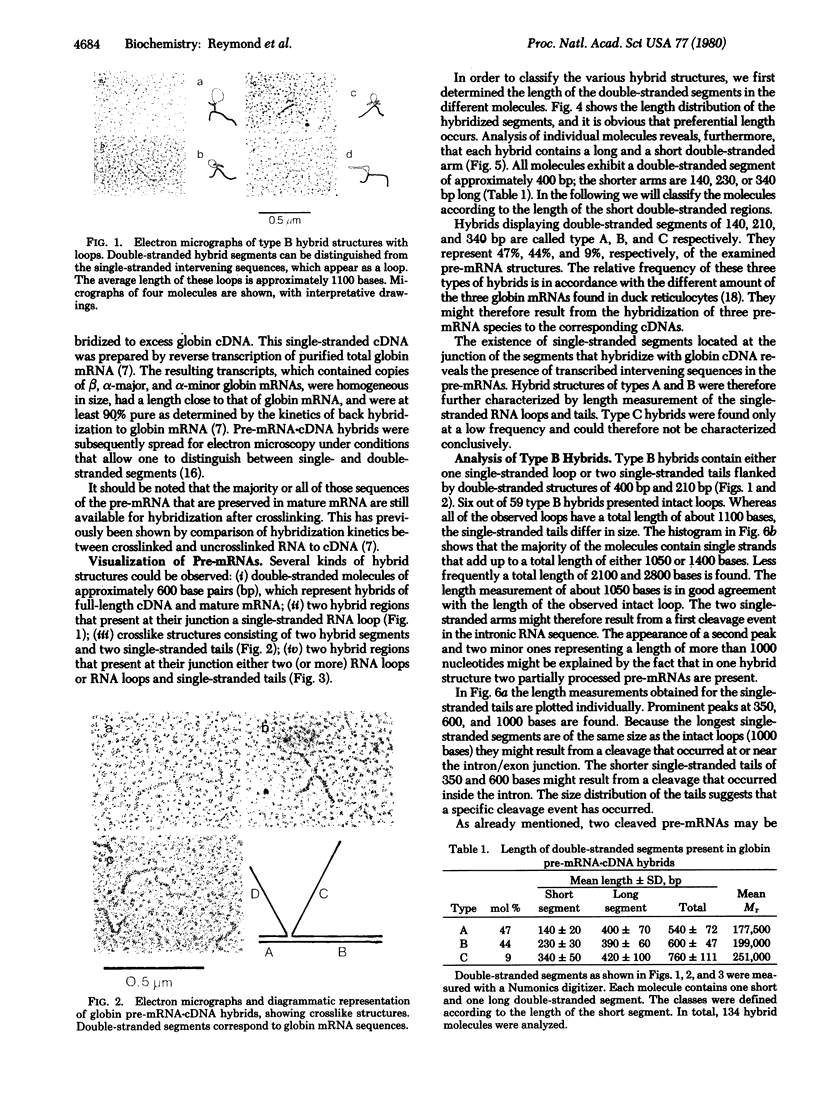
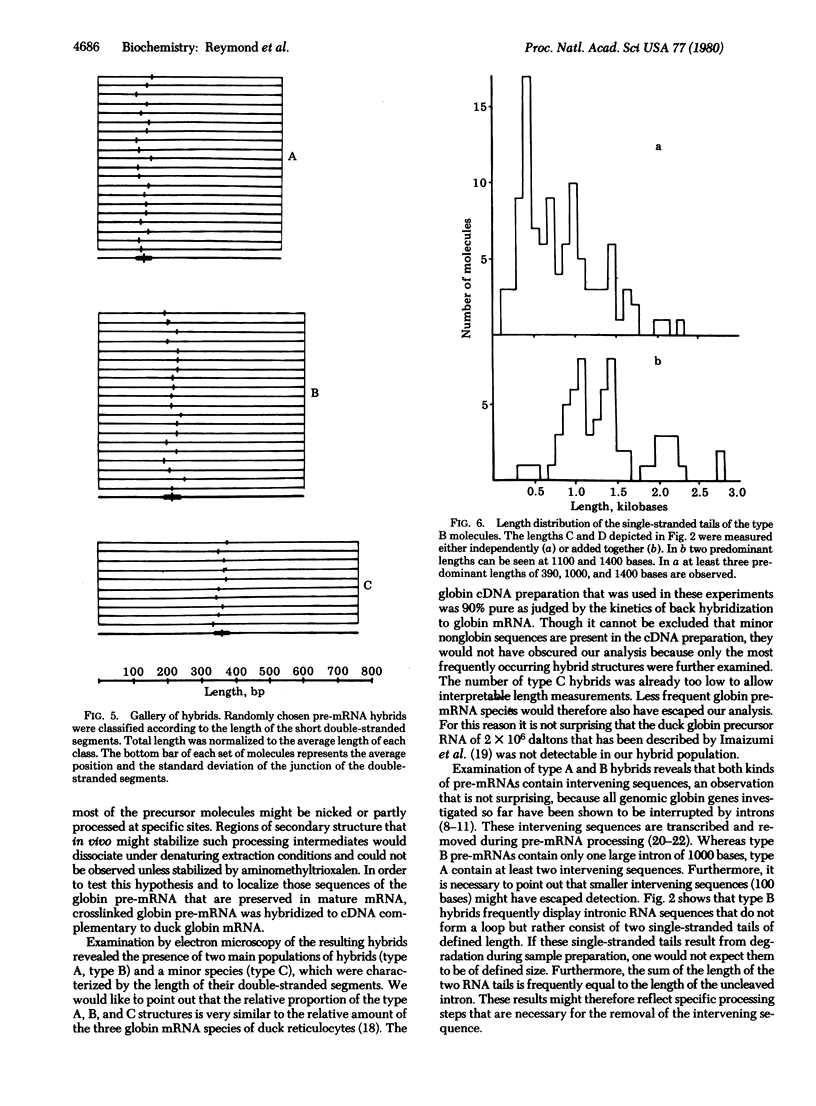
Abstract
Double-stranded RNA segments present in duck globin pre-mRNA were crosslinked in situ with aminomethyltrioxalen and UV light. The secondary structure of the crosslinked pre-mRNA was then studied by electron-microscopic analysis of pre-mRNA . cDNA hybrids. The data suggest that duck globin pre-mRNAs contain intervening sequences that are excised stepwise. Excision and subsequent ligation appears to occur on precursor molecules that are stabilized by base-paired regions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aglianó A. M., Nacci A., Reymond C., Appleby D., Spohr G. Globin mRNA precursor. Cross-linking in situ of double-stranded segments with aminomethyltrioxalen. Eur J Biochem. 1979 Mar 15;95(1):203–213. doi: 10.1111/j.1432-1033.1979.tb12955.x. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Secondary structure of heterogeneous nuclear RNA: two classes of double-stranded RNA in native ribonucleoprotein. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3705–3709. doi: 10.1073/pnas.74.9.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Fedoroff N., Wellauer P. K., Wall R. Intermolecular duplexes in heterogeneous nuclear RNA from HeLa cells. Cell. 1977 Apr;10(4):597–610. doi: 10.1016/0092-8674(77)90092-7. [DOI] [PubMed] [Google Scholar]
- Imaizumi T., Diggelmann H., Scherrer K. Demonstration of globin messenger sequences in giant nuclear precursors of messenger RNA of avian erythroblasts. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1122–1126. doi: 10.1073/pnas.70.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. The rabbit beta-globin gene contains a large large insert in the coding sequence. Cell. 1977 Dec;12(4):1097–1108. doi: 10.1016/0092-8674(77)90172-6. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R. Inverted repeated DNA from Chinese hamster ovary cells studied with cloned DNA fragments. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2679–2683. doi: 10.1073/pnas.75.6.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Molloy G., Fernandez-Munoz R., Salditt M., Darnell J. E. Secondary structure in heterogeneous nuclear RNA: involvement of regions from repeated DNA sites. J Mol Biol. 1974 Jan 25;82(3):361–370. doi: 10.1016/0022-2836(74)90597-x. [DOI] [PubMed] [Google Scholar]
- Kinniburgh A. J., Mertz J. E., Ross J. The precursor of mouse beta-globin messenger RNA contains two intervening RNA sequences. Cell. 1978 Jul;14(3):681–693. doi: 10.1016/0092-8674(78)90251-9. [DOI] [PubMed] [Google Scholar]
- Kinniburgh A. J., Ross J. Processing of the mouse beta-globin mRNA precursor: at least two cleavage-ligation reactions are necessary to excise the larger intervening sequence. Cell. 1979 Aug;17(4):915–921. doi: 10.1016/0092-8674(79)90331-3. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Jelinek W. Determination of nucleotide sequences from double-stranded regions of HeLa cell nuclear RNA. J Mol Biol. 1977 Oct 5;115(4):571–589. doi: 10.1016/0022-2836(77)90103-6. [DOI] [PubMed] [Google Scholar]
- Ryskov A. P., Saunders G. F., Farashyan V. R., Georgiev G. P. Double-helical regions in nuclear precursor of mRNA (pre-mRNA). Biochim Biophys Acta. 1973 Jun 8;312(1):152–164. doi: 10.1016/0005-2787(73)90060-9. [DOI] [PubMed] [Google Scholar]
- Spohr G., Dettori G., Manzari V. Globin mRNA sequences in polyadenylated and nonpolyadenylated nuclear precursor-messenger RNA from avian erythroblasts. Cell. 1976 Aug;8(4):505–512. doi: 10.1016/0092-8674(76)90218-x. [DOI] [PubMed] [Google Scholar]
- Spohr G., Imaizumi T., Stewart A., Scherrer K. Identification of free cytoplasmic globin mRNA of duck erythroblasts by hybridization to anti-messenger DNA and by cell-free protein synthesis. FEBS Lett. 1972 Dec 1;28(2):165–168. doi: 10.1016/0014-5793(72)80702-6. [DOI] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Polsky F. I., Seidman J. G., Leder A., Edgell M. H., Leder P. A comparison of two cloned mouse beta-globin genes and their surrounding and intervening sequences. Cell. 1978 Jun;14(2):237–245. doi: 10.1016/0092-8674(78)90110-1. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Curtis P. J., Tiemeier D. C., Leder P., Weissmann C. The intervening sequence of a mouse beta-globin gene is transcribed within the 15S beta-globin mRNA precursor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1309–1313. doi: 10.1073/pnas.75.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of RNA: processing of HeLa ribosomal RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2827–2831. doi: 10.1073/pnas.70.10.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L., Hogness D. S. R loop mapping of the 18S and 28S sequences in the long and short repeating units of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):177–192. doi: 10.1016/0092-8674(77)90213-6. [DOI] [PubMed] [Google Scholar]
- Wood T. G., Lingrel J. B. Purification of biologically active globin mRNA using cDNA-cellulose affinity chromatography. J Biol Chem. 1977 Jan 25;252(2):457–463. [PubMed] [Google Scholar]