conducted a 48-week randomized, double-blind, placebo-controlled trial of extended-release (XR) metformin therapy in nondiabetic obese adolescents, followed by a 48-week monitoring period after completion of treatment.
METHODS
HYPOTHESIS
We hypothesized that treatment of obese adolescents with metformin XR coupled with a lifestyle intervention would decrease BMI as compared with treatment with placebo and the same lifestyle intervention.
SETTING
The study was conducted from October 2003 to August 2007 at the 5 clinical sites of the Glaser Pediatric Research Network, along with the Data Coordinating Center located at Children’s Hospital Boston. The study was approved by the institutional review boards at each of the 6 centers; informed parental consent and subject assent were obtained. An external Data and Safety Monitoring Board was involved throughout the study.
SUBJECTS
Adolescents aged 13.00 years to younger than 18 years were eligible if they were obese (BMI≥95th percentile for age and sex5) but weighed less than 136 kg (the weight limit for the dual-emission x-ray absorptiometry [DXA] table). Subjects were excluded if they had a previous diagnosis of diabetes mellitus, had ever used a medication to treat diabetes mellitus or insulin resistance, had ever used a medication to aid in weight loss, were taking any medications known to increase metformin levels (eg, cimetidine), received recent glucocorticoid therapy, had any identified syndrome or medical disorder predisposing to obesity, had surgical therapy for obesity, had attended a formal weight loss program within the previous 6 months, admitted to significant alcohol use in the past 6 months, had elevated creatinine (>1.2 mg/dL [to convert to micromoles per liter, multiply by 88.4]) or liver enzymes (aspartate aminotransferase or alanine aminotransferase >80 U/L [to convert to microkatals per liter, multiply by 0.0167]) levels, had untreated disorders of thyroid function, had impaired ambulation or mobility, or had ever been pregnant.
STUDY DESIGN
After clinical eligibility was confirmed, diabetes mellitus was excluded at a baseline visit (study day 0) using an oral glucose tolerance test (OGTT). Other fasting laboratory studies, DXA, and abdominal computed tomography (CT) were also performed at baseline.
The study sample was enriched for subjects with a higher likelihood of complying with the protocol using a 4-week placebo run-in phase, during which subjects were required to attend at least 2 of 3 scheduled lifestyle modification sessions and demonstrate 80% compliance with daily placebo treatment (pill count) for subsequent randomization. Subjects were then randomized 1:1 to treatment with either metformin XR (Glucophage XR) or identical placebo tablets and instructed to take 1 tablet/d (metformin hydrochloride XR 500 mg or placebo) orally before dinner for 2 weeks, then 2 tablets/d for 2 weeks, then 4 tablets/d from week 8 to week 52. Investigators were permitted to adjust the dose of study drug as follows. If symptoms were mild and tolerable, study drug was continued. Persistent or severe gastrointestinal or other symptoms could lead to a reduction from 4 tablets/d to 1 tablet/d; the dose was then increased by 1 tablet/d in weekly intervals until the subject achieved a tolerable dose level of up to 4 tablets/d. Compliance was assessed at each study visit by asking the patient and parent(s) how many doses were missed during the preceding 7 days. Adverse events were recorded at each study visit, with investigator grading of relatedness and severity.
While healthy eating was a major component of the lifestyle modification program (described later), no specific calorie goal was assigned to the subjects. To mitigate the possible impact of diet modification on vitamin and calcium intake, as well as possible effects of metformin on vitamin B metabolism and excretion,6 subjects were also instructed to take a multivitamin tablet and 1000 mg of calcium carbonate daily.7 After the baseline visit (day 0) and randomization at week 4, subjects returned at 16, 28, 40, 52, 64, 76, 88, and 100 weeks for a physical examination, anthropometry, and safety laboratory studies, including a pregnancy test for girls. The OGTT, DXA, and abdominal CT were performed at baseline, then at 52 weeks (last dose of study drug) and 100 weeks (completion of study).
Subjects were asked to self-identify race from the following categories: American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander, black or African American, white, or other. Hispanic or Latino ethnicity was also voluntarily self-identified.
LIFESTYLE INTERVENTION
All subjects were prescribed a lifestyle intervention program to increase physical activity level and optimize dietary intake. To decrease variability across sites, we selected the Weigh of Life LITE8 program developed at Texas Children’s Hospital, Houston. Beginning with the run-in period, subjects were expected to attend 10 individualized “intensive” sessions at weekly intervals, following a specific curriculum. Monthly follow-up sessions were conducted for the remainder of the study. A trained health specialist led the sessions and parents/guardians were invited to attend.
ANTHROPOMETRY
At each visit, height was measured twice using a calibrated, wall-mounted stadiometer and weight was measured twice using a calibrated electronic scale. A third reading was taken if the difference between the first 2 readings was more than 0.5 cm for height or more than 0.3 kg for weight. Body mass index was calculated as the mean weight in kilograms divided by the mean height in meters squared2 and converted to a sex- and age-specific z score.5 Waist circumference was measured as the smallest circumference below the rib cage and above the umbilicus.9 Tanner breast (female), genital (male), and pubic hair (both sexes) staging was assessed by an experienced clinician at each visit.
RADIOLOGICAL PROCEDURES
Abdominal CT scans were performed to evaluate abdominal fat content and distribution, using a modification of published methods.10 The slice was aligned with the L4–L5 intervertebral disc to the nearest millimeter using a low-dose abdominal scout radiograph, and cross-sectional areas (in centimeters squared) for intraperitoneal and subcutaneous fat were determined using software available on the CT review console. Percentage of body fat and lean body mass were measured by whole-body DXA.11
LABORATORY STUDIES
A 3-hour OGTT (75-g glucose) was performed after 3 days of a 150 g/d or more carbohydrate diet and a 10-hour overnight fast. Plasma insulin and glucose levels were measured at 0 (before glucose bolus), 15, 30, 60, 90, 120, and 180 minutes. Lipid profiles and other laboratory test levels were measured in the fasting sample. Insulin was measured by 2-site immunochemiluminometric assays with sensitivities of 0.6 µU/mL. Safety laboratory tests included hematology and chemistry panels. All assays were performed at Esoterix Clinical Trials Services (Calabasas Hills, California).
CALCULATED INSULIN INDICES
The homeostasis model assessment–insulin resistance (HOMA-IR) was calculated as [Fasting Glucose Level (in millimoles) × Fasting Insulin Level (in microunits per deciliter)]/22.5.12 The composite insulin sensitivity index13 was calculated as
where FI is the fasting insulin level, FBG is the fasting glucose level, and MI and MG are the mean insulin and glucose levels measured between 0 and 120 minutes during the OGTT.
Beta-cell activity was estimated using the corrected insulin release at the glucose peak14 calculated as
where Ggp is the peak glucose level (maximum of all 7 measures [0–180 minutes]) and Igp is the insulin concentration at the time of the glucose peak.
RANDOMIZATION AND BLINDING
Subjects who successfully completed the run-in period were randomized to metformin XR or placebo treatment according to random sequences constructed at the Data Coordinating Center. To ensure balance across major factors, the randomization was stratified by site and sex. Subjects and study personnel were blinded to assignment throughout the entire study. To ensure nonpredictability of assignment, the randomization sequence was grouped in randomly permuted blocks of 2 and 4, and assignments were randomly permuted within block. Study drugs were prepared so as to be indistinguishable and labeled with a unique but uninformative code. The Data Coordinating Center maintained the key to drug codes for use during unblinding as needed for safety concerns (eg, in 2 cases of pregnancy) and for the data analyses.
STATISTICAL ANALYSIS
The intention-to-treat principle was used, analyzing each subject as part of his or her assigned treatment group, regardless of compliance. All analyses used 2-tailed tests with P = .05 as the critical value for statistical significance. SAS software (version 9.1; SAS Institute Inc, Cary, North Carolina) was used for all computations.
Between-group comparisons of baseline characteristics used the χ2 test for dichotomous and polytomous variables, corroborated in cases of sparse data by the Fisher exact test, and the 2-sample t test for continuous measures, corroborated in cases of severely skewed distribution or markedly unequal variance by the Wilcoxon 2-sample test. The same methods were used to compare baseline characteristics between those who completed the 52-week primary assessment and those who dropped out.
Repeated-measures analysis of variance was used to assess the effect of treatment on the primary and secondary end point measures. For BMI, the analysis comprised 10 repeated measures over 100 weeks and for the secondary end points, 3 measures, done at baseline, 52 weeks, and 100 weeks. The independent variables were treatment (1 df), time (9 df for BMI, 2 df for the other end points), and time × treatment interaction, which addressed the question of treatment efficacy. The analysis was adjusted for site, sex, race, ethnicity, and age and assumed a compound-symmetric covariance structure (equal correlation among data from each subject, equivalent to a random-subject effect). Contrasts from parameters of the fitted model were formed to estimate effects of particular interest, including adjusted means, in each treatment arm at baseline, 52 weeks, and 100 weeks (eg, Ŷ52; changes over those intervals in each treatment arm [eg, Ŷ52-0 = Ŷ52 − Ŷ0]; and differential change between the 2 treatment arms [eg, Δ52-0 = Ŷ52-0,Metformin − Ŷ52-0,Placebo]). To test for effect modification, we added preplanned interaction terms and formed corresponding contrasts (eg, change in Δ52-0 per unit HOMA-IR, tested by HOMA-IR × time × treatment interaction, or Δ52-0,Male − Δ52-0,Female, tested by sex × time × treatment interaction).
To test for biased dropout, we performed a logistic regression analysis to test whether BMI on a particular visit was associated with dropout before the following visit. In a second set of analyses, we tested for association between baseline variables (including BMI) and completion of the week 52 visit.
The repeated-measures analysis comprised all available measurements on all randomized subjects, including withdrawals and dropouts as well as completers, through the last visit for those who withdrew or were lost to follow-up. This analysis is unbiased under the assumption of missingness at random, ie, likelihood of missing data related only to variables included in the model.15 For corroboration, we imputed the missing data in 2 ways, both conservatively biased toward the null hypothesis of no drug effect: return to baseline BMI or last observation carried forward. In both cases, intermittent missing values were imputed with the last prior observation.
An interim analysis was performed and presented to the Data and Safety Monitoring Board after 50% of subjects had reached the 52-week primary evaluation point, for purposes of assessing safety and progress. Unblinded data were seen only by the Data and Safety Monitoring Board and study statistician. There were no plans to stop the study for early success or lack of power based on the interim results, as it was expected that all subjects would be enrolled by that time. Consequently, no adjustment was made to the critical P value for final analysis of the primary end point.
POWER AND SAMPLE SIZE
To estimate power, we analyzed a simulated sample with 15% African American and 15% Hispanic subjects, balanced by sex, with 20% attrition and a bias induced by selective dropout.16 Assuming an SD of 1.9 for BMI change,17 an enrolled sample of 72 provided 80% power to detect a differential of 1.46 between treatment arms or between sexes and 1.75 between white subjects and others. The final randomized sample was 77, owing to simultaneous successful run-ins at different sites in the final weeks of recruitment.
RESULTS
SUBJECT DISPOSITION
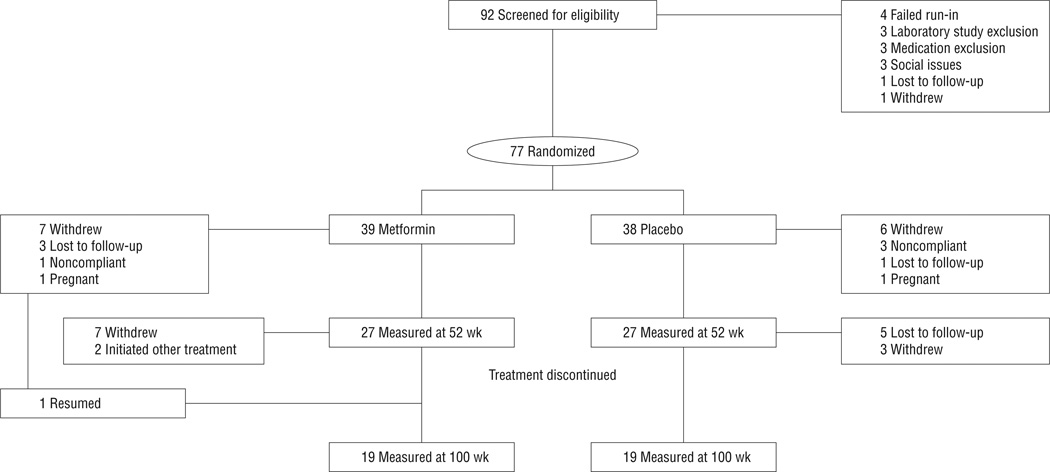
Ninety-two subjects were screened and 77 were randomized, 39 to metformin XR, 38 to placebo; 27 and 19 in each group were measured at weeks 52 and 100, respectively (Figure 1). For the randomized participants, there were no between-group differences in baseline characteristics (Table 1). During the treatment period, the odds of dropping out after any particular visit increased by a factor of 1.15 per unit BMI at that visit, but that rate did not differ between metformin and placebo subjects. The 23 subjects not measured at week 52 had a higher mean (SE) baseline BMI compared with the remaining 54 subjects (37.8 [1.1] vs 35.1 [0.7]; P = .04); however, the influence of BMI on dropout did not differ between the 2 treatment arms (P = .63) for treatment × completion interaction and no other baseline characteristic had an influence on the likelihood of dropout. One subject withdrew from the study after week 16 but returned for measurement at week 100. There were 2 pregnancies (1 each, metformin and placebo groups) resulting in discontinuation from study.
Figure 1.
Disposition of subjects. “Withdrew” refers to withdrawal of consent. One subject in the metformin hydrochloride extended release group withdrew consent at week 16 but returned for a measurement at week 100 (end of study). See text for further details.
Table 1.
Subject Characteristics at Baselinea
| No. (%) | ||
|---|---|---|
| Metformin | Placebo | |
| Sample size | 39 | 38 |
| Study site | ||
| Baylor | 10 (26) | 9 (24) |
| Harvard | 5 (13) | 4 (11) |
| Stanford | 12 (31) | 11 (29) |
| UCLA | 6 (15) | 6 (16) |
| UCSF | 6 (15) | 8 (21) |
| Sex | ||
| F | 26 (67) | 25 (66) |
| M | 13 (33) | 13 (34) |
| Race | ||
| White | 22 (56) | 27 (71) |
| African American | 8 (21) | 6 (16) |
| Asian | 3 (8) | 0 |
| Other | 6 (15) | 5 (13) |
| Hispanic ethnicity | 7 (18) | 11 (29) |
| Tanner stage, breast (females) | ||
| III | 1 (4) | 6 (24) |
| IV | 9 (35) | 6 (24) |
| V | 16 (62) | 13 (52) |
| Tanner stage, genital (males) | ||
| II | 1 (8) | 2 (15) |
| III | 6 (50) | 4 (31) |
| IV | 5 (42) | 3 (23) |
| V | 0 | 4 (31) |
| Tanner stage, pubic hair (all) | ||
| I | 1 (3) | 0 |
| II | 1 (3) | 2 (5) |
| III | 7 (18) | 5 (13) |
| IV | 12 (31) | 13 (34) |
| V | 18 (46) | 18 (47) |
| History of diabetes mellitus, biological mother | 3 (8) | 1 (3) |
| History of diabetes mellitus, biological father | 4 (10) | 5 (13) |
|
Mean (SD) |
||
| Metformin | Placebo | |
| Age, y | 14.8 (1.3) | 15.0 (1.5) |
| Waist circumference, cmb | 103.9 (13.1) | 104.7 (9.1) |
| Weight, kg | 95.9 (16.6) | 101.8 (15.7) |
| BMI | 35.9 (5.7) | 35.9 (4.7) |
| BMI z score | 2.28 (0.31) | 2.31 (0.21) |
| Systolic blood pressure, mm Hg | 121 (14) | 125 (16) |
| Diastolic blood pressure, mm Hg | 67 (9) | 66 (7) |
| 3-h OGTT, glucose level | ||
| Fasting, mg/dL | 91 (9) | 92 (9) |
| At 2 h, mg/dL | 119 (21) | 120 (25) |
| AUC, mmol/L × h | 19.7 (2.4) | 20.2 (3.1) |
| 3-h OGTT, insulin level | ||
| Fasting, µU/mL | 17 (12) | 21 (14) |
| At 2 h, µU/mL | 96 (99) | 124 (136) |
| AUC, pmol/L × h | 1815 (1395) | 2349 (2101) |
| HOMA-IR index, mmol/L × µU/mL | 3.8 (2.8) | 5.0 (3.5) |
| CISI, [mg/dL × µU/mL]−1 | 4.4 (4.0) | 2.9 (1.7) |
| CIRgp, µU/L × [mg/dL]−2 | 1.07 (0.83) | 1.25 (0.79) |
| Hemoglobin A1c level, % | 5.4 (0.3) | 5.3 (0.3) |
| TSH level, µU/mL | 1.81 (1.25) | 1.72 (1.23) |
| Leptin level, ng/mL | 40 (23) | 32 (17) |
| ALT level, U/L | 22 (13) | 24 (14) |
| AST level, U/L | 24 (5) | 24 (7) |
| DXA fat mass, kg | 38.9 (10.6) | 41.1 (9.1) |
| DXA trunk fat mass, kg | 17.9 (5.7) | 19.3 (4.6) |
| DXA lean mass, kg | 54.2 (8.2) | 58.4 (9.1) |
| CT fat area, cm2 | 572 (162) | 593 (134) |
| CT intraperitoneal fat area, cm2 | 70 (40) | 78 (32) |
| CT subcutaneous fat area, cm2 | 502 (141) | 516 (116) |
| TC level, mg/dL | 163 (34) | 172 (42) |
| LDL-C level, mg/dL | 102 (26) | 110 (35) |
| HDL-C level, mg/dL | 40 (9) | 39 (8) |
| Triglycerides level, mg/dL | 121 (118) | 118 (77) |
| Daily energy intake, kcal | 1711 (603) | 1900 (1058) |
| Mother’s highest grade level | 14 (2) | 14 (2) |
| Father’s highest grade level | 14 (3) | 14 (3) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC, area under the curve; BMI, body mass index (calculated as the mean weight in kilograms divided by the mean height in meters squared); CIRgp, corrected insulin release at the glucose peak; CISI, composite insulin sensitivity index; CT, computed tomography; DXA, dual-emission x-ray absorptiometry; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment–insulin resistance; LDL-C, low-density lipoprotein cholesterol; OGTT, oral glucose tolerance test; TC, total cholesterol; TSH, thyrotropin; UCLA, University of California, Los Angeles; UCSF, University of California, San Francisco.
SI conversion factors: To convert glucose to millimoles per liter, multiply by 0.0555; insulin to picomoles per liter, multiply by 6.945; hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01; ALT and AST to microkatals per liter, multiply by 0.0167; TC, LDL-C, and HDL-C to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113.
Metformin was given as metformin hydrochloride extended release.
Wang et al.9
PRIMARY OUTCOME
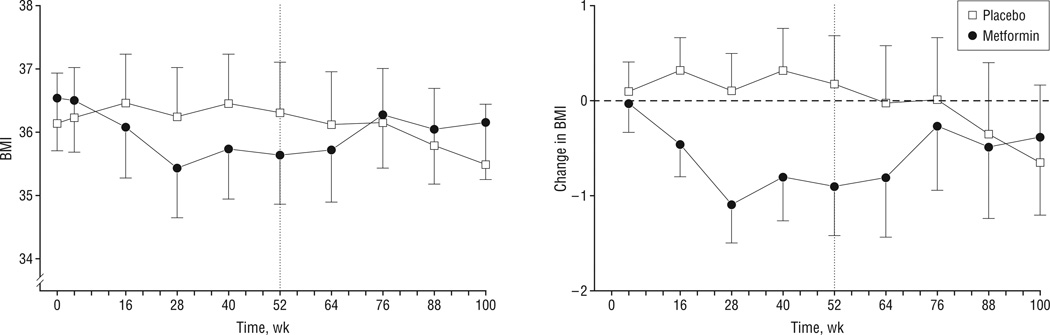
Metformin XR had a small but statistically significant impact on BMI over the initial 52 weeks of the study (Table 2) (Figure 2). The mean (SE) BMI (adjusted for site, sex, race, ethnicity, and age) increased 0.2 (0.5) in the control group and decreased 0.9 (0.5) in the metformin XR group. Repeated-measures analysis showed significant time × treatment interaction (P < .05) and treatment contrast between baseline and 52 weeks (P = .03). The mean (SE) BMI difference of −1.1 (0.5) represents an approximately 3-kg weight difference at a height of 165 cm. The difference in mean adjusted BMI was fully established by week 28 (32 weeks of study drug treatment) (Figure 2A). Imputation of missing data by last observation carried forward left the week 52 results unchanged in each arm (−0.9 for metformin, +0.5 for placebo) and the treatment contrast slightly enhanced (−0.09) and significant at P = .02. Imputation by return to baseline slightly attenuated the treatment contrast (−0.07; P = .05).
Table 2.
Primary and Secondary Outcomesa
| Adjusted Mean (SE) | Change (SE) | Change (SE) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Trial Arm | Baseline | 52 wk | 100 wk | Baseline to 52 wk |
Metformin −Placebo, Baseline to 52 wk |
P Value |
52–100 wk | Metformin −Placebo, 52–100 wk |
P Value |
| BMI | Metformin | 36.5 (0.8) | 35.6 (0.8) | 36.2 (0.9) | −0.9 (0.5) | −1.1 (0.5) | .03 | +0.5 (0.5) | 1.3 (0.6) | .02 |
| Placebo | 36.2 (0.9) | 36.3 (0.9) | 35.5 (0.9) | +0.2 (0.5) | −0.8 (0.5) | |||||
| BMI z score | Metformin | 2.28 (0.05) | 2.19 (0.05) | 2.24 (0.06) | −0.09 (0.04) | −0.08 (0.05) | .09 | +0.05 (0.05) | 0.13 (0.06) | .02 |
| Placebo | 2.29 (0.05) | 2.28 (0.05) | 2.20 (0.06) | −0.01 (0.04) | −0.08 (0.05) | |||||
| DXA fat mass, kg | Metformin | 39.5 (1.6) | 38.5 (1.6) | 38.8 (1.9) | −1.0 (1.5) | −2.4 (1.8) | .19 | +0.4 (1.6) | 3.5 (2.1) | .10 |
| Placebo | 40.9 (1.5) | 42.3 (1.6) | 39.2 (1.9) | +1.4 (1.5) | −3.1 (1.6) | |||||
| DXA lean mass, kg | Metformin | 55.7 (1.3) | 55.6 (1.4) | 58.1 (1.6) | −0.1 (1.1) | −1.5 (1.3) | .26 | +2.5 (1.2) | 1.2 (1.5) | .44 |
| Placebo | 59.0 (1.3) | 60.4 (1.4) | 61.8 (1.6) | +1.4 (1.1) | +1.3 (1.2) | |||||
| CT IP fat area, cm2 | Metformin | 74.2 (5.6) | 66.6 (6.3) | 66.8 (7.5) | −7.7 (6.8) | 0.5 (8.7) | .95 | +0.2 (7.5) | 5.1 (10.0) | .61 |
| Placebo | 77.5 (5.5) | 69.4 (6.2) | 64.5 (7.5) | −8.2 (6.6) | −4.8 (7.3) | |||||
| CT SQ fat area, cm2 | Metformin | 512 (23) | 500 (23) | 524 (27) | −12 (21) | −11 (24) | .67 | +24 (22) | 31 (28) | .26 |
| Placebo | 518 (22) | 517 (24) | 509 (28) | −1 (20) | −8 (21) | |||||
| CT IP fat, % of abdominal fat | Metformin | 12.4 (0.7) | 11.5 (0.8) | 11.7 (0.9) | −1.0 (0.8) | 0.0 (1.1) | .98 | +0.2 (0.9) | 1.0 (1.2) | .44 |
| Placebo | 12.8 (0.7) | 11.8 (0.8) | 11.1 (0.9) | −1.0 (0.8) | −0.7 (0.9) | |||||
| CT IP:SQ ratio | Metformin | 0.14 (0.01) | 0.13 (0.01) | 0.13 (0.01) | −0.01 (0.01) | 0.00 (0.02) | .94 | +0.00 (0.01) | 0.01 (0.02) | .52 |
| Placebo | 0.15 (0.01) | 0.14 (0.01) | 0.13 (0.01) | −0.01 (0.01) | −0.01 (0.01) | |||||
| HOMA-IR index | Metformin | 3.7 (0.5) | 3.6 (0.6) | 3.2 (0.8) | −0.1 (0.8) | 0.7 (1.0) | .48 | −0.4 (0.9) | −0.9 (1.2) | .44 |
| Placebo | 4.8 (0.5) | 4.0 (0.6) | 4.5 (0.8) | −0.8 (0.7) | +0.5 (0.8) | |||||
| Area under the insulin curve, nmol/Lb | Metformin | 1.8 (0.3) | 1.4 (0.3) | 1.3 (0.3) | −0.4 (0.2) | 0.0 (0.3) | .98 | −0.1 (0.3) | 0.2 (0.3) | .61 |
| Placebo | 2.3 (0.3) | 1.9 (0.3) | 1.7 (0.3) | −0.4 (0.2) | −0.2 (0.3) | |||||
| Area under the glucose curve, mmol/Lb | Metformin | 19.6 (0.4) | 19.1 (0.5) | 19.3 (0.6) | −0.5 (0.6) | 0.8 (0.7) | .30 | +0.1 (0.6) | 0.1 (0.9) | .95 |
| Placebo | 20.1 (0.4) | 18.9 (0.5) | 18.9 (0.6) | −1.2 (0.5) | +0.0 (0.6) | |||||
| CISI, [mg/dL × U/mL]−1 | Metformin | 4.1 (0.5) | 5.0 (0.5) | 4.6 (0.6) | +0.9 (0.6) | 0.1 (0.7) | .85 | −0.4 (0.6) | −1.0 (0.8) | .20 |
| Placebo | 2.9 (0.5) | 3.6 (0.5) | 4.3 (0.6) | +0.7 (0.5) | +0.7 (0.6) | |||||
| CIRgp, U/L × [mg/dL]–2 | Metformin | 1.0 (0.1) | 0.9 (0.1) | 1.0 (0.2) | −0.2 (0.2) | −0.3 (0.2) | .21 | +0.2 (0.2) | 0.5 (0.3) | .06 |
| Placebo | 1.2 (0.1) | 1.3 (0.1) | 1.0 (0.2) | +0.1 (0.2) | −0.4 (0.2) | |||||
| LDL-C level, mg/dL | Metformin | 102 (5) | 102 (5) | 107 (6) | 0 (4) | 0 (5) | .97 | +5 (5) | 3 (5) | .63 |
| Placebo | 110 (5) | 111 (5) | 113 (6) | 0 (4) | +2 (4) | |||||
| Triglycerides level, mg/dL | Metformin | 121 (15) | 119 (15) | 109 (18) | −2 (12) | −3 (14) | .80 | −10 (13) | 1 (16) | .94 |
| Placebo | 116 (14) | 118 (15) | 107 (18) | +1 (12) | −11 (12) | |||||
| HDL-C level, mg/dL | Metformin | 39 (1) | 41 (1) | 40 (2) | +1 (1) | 2 (2) | .38 | 0 (2) | −1 (2) | .81 |
| Placebo | 39 (1) | 38 (1) | 38 (2) | 0 (1) | 0 (2) | |||||
| Triglycerides:HDL-C ratio | Metformin | 3.7 (0.7) | 3.6 (0.7) | 3.4 (0.8) | 0.0 (0.5) | −0.2 (0.4) | .58 | −0.2 (0.5) | −0.1 (0.5) | .90 |
| Placebo | 3.2 (0.7) | 3.4 (0.7) | 3.2 (0.8) | +0.2 (0.4) | −0.1 (0.4) | |||||
Abbreviations: BMI, body mass index (calculated as the mean weight in kilograms divided by the mean height in meters squared); CIRgp, corrected insulin release at the glucose peak; CISI, composite insulin sensitivity index; CT, computed tomography; DXA, dual-emission x-ray absorptiometry; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment–insulin resistance; IP, intraperitoneal; LDL-C, low-density lipoprotein cholesterol; SQ, subcutaneous.
SI conversion factors: To convert LDL-C and HDL-C to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113.
Adjusted mean and change, with standard errors, from repeated-measures analysis of variance, adjusted for site, sex, race, ethnicity, and age. P tests for equal change in the metformin and placebo arms over indicated interval. Sample size was 39 for the metformin group and 38 for the placebo group at baseline; there were 27 in each arm at week 52 and 19 in each arm at week 100. Metformin was given as metformin hydrochloride extended release.
From oral glucose tolerance test, × 3 hours.
Figure 2.
Body mass index (BMI) (calculated as mean weight in kilograms divided by mean height in meters squared) (A) and adjusted change in BMI from baseline (B) (see text for further details). Data are plotted as the mean and 1 SE. Vertical dotted lines separate the study drug treatment (4–52 weeks) and post–study drug treatment (52–100 weeks) monitoring periods. Part A includes data for the run-in period (0–4 weeks). Metformin was given as metformin hydrochloride extended release.
SUBGROUP ANALYSIS
Neither sex, race, nor ethnicity significantly modified the metformin effect on BMI found in the entire group (P > .20 for interaction in repeated-measures analysis). The treatment effect did not vary by study center, parental education, or family history of type 1 or type 2 diabetes (P > .10). Likewise, baseline fasting insulin level, OGTT insulin response, composite insulin sensitivity index, and HOMA-IR did not modify the effect in the full sample or when restricted to white race.
SECONDARY OUTCOMES
Among the secondary measures of obesity, BMI z score and DXA fat mass revealed similar changes to BMI, although the mean adjusted difference between the 2 groups did not reach statistical significance. Only BMI z score showed a P value less than .10. Metformin XR treatment had no significant impact on DXA fat mass, DXA lean mass, CT intraperitoneal fat area, CT subcutaneous fat area, CT intraperitoneal fat, abdominal fat by CT, or CT intraperitoneal fat to subcutaneous fat ratio (Table 2). Likewise, metformin XR had no significant impact on HOMA-IR; the area under the insulin curve; the area under the glucose curve; composite insulin sensitivity index; corrected insulin release at the glucose peak; levels of low-density lipoprotein cholesterol, triglycerides, or high-density lipoprotein cholesterol; or the triglycerides to high-density lipoprotein cholesterol ratio.
SECOND YEAR
The BMI difference between the groups persisted for 12 to 24 weeks after cessation of study drug (Figure 2) (Table 2). Thereafter, the mean BMI in the metformin group increased toward that in the control group.
COMPLIANCE WITH INTERVENTION
Compliance with medications was good and similar in both groups (mean [SD] number of missed doses per week, 1.2 [1.7] metformin vs 1.3 [3.5] control; P = .29). Likewise, the mean [SD] number of the lifestyle modification sessions attended was similar in both groups (6.3 [3.1] metformin vs 6.7 [3.3] control; P = .38). Neither the estimate of the number of missed doses nor the number of lifestyle sessions attended were associated with the change in BMI in the metformin group.
SAFETY
During weeks 4 to 52, the safety population consisted of all subjects who received at least 1 dose of study drug. During weeks 52 to 100, the safety population included all subjects who had at least 1 visit during this period.
During weeks 4 to 52, the following adverse events occurred at least once in 5% or more of subjects in either group and 5 or more percentage points greater in 1 group relative to the other (metformin vs placebo): headache (n = 12 [31%] vs 8 [21%]), nausea (n = 9 [23%] vs 3 [8%]), vomiting (n = 6 [15%] vs 1 [3%]), upper respiratory tract infection (n = 18 [46%] vs 23 [61%]), and musculoskeletal complaints (n = 5 [3%] vs 7 [18%]). There was no statistically significant difference between the metformin and placebo groups in the incidence of any particular class of adverse events. Two events of nausea in 2 metformin-treated subjects were considered probably related; 1 subject discontinued taking the study drug. Two subjects in the metformin group and 1 in the placebo group had elevated alanine aminotransferase levels before week 52 and discontinued taking the study drug. There was 1 severe adverse event (appendectomy, metformin group) considered unrelated to the study drug; all other adverse events were mild or moderate. In total, the dose of study drug was decreased during weeks 4 to 52 for 6 subjects in the metformin group and 3 in the placebo group.
During weeks 52 to 100, headache was more frequent in the group previously treated with metformin XR (n = 6 [30%] vs 5 [24%]; P = .73), and there was 1 severe adverse event (leg vein thrombosis) considered unrelated to previous study drug (metformin) treatment.
COMMENT
To our knowledge, this is the first multicenter, double-blind, randomized, placebo-controlled trial reporting the effects of metformin XR on BMI in obese adolescents over a 1-year treatment period and in posttreatment follow-up. We found that the addition of metformin to a lifestyle change intervention for a period of 12 months resulted in a significant improvement of BMI, regardless of baseline fasting insulin levels, that persisted for 12 to 24 weeks after cessation of drug treatment. We found no evidence of selective attrition differing between arms of the trial, nor did conservative methods of imputation for missing data substantially attenuate the estimated effect. The mean (SE) reduction in BMI of −1.1 (0.5) at 1 year was comparable with that observed in other randomized controlled trials of metformin treatment in obese adolescents,16,18–20 although these randomized controlled trials involved shorter treatment duration (about 6 months), targeted obese children with additional diabetes risks, and had smaller sample sizes.
The major contributory factor to childhood type 2 diabetes mellitus is obesity.21 Metformin reduces the incidence of type 2 diabetes mellitus and lowers body weight in overweight and obese adults.22 The mechanisms of action for these effects have not fully been elucidated but may involve beneficial effects on carbohydrate and lipid metabolism, mediated through adenosine monophosphate kinase.23
As has been reported in shorter-term studies in obese adolescents,18,24 we did not find significant changes in central adiposity, insulin indices, or lipid indices during metformin therapy.16,19,20,24 However, we did not specifically power this study to evaluate the effect of metformin on insulin and lipid indices. Post hoc power (probability of demonstrating statistical significance for an intervention effect of the magnitude observed) did not exceed 26% for any of the measures of central adiposity listed in Table 2; 24% for insulin indices; or 14% for the lipids.
Gastrointestinal complaints are not uncommon during initiation of metformin treatment and were also noted in our study; only 1 subject discontinued therapy because of nausea. Two subjects became pregnant during study treatment (1 each in the metformin and placebo groups). The design of this study is robust, a randomized controlled trial with an intention-to-treat analysis including an adequate number of participants.25 In addition, the racial and ethnic distribution is similar to the background US adolescent population.
Metformin, in combination with lifestyle modification, had a small but statistically significant effect to reduce BMI in obese adolescents; this effect waned within 12 to 24 weeks of discontinuing metformin treatment. Metformin was safe and tolerated in this population. These results indicate that metformin may have an important role in the treatment of adolescent obesity. Longer-term studies will be needed to define the effects of metformin treatment on obesity-related disease risk in this population.
Acknowledgments
Funding/Support: The Glaser Pediatric Research Network (GPRN) consists of 5 clinical research centers and a Data Coordinating Center devoted to clinical research involving disorders important in pediatrics. The GPRN is funded by the Elizabeth Glaser Pediatric Research Foundation, a program of the Elizabeth Glaser Pediatric AIDS Foundation. The study was supported by the Elizabeth Glaser Pediatric Research Foundation and the National Institutes of Health–supported Clinical Research Centers (Stanford University, grant MO1-RR00070; Baylor College of Medicine, grant MO1-RR00188; University of California, San Francisco, grant UL-RR024131-01; University of California, Los Angeles, grant MO1-RR00865; Harvard Medical School, grant MO1-RR02172).
Glaser Pediatric Research Network Obesity Study Group
Authors
Darrell M. Wilson, MD (chair); Stephanie H. Abrams, MD; Tandy Aye, MD; Phillip D. K. Lee, MD; Carine Lenders, MD, MS, ScD; Robert H. Lustig, MD; Stavroula V. Osganian, MD, ScD; Henry A. Feldman, PhD.
Principal Investigator
Darrell M. Wilson, MD, Lucile Salter Packard Children’s Hospital, Stanford University School of Medicine.
Participating Institutions, Lead Site Investigators, and Coinvestigators (Clinical Sites Are Listed in the Order of the Number of Subjects Enrolled Into This Study)
Lucile S. Packard Children’s Hospital, Stanford University School of Medicine: lead site investigator: Patricia Fechner, MD; coinvestigators: Tandy Aye, MD, Thomas Robinson, MD, Bruce Buckingham, MD; coordinators: Trudy Esrey, RD, Keniki McNeil, RN, Beatrice Sorensen, Kirsten Wilson, Jeanne Davis, RN, FNP; Texas Children’s Hospital, Baylor College of Medicine: lead site investigator: William Klish, MD; coinvestigator: Stephanie Abrams, MD; coordinators: Pam Holt, RN, Cynthia Edwards, Linda Howard, RN; Children’s Medical Center, University of California, San Francisco: lead site investigator: Stephen Gitelman, MD; coinvestigator: Robert Lustig, MD; coordinators: Marcia Wertz, RN, Jessica Breland, Tania Lihatsh; Mattel Children’s Hospital, University of California, Los Angeles: lead site investigator: Phillip D. K. Lee, MD; coinvestigators: Anna Haddal, MD, Pinchas Cohen, MD; coordinators: Sally Shupien, Janet Mooney, RN, Elena Khanukhova, Helene Cohen, RN; Children’s Hospital Boston and Harvard Medical School: lead site investigator: Carine Lenders, MD, MS, ScD; coinvestigators: George Taylor, MD, Christopher Duggan, MD, MPH, Sam Nurko, MD, MPH; coordinators: Carol Sweeney, RN, Katie Zhang.
Coordinating Center
Children’s Hospital Boston, Clinical Research Program: lead investigator: Stavroula Osganian, MD, ScD; coinvestigator: Henry Feldman, PhD; project director: Maggie McCarthy, MS, MPH; data managers: Michael Wake, Rajna Filip-Dhima.
Glaser Pediatric Research Network Central Office
Scientific director: Charles Prober, MD; programs manager: Karen Urbanek, MS; research project manager: Alisa Kim; programs coordinators: Anita Kelley, Christine Crabtree.
Data and Safety Monitoring Board
Dennis Styne, MD (chair), Rumsey Chair of Pediatric Endocrinology, University of California, Davis; Michael Gottschalk, MD, PhD, chief, Division of Pediatric Endocrinology, University of California, San Diego; Daniel Hale, MD, chief, Division of Pediatric Endocrinology and Diabetes, University of Texas Health Science Center at San Antonio; Heidi Krause-Steinrauf, MS, lead research scientist, The Biostatistics Center, George Washington University.
Footnotes
Author Contributions: The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Wilson, Lee, Lenders, Lustig, Osganian, and Feldman. Acquisition of data: Wilson, Abrams, Aye, Lee, Lenders, Lustig, and Feldman. Analysis and interpretation of data: Wilson, Abrams, Aye, Lee, Lenders, Lustig, Osganian, and Feldman. Drafting of the manuscript: Wilson, Abrams, Aye, Lee, Lenders, Lustig, and Feldman. Critical revision of the manuscript for important intellectual content: Wilson, Abrams, Aye, Lee, Lustig, Osganian, and Feldman. Statistical analysis: Feldman. Obtained funding: Wilson and Osganian. Administrative, technical, and material support: Wilson, Abrams, Lee, Lenders, and Osganian. Study supervision: Wilson, Lee, and Lustig.
Financial Disclosure: Bristol-Myers Squibb provided active drug (Glucophage XR) and both placebos.
Additional Information: The GPRN approved the design of the trial. Journal Club slides are available at http://archpediatrics.com.
REFERENCES
- 1.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 2.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 3.August GP, Caprio S, Fennoy I, et al. Endocrine Society. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab. 2008;93(12):4576–4599. doi: 10.1210/jc.2007-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boon CS, Clydesdale FM. A review of childhood and adolescent obesity interventions. Crit Rev Food Sci Nutr. 2005;45(7–8):511–525. doi: 10.1080/10408690590957160. [DOI] [PubMed] [Google Scholar]
- 5.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 6.Ting RZ, Szeto CC, Chan MH, Ma KK, Chow KM. Risk factors of vitamin B(12) deficiency in patients receiving metformin. Arch Intern Med. 2006;166(18):1975–1979. doi: 10.1001/archinte.166.18.1975. [DOI] [PubMed] [Google Scholar]
- 7.Bauman WA, Shaw S, Jayatilleke E, Spungen AM, Herbert V. Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care. 2000;23(9):1227–1231. doi: 10.2337/diacare.23.9.1227. [DOI] [PubMed] [Google Scholar]
- 8.Mikhail C, Raynaud AS, Shepard V, Nieman P, Arceo D, Klish W. Predictors of success in a pediatric cognitive-behavioral weight control program. Tex Med. 2009;105(2):25–32. [PubMed] [Google Scholar]
- 9.Wang J, Thornton JC, Bari S, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutr. 2003;77(2):379–384. doi: 10.1093/ajcn/77.2.379. [DOI] [PubMed] [Google Scholar]
- 10.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36(1):172–177. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 11.von Scheven E, Gordon CM, Wypij D, Wertz M, Gallagher KT, Bachrach L. Variable deficits of bone mineral despite chronic glucocorticoid therapy in pediatric patients with inflammatory diseases: a Glaser Pediatric Research Network study. J Pediatr Endocrinol Metab. 2006;19(6):821–830. doi: 10.1515/jpem.2006.19.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 14.Sluiter WJ, Erkelens DW, Reitsma WD, Doorenbos H. Glucose tolerance and insulin release, a mathematical approach, I: assay of the beta-cell response after oral glucose loading. Diabetes. 1976;25(4):241–244. doi: 10.2337/diab.25.4.241. [DOI] [PubMed] [Google Scholar]
- 15.Gadbury GL, Coffey CS, Allison DB. Modern statistical methods for handling missing repeated measurements in obesity trial data: beyond LOCF. Obes Rev. 2003;4(3):175–184. doi: 10.1046/j.1467-789x.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 16.Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. 2001;107(4):E55. doi: 10.1542/peds.107.4.e55. [DOI] [PubMed] [Google Scholar]
- 17.Zucker DM, Lakatos E, Webber LS, et al. Statistical design of the Child and Adolescent Trial for Cardiovascular Health (CATCH): implications of cluster randomization. Control Clin Trials. 1995;16(2):96–118. doi: 10.1016/0197-2456(94)00026-y. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan S, Ambler GR, Baur LA, et al. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J Clin Endocrinol Metab. 2006;91(6):2074–2080. doi: 10.1210/jc.2006-0241. [DOI] [PubMed] [Google Scholar]
- 19.Love-Osborne K, Sheeder J, Zeitler P. Addition of metformin to a lifestyle modification program in adolescents with insulin resistance. J Pediatr. 2008;152(6):817–822. doi: 10.1016/j.jpeds.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyperinsulinemia: a 6-month, randomized, double-blind, placebo-controlled clinical trial. J Pediatr Endocrinol Metab. 2008;21(4):339–348. doi: 10.1515/jpem.2008.21.4.339. [DOI] [PubMed] [Google Scholar]
- 21.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 22.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgert TS, Duran EJ, Goldberg-Gell R, et al. Short-term metabolic and cardiovascular effects of metformin in markedly obese adolescents with normal glucose tolerance. Pediatr Diabetes. 2008;9(6):567–576. doi: 10.1111/j.1399-5448.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 25.Oude Luttikhuis H, Baur L, Jansen H, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009;(1) doi: 10.1002/14651858.CD001872.pub2. CD001872. [DOI] [PubMed] [Google Scholar]