Abstract
Injuries to connective tissues are painful and disabling and result in costly medical expenses. These injuries often require re-attachment of an unmineralized connective tissue to bone. The uninjured tendon/ligament-to-bone insertion (enthesis) is a functionally graded material that exhibits a gradual transition from soft tissue (i.e., tendon or ligament) to hard tissue (i.e., mineralized bone) through a fibrocartilaginous transition region. This transition is believed to facilitate force transmission between the two dissimilar tissues by ameliorating potentially damaging interfacial stress concentrations. The transition region is impaired or lost upon tendon/ligament injury and is not regenerated following surgical repair or natural healing, exposing the tissue to risk of re-injury. The need to regenerate a robust tendon-to-bone insertion has led a number of tissue engineering repair strategies. This review treats the tendon-to-bone insertion site as a tissue structure whose primary role is mechanical and discusses current and emerging strategies for engineering the tendon/ligament-to-bone insertion in this context. The focus lies on strategies for producing mechanical structures that can guide and subsequently sustain a graded tissue structure and the associated cell populations.
Keywords: Engineering of graded tissues, tendon-to-bone insertion site, fibrocartilage, mineralized fibrocartilage, enthesis, mechanical conditioning of engineered tissues
Introduction
Connective tissue injuries occurring at or near the interface between soft tissue and bone commonly result in pain, disability and significant medical costs [1]. Tears of the rotator cuff tendons of the shoulder and the cruciate ligaments of the knee, for example, normally require surgical attachment of a tendon to its bony insertion. Uninjured insertions possess a transitional region between soft tissue and bone exhibiting gradations in cell phenotype, tissue organization, tissue composition, and tissue mechanical properties. The gradations facilitate the effective transfer of load between two materials with vastly different mechanical properties (soft, compliant tendon/ligament to hard, stiff bone) by reducing the potentially damaging stress concentrations that would otherwise form at the interface [2–5]. Following surgical or natural repair processes, this transitional region is not regenerated. Rather, tendon-to-bone healing results in a mechanically inferior fibrovascular tissue at the repair site [6–9]. This exposes the insertion to high stresses and an increased risk for failure. As a result, current surgical repair techniques have high re-injury rates; up to 94% of repaired massive rotator cuff tears fail [10] and 56% of ACL reconstruction patients experience knee pain 1 year post-surgery [11]. Multiple biological and mechanical factors influence soft tissue healing, but regardless, many patients experience less than satisfactory outcomes. Researchers have begun to focus on tissue engineered constructs for tendon/ligament-to-bone repair. This review will discuss several current and developing strategies for producing constructs possessing biological and mechanical properties suitable for insertion site repair [12–17].
Tendon/ligament-to-bone insertion site healing: surgical challenges
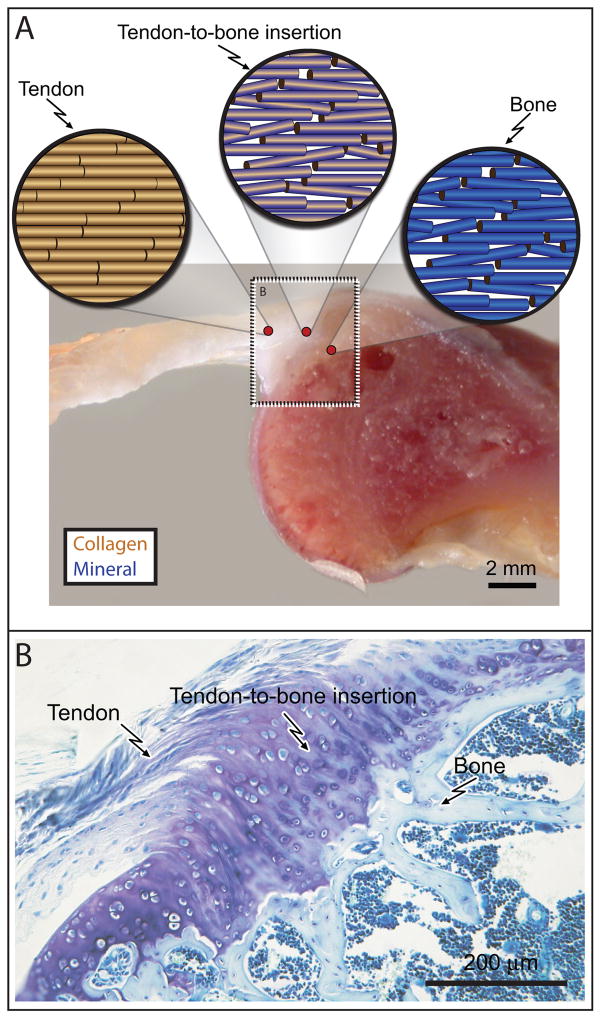
The tendon/ligament-to-bone insertion site presents a functionally graded material system exhibiting a gradual transition from unmineralized tissue (i.e., tendon or ligament) to unmineralized fibrocartilage to mineralized fibrocartilage to mineralized tissue (i.e., bone), exhibiting no distinct boundaries between tissue regions (Figure 1) [18–20]. In tendon and ligament, collagen type I and tendon/ligament cells are aligned in highly ordered arrays oriented in the direction of tensile force. Fibrocartilage is rich in collagen type II (normally observed in articular cartilage) with high levels of pericellular collagen type III and small amounts of collagen types I and X, decorin, and aggrecan [2, 21]. The mineralized fibrocartilage is primarily comprised of collagen type II, significant amounts of collagen type X and substantial levels of aggrecan [22]. Fibril alignment in mineralized fibrocartilage is appreciably less organized than fibrocartilage and tendon proper [2]. Finally, bone is comprised of about 50% by volume of a relatively stiff carbonated hydroxyl apatite mineral within a scaffold of type I collagen. Accordingly, the insertion site exhibits a graded increase in mineral content with a corresponding graded decrease in tissue organization from tendon/ligament to bone [2]. These competing gradations result in a region of tissue between tendon and bone that is more compliant than either tendon or bone [4, 23–25]. The associated gradations in mechanical properties of the insertion site tissue is believed to ameliorate stress concentrations [5, 23], facilitating safe force transmission.
Figure 1.
(A) A schematic of collagen fibers is shown above a cross-sectional view of a rat supraspinatus tendon-to bone insertion. Blue shading indicates the concentration of mineral within each fiber. Note that the collagen fiber orientation distribution becomes less organized from tendon to bone. (B) A histologic section of a rat supraspinatus tendon-to-bone insertion is shown, demonstrating the change in cell morphology and matrix composition from tendon to bone (Toluidine blue stain).
This highly effective grading and its associated effective stress transfer is in contrast to the less organized tissue and presumably higher localized stresses that arise at abrupt interfaces, seen between repaired tendon/ligament and bone [8, 26]. Two examples follow of tendon-to-bone attachments that are injured routinely, heal poorly, and are difficult to repair surgically.
Rotator Cuff
The rotator cuff is a group of muscles in the shoulder that provides stability and motion to the glenohumeral joint [27]. Rotator cuff injuries can involve one or more tendon tears from the humeral head, leading to loss of function, instability and pain. The prevalence of tears is high; approximately 30% of the population over 60 years of age has a rotator cuff tear [28, 29]. Repair to recover shoulder function is a common orthopedic surgical procedure, with over 75,000 repairs performed each year in the United States [30]. Although the tendons are effectively re-apposed to their pre-injury anatomic footprint, the functionally graded transitional tissue at the uninjured tendon-to-bone interface is not regenerated. The quality of the resulting tissue differs significantly from normal, with inferior structural and viscoelastic properties [26]. Poor anatomic restoration of a robust attachment after surgical rotator cuff repair is a well-known challenge, with clinical studies showing failure rates ranging from 30–94% [10, 31]. Further complicating surgical outcomes is the presentation of chronic injuries; chronicity often results in tendon retraction [32], tendon stump fraying, and osteolysis [6] at the insertion site.
Anterior Cruciate Ligament
The most commonly injured ligament in the knee is the anterior cruciate ligament (ACL), resulting in approximately 100,000 ACL reconstructions in the US each year [33]. Injury to the ACL reduces knee stability and predisposes the patient to osteoarthritis later in life. Difficulty in primary repair of the torn ACL requires that auto- and allo-grafts are used to reconstruct the ligament. The grafts are either resected from cadavers or from the patient’s own donor sites. Bone-tendon-bone (BTB) tissue, taken from the central third of the patellotibial junction of the uninjured knee, is commonly used as a graft for ACL reconstruction. The ends of the BTB are secured into tibial and femoral bone tunnels, replacing the function of the torn ACL. This method facilitates bone-to-bone integration of the graft to the lumen of each bone tunnel. Additionally, this BTB grafting keeps the graft insertions intact, maintaining the natural tendon-to-bone architecture and moving the critical point of failure from the tendon-to-bone interface to the interluminal bone-to-bone interface. However, BTB graft harvest damages an otherwise healthy donor site, contributing to patient morbidity [11], chronic joint pain [11], and osteoarthritis [34] at the donor site.
A second common ACL reconstruction procedure involves fixation of a hamstring or gracilis tendon between the tibia and femur. First, bone tunnels are drilled into the distal femur and proximal tibia. One end of the graft tendon is inserted into each tunnel and an interference screw is used to secure the graft to the side of each tunnel. While use of this method minimizes donor site complications, interference screw threads are at risk of becoming failure points due to graft impingement and subsequent laceration [35, 36]. Graft slippage between the screw and bone tunnel can also occur, resulting in joint laxity and subsequent instability [37].
The utility of allograft sourced BTB, hamstring, and gracilis grafts is reduced by graft availability, sterilization processing costs, reduced biological and mechanical integrity due to the associated processing [38, 39], and a risk of transmitting donor pathogens. The drawbacks of existing graft repair techniques motivate the need for a tissue construct capable of bearing load while minimizing stress concentrations and donor site morbidity. Accordingly cell- and biomaterial- based strategies for tendon-to-bone insertion engineering are an area of growing interest and research activity.
Tissue-engineered healing strategies
The challenges associated with tissue engineered solutions to tendon-to-bone attachment are many and varied. From the perspective of the mechanical role that defines the function of the tendon-to-bone insertion site, three central challenges emerge. First, the tendon-to-bone insertion site must be stabilized mechanically during healing. Second, the structure present during healing must guide reconstruction of a natural, graded tissue. Third, it must culminate in a tissue system in which, recursively, a spatially-varying population of cells is sustained that maintains a graded mechanical structure, and a graded mechanical structure is maintained that sustains a spatially-varying population of cells. The focus here is the second challenge, and the review is presented from the perspective of cell populations and mechanical functions and stimuli.
Two basic paradigms have been established in tendon/ligament-to-bone construct design (Figure 2). The first approach utilizes stratified constructs in which each biomaterial stratum is seeded with a separate cell type relevant to a certain region of the insertion and the strata are held together in series [14, 40–46]. In this paradigm, it is hypothesized that multiple cell phenotypes in close proximity to each other will initiate cell-mediated metaplasia, resulting in an insertion-site like graded tissue. The second approach utilizes a single pluripotent cell type that is stimulated by gradations in local stimuli [17, 47–49]. In this paradigm, it is hypothesized that biochemical and mechanical stimuli will promote local cell differentiation, resulting in graded changes in cell and tissue type. Typically, adult mesenchymal stem cells (MSCs) are used since they can be isolated from a range of tissues and then differentiated into insertion-relevant cell types [50–52].
Figure 2.
Two basic paradigms have been established in tendon/ligament-to-bone tissue engineering. In the stratified construct approach, each biomaterial stratum is seeded with a separate cell type and the strata are held together in series. In this paradigm, communication between cell phenotypes in close proximity to each other will initiate cell-mediated metaplasia, resulting in an insertion-site like graded tissue. In the gradient construct approach, a single pluripotent cell type seeded onto a functionally graded scaffold. In this paradigm, local stimuli promote local cell differentiation, resulting in graded changes in cell and tissue type.
Stratified insertion constructs
Stratified constructs for tissue engineering have been developed for a number of tissues, including tendon/ligament-to-bone interfaces [40, 41, 53] and osteochondral interfaces [44–46, 54–56]. Wang et al., and Spallazzi et al. studied heterotypic interactions between different mature cell populations seeded adjacent to each other in a series of strata representing the tissue phases of the insertion [14, 42]. They hypothesized that fibroblast-osteoblast interaction would result in fibrocartilage cell transdifferentiation at the interface of the two cell types. In a two-dimensional co-culture study, fibroblasts and osteoblasts were plated opposite to each other in a tissue culture dish with a gate separating the two cell populations [42]. Migrating cells at the co-culture interface demonstrated increased cartilage specific type II collagen and COMP gene expression, indicating that insertion related transdifferentiation may be stimulated by fibroblast-osteoblast interactions. Spalazzi et al. then investigated the role of cell-cell interactions in a 3-dimensional 3-phase co-culture model [14]. Phase A was a porous poly (lactic-co glycolic acid) (PLGA) (10:90) matrix seeded with fibroblasts and served as the tendon formation region. Phase C was composed of sintered PLGA (85:15) and 45S5 glass microspheres and was seeded with osteoblasts to represent the bone phase. The unseeded fibrocartilage phase (phase B) was composed of PLGA (85:15). Both fibroblasts and osteoblasts migrated into phase B, significant collagen type I deposition was observed in phases A and B, and phase C was highly mineralized. Despite tendon-like and bone-like formation in the extremities of the stratified scaffold, a fibrocartilaginous region in phase B was not realized. In order to produce a fibrocartilage region, chondrocytes were included in Phase B and examined in a subcutaneous co-culture system [40]. Using three cell populations resulted in tissue continuity across the three phases, mineralization in phase C, and fibrocartilage between phases A and B. These studies indicated that a representative ligament-to-bone construct could be created with the appropriate cell types and extracellular matrix components using a stratified approach.
Seeding multiple cell types into a stratified tissue composite poses practical challenges for clinical use. Isolating mature fibroblasts, chondrocytes, and osteoblasts from three separate sites involves considerable cost and possibly prohibitive levels of pain. Research led by Ma et al. presented a possible solution to the difficulties of seeding multiple cell types by culturing tissue phases separately with MSCs and allowing them to self assemble [41]. Compared to primary cell isolations, this approach requires only one extraction site with the potential for higher extraction yields and faster cell proliferation. MSC populations were separately cultured into sheets of bone- and ligament-like cells. The osteoblast sheet was then layered onto the ligament sheet and the layered composite was rolled upon itself into a 3 dimensional bone-ligament-bone construct. In vivo, these 3-D composites formed viable ligament replacements with bimodal changes in tissue properties. However, cell-cell interaction between osteoblast-like and fibroblast-like cells did not generate a fibrocartilage transition region, despite long term implantation. From the mechanical perspective, constructs of this character must achieve mechanical integrity and an advanced structural form prior to implantation. The specific challenge that must be overcome is that layered solids are inherently unstable and prone to stress concentrations [57, 58]. Sharp interfaces must therefore be attenuated prior to implantation.
Graded insertion constructs
Functional gradations in cell response relevant to the insertion can be synthesized using a variety of approaches. Local variations in stimulus cues can be used to promote a gradation in cell differentiation. Ideally, the construct produced should have a continuous change in properties, thereby generating a graded tissue serving to reduce stress concentrations at the interface.
At the tendon-to-bone insertion, spatial changes in biological factors correlate to spatial changes in tissue composition and structure. This serves to maintain cell phenotype and therefore region-specific tissue integrity [59–62]. Similarly, it may be necessary for engineered insertion constructs to present gradients in biological factors that spatially regulate cell/tissue type. Using this approach, Phillips et al. seeded fibroblasts on a substrate that had been conjugated with a linearly graded increase in Runx2, an osteoblastic transcription factor [47]. A roughly graded increase in osteoblastic transdifferentiation, positively correlated to Runx2 concentration, was observed, indicating that a graded interface between tendon-like and bone-like properties could be accomplished. In a separate study, MSCs were cultured on the surface of silk constructs adsorbed with BMP-2 and IGF-I in osteochondral medium [49]. Increasing BMP-2 content while simultaneously decreasing IGF-I content resulted in graded increases in calcium and GAG deposition and increases in collagen type I, II, and X gene transcription. The gradient achieved closely resembled the transition from unmineralized fibrocartilage to mineralized fibrocartilage to bone found in the natural insertion. Therefore, there is potential for using biochemical gradients to control cellular differentiation relevant to the engineering a tendon/ligament-to-bone transition region. Moreover, proper selection and spatial distribution of biochemical factors can lead to a tissue composite exhibiting insertion-like tissue transitions.
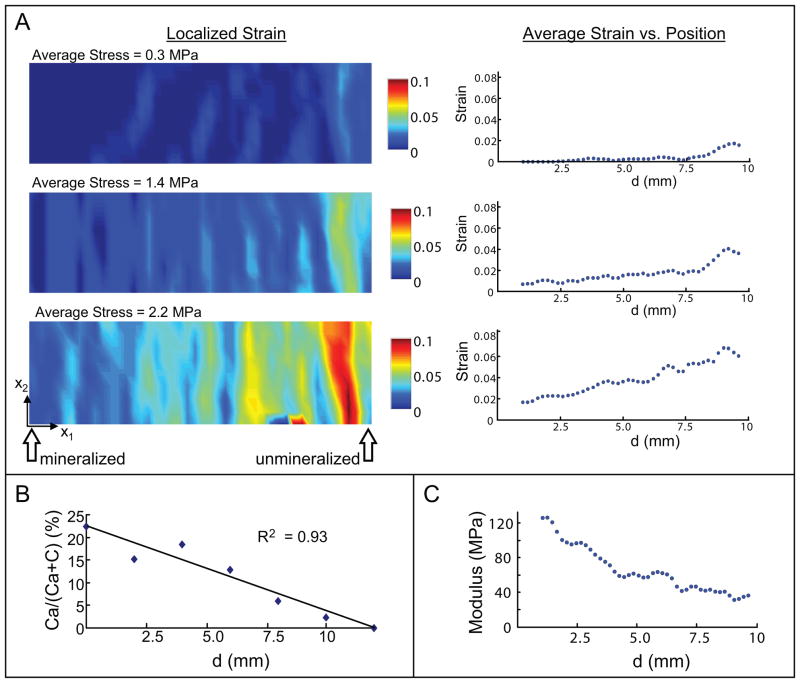
In a study by Li et al, simulated body fluid was used to generate a mineral gradient on electrospun nanofibers of PLGA [17]. Tensile tests indicated that mineral content was positively correlated with the local elastic modulus (Figure 3). Both increased mineral content and increased matrix stiffness have been positively correlated with osteogenesis in MSCs [63]. Culturing MSCs on a mineral-rich construct is sufficient to stimulate osteogenesis in lieu of osteogenic culture medium [64]. Therefore, though the influences of mineral content and substrate stiffness have not been decoupled, it is possible that the gradation in mineral content can serve to mitigate interfacial stress concentrations and promote graded stem cell differentiation.
Figure 3.
Nanofiber scaffolds were synthesized with gradations in mineral to mimic the natural tendon-to-bone insertion. There was a gradation in mechanical properties along the length of the scaffolds (a representative PLGA scaffold is shown). (A) The strains in the x1 direction for three values of stress are shown. Localized strains are shown on the left and average strains are shown on the right. Strain increased with increasing stress and was highest on the unmineralized side of the scaffold. (B) There was a linear decrease in calcium phosphate along the length of the scaffold. (C) Young’s modulus decreased with decreasing calcium phosphate content. [Reproduced, with permission, from: Li X, Xie J, Lipner J, Yuan X, Thomopoulos S, Xia Y. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Letters. 2009;9(7):2763–8.]
Photolithography techniques were used in a recent study to generate biomolecular spatial patterns on collagen-based scaffolds [65]. To demonstrate the technique, fibronectin was immobilized within the scaffolds. This led to increased speed of cell attachment relative to unmodified scaffolds. Various spatial patterns were also created using fluorescently labeled molecules. This methodology for incorporating biomolecules into collagen scaffolds has broad application for tendon/ligament tissue engineering, where spatial control of cell activity is critical.
Mechanics as a driving force for differentiation
In both of the above-mentioned strategies, the differentiation and sustenance of a cell population is a key challenge. While many factors play a role in these processes, regulation of cell behavior via mechanics is an important factor at the insertion site. We focus here on mechanical cues that are emerging as potentially dominant factors in cell differentiation, morphology, and homeostasis.
Researchers have postulated that zonal variations in stress at the insertion may be partially responsible for the graded changes in cell differentiation, cell morphology, tissue composition and subsequent tissue properties, indicating an interdependent relationship between mechanical stress and tissue formation [18, 60, 66–68]. For example, the tendinous region distal to the insertion interface is subjected to uniaxial tension and exhibits spindle-like cellular alignment along the axis of loading and high tensile stiffness [69]. In contrast, the parts of the insertion subjected to compressive and/or shear stresses exhibit cartilage-specific extracellular matrix and cellular organization [60]. In order to engineer an insertion possessing spatial changes in tissue properties capable of mitigating stress concentrations, researchers have sought to exploit mechanically regulated tissue differentiation and organization to produce insertional tissue components. Initial studies, though normally focused on one stress component or tissue type, have nonetheless provided key insights into the influences of mechanical stress on insertion engineering.
In a study by Kuo et al., tensile stress applied to MSCs embedded in type I collagen exhibited scleraxis upregulation [70]. Scleraxis is a transcription factor necessary for tenogenesis. Tendon related matrix metalloproteinase and collagen type I upregulation were also observed, indicating that tissue remodeling and matrix deposition were increased due to tensile stress. In related studies, tensile stress applied to MSCs in vitro resulted in increases in tendon-related matrix deposition and cell alignment [12, 71]. In a study by Juncosa-Melvin et al, MSC-seeded collagen type I sponges were loaded in cyclic tension for up to two weeks. Compared to unstimulated constructs, mechanically loaded constructs exhibited 3–4 times greater collagen type gene expression and a 4-fold increase in linear modulus [71]. The tension-regulated tenogenic differentiation demonstrates that mechanical load can be used to promote the formation tendon.
Regions of tendon under compression in vivo generate sesamoid fibrocartilage [60, 68]. This has led investigators to suggest that fibrocartilage at the tendon-to-bone interface develops due to a local transdifferentiation by tendon fibroblasts in response to compressive and bending forces [66]. Beaupre et al. observed that compression of an MSC-laden collagen matrix prompted the expression of Sox9, a chondrogenic transcription factor [16]. A collagen matrix similarly seeded and subjected to tension did not exhibit chondrogenic behavior. Related studies of MSCs under compression in vitro have demonstrated biochemically-determined increases of cartilage-specific collagens and qPCR-determined upregulation of glycosaminoglycans and transcription factors [72–77]. Studies have further indicated that MSC compression in vitro can generate chondrogenesis on par with biochemically generated chondrogenesis [73].
Mechanically regulated differentiation is advantageous because different loading motifs can be applied in close proximity to one another; one region can be stimulated for tenogenesis and another for chondrogenesis [78], thereby generating two critical phases of the insertion. Thomopoulos et al. investigated fibrocartilage generation with multipotent cells by observing how biochemical and mechanical (tensile, compressive, static, and cyclic) cues together modulate differential and phenotypic behavior over a 7 day culture period [78]. MSCs in a 3D collagen matrix were subjected to compressive and tensile stresses as a result of externally applied static or cyclic hydrostatic tension. Cyclic stresses applied in pure tension promoted spindle-shaped cells aligned with the direction of tension and upregulation of scleraxis and type one collagen mRNA expression. Cells experiencing tension with a single component of compressive stress exhibited randomly oriented cells with rounded morphology. In general, significant differences in Sox9 and aggrecan mRNA expression were not observed when comparing cyclic and static loading unless TGF-β3 was present in the culture medium. Compared to cyclically loaded cultures, TGF-β3 supplementation of static cultures resulted in upregulated Sox9 mRNA expression while cyclically loaded cultures resulted in the upregulation of aggrecan mRNA when compared to statically loaded cultures. The authors concluded that mechanical stimuli alone could produce the fibrous component of fibrocartilage while biochemically coupled mechanical cues were required for production of the cartilaginous component of fibrocartilage.
Lima et al. developed a culture model comprised of a hydrogel contiguous with a hydrogel/cancellous bone, roughly duplicating the interface of cartilage with bone [54]. Despite a biphasic change in stiffness, compressing the construct generated a graded decrease in axial stress from the gel surface to the gel interface with bone. Cells near the interface exhibited upregulated chondrogenesis compared to cells more distal to the interface, demonstrating modulated cell response to graded changes in strain.
Recent work indicates that mechanically coupled differentiation may be more complex/nuanced than applying biochemical and mechanical stimuli simultaneously [79]. Connelly et al. compared free-swelling MSC-seeded fibrin constructs cultured for 21 days to free-swelling constructs cultured for 7 days followed by 14 days of cyclic tension [80]. Both groups were cultured in chondrogenic TGF-β supplemented medium for the duration of the experiment. From day 14 to 21, both groups exhibited similar increases in chondrogenic gene activity compared to day 7 free-swelling controls. However, collagen I expression was significantly increased in the cyclically loaded group compared to same day free-swelling groups. Modulation of growth factor-regulated chondrogenesis by cyclic tension can therefore generate fibrous tissue components, resulting in a fibrocartilage-like differentiation.
Thorpe et al. observed that agarose embedded MSCs subjected to 3 weeks of culture in TGF-β3-enriched medium followed by 3–6 weeks of culture in TGF-β3 plus cyclic compression had enhanced glysosaminoglycan content compared to constructs subjected to both TGF-β3 and compression from the start of culture [79]. These recent results imply that the sequence of stimulus application (mechanical and biochemical) can influence the formation of fibrobcartilage.
However, despite this progress, major challenges remain. The fundamental mechanisms by which cells in general and MSCs in particular respond to mechanical loading are active areas of inquiry [81], with hypotheses including strictly mechanical pathways, such as signalling cascades triggered by force-induced unfolding of cryptic domains [82, 83], stress-induced fluidization and rebuilding of cytoskeletal features [84], and stress-induced changes to cell nuclei [85]. While cell differentiation can be directed effectively in some cases by a combination of mechanical and soluble factors, progress must also be made in understanding the fundamental mechanisms for mechanical interaction between cells and their environment.
Cell Sources
The majority of stem cell studies in musculoskeletal tissue engineering use mesenchymal stem cells derived from bone marrow (MSCs). These cells respond to biochemical and mechanical stimuli in a predictable manner to differentiate into various musculoskeletal tissue cell types [86]. Despite thorough research and optimization of bone marrow derived stem cells for tissue engineering, adipose derived mesenchymal stem cells (ASCs) offer an alternative cell source for tendon/ligament-to-bone tissue engineering with some advantages over MSCs [51, 52, 87–89]. For example, ASCs can be isolated from adipose tissue harvested by lipoaspiration or block dissection, registering lower on the pain scale than bone marrow isolation. Monolayer studies have demonstrated that ASCs may proliferate faster than bone marrow derived MSCs and continue to proliferate after many population doublings. The ASC senescence ratio stays stable at 5.6% from 2 to passage 8, compared to 23% and 9.5% for MSCs at passages 2 and 7 respectively [52]. Grimes et al. also observed significant genomic ASC stability, with total ASC chromosomal abnormality below 5% after 35 population doublings [90]. Due to the isolation and proliferation characteristics of ASCs described above, the delay between injury and treatment can be minimized. However, their responses in vivo with regard to tendon-to-bone insertion tissue engineering have not yet been characterized.
Accordingly, researchers have begun exploring ASCs and their multipotent potential to engineer components of the tendon-to-bone insertion. James et al. demonstrated tenogenic ASC differentiation on electrospun nanofiber PLGA scaffolds using GDF-5 enriched culture medium [91]. Specifically, after 14 days in culture, ASCs cultured in 100 ng/ml GDF-5 supplemented medium exhibited a early 3-fold increase in collagen type I and scleraxis gene expression compared to controls. Similar results were reported by Park et al. [92].
Awad et al. were able to modulate chondrogenic ASC differentiation based on extracellular matrix cues [93]. ASCs in algae derived matrices (agarose and alginate) were compared to ASCs in mammal derived matrix (gelatin) using identical seeding density and culture conditions. While outcomes characteristic of chondrogenesis (e.g., collagen II, chondroitin sulfate deposition) were observed in all three cultures, gelatin cultures induced the best response. This suggests that biologically active matrices such as gelatin are capable of inducing differentiation in mesenchymal stem cells.
Erisken et al. seeded ASCs on a PCL construct containing opposing IGF and Beta-glycerophosphate (BGP) gradients [94]. The authors observed chondrogenic differentiation as a function of IGF concentration as well as increased ALK-1 and mineral deposition in proportion to BGP concentration, demonstrating the ability of ASCs to develop graded osteogenic and chondrogenic components representative of the tendon-to-bone insertion.
Conclusions: challenges and opportunities
The tendon/ligament-to-bone insertion is a functionally graded tissue with multiple cell types and extracellular matrix components. Current treatment approaches for injured insertions do not regenerate the grade tissue interface. Poor clinical outcomes and sub-optimal tissue sources for grafts motivate the need for biologically active tissue engineering solutions. Scaffold design should take into consideration the continuous, graded nature of the native insertion. Strategies involving multipotent mesenchymal stem cells are attractive, as these cells can be stimulated by local factors to regulate cell differentiation to form functionally graded tissues. Although most orthopaedic tissue engineering studies using stem cells has been performed with MSCs, ASCs may eventually present a more attractive source of cells clinically. The development of a successful tissue engineered tendon/ligament-to-bone insertion will require a functionally graded construct design, thoughtful recapitulation of the natural insertion’s temporally and spatially resolved cues (both mechanical and biochemical), and careful selection of an clinically attractive and plentiful source of pluripotent cells.
Many scientific hurdles remain before these goals can be achieved. The basic mechanics leading to a robust tendon-to-bone attachment is still an active area of inquiry. It is unclear what the minimum design criteria are for an effective tissue replacement for the tendon-to-bone insertion site. If these properties are achieved in vitro, how will the system, which contains a spatially-varying population of cells maintaining a graded mechanical structure, be sustained in vivo? While we have reviewed several specific examples of success, a fundamental understanding of how cells interact mechanically with their environment is lacking. Models relating mechanical environment with cell differentiation, morphology, and function are needed. Future studies should combine applied tissue engineering efforts with mechanistic basic science efforts to achieve a successful replacement for the tendon/ligament-to-bone insertion.
Acknowledgments
National Institutes of Health (AR055184, AR060820) and National Science Foundation (CAREER 844607).
References
- 1.Praemer A, Furner S, Rice D, editors. Musculoskeletal conditions in the United States. Park Ridge IL: American Academy of Orthopaedic Surgeons; 1992. [Google Scholar]
- 2.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. Journal of Orthopaedic Research. 2003;21(3):413–9. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 3.Thomopoulos S, Marquez JP, Weinberger B, Birman V, Genin GM. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J Biomech. 2006;39(10):1842–51. doi: 10.1016/j.jbiomech.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Genin GM, Kent A, Birman V, Wopenka B, Pasteris JD, Marquez PJ, Thomopoulos S. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys J. 2009;97(4):976–85. doi: 10.1016/j.bpj.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Birman V, Chen C, Thomopoulos S, Genin GM. Mechanisms of bimaterial attachment at the interface of tendon to bone. Journal of Engineering Materials and Technology. 2011;133(1) doi: 10.1115/1.4002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galatz LM, Rothermich SY, Zaegel M, Silva MJ, Havlioglu N, Thomopoulos S. Delayed repair of tendon to bone injuries leads to decreased biomechanical properties and bone loss. Journal of Orthopaedic Research. 2005;23(6):1441–7. doi: 10.1016/j.orthres.2005.05.005.1100230629. [DOI] [PubMed] [Google Scholar]
- 7.Newsham-West R, Nicholson H, Walton M, Milburn P. Long-term morphology of a healing bone–tendon interface: a histological observation in the sheep model. Journal of Anatomy. 2007;210(3):318–27. doi: 10.1111/j.1469-7580.2007.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva MJ, Thomopoulos S, Kusano N, Zaegel MA, Harwood FL, Matsuzaki H, Havlioglu N, Dovan TT, Amiel D, Gelberman RH. Early healing of flexor tendon insertion site injuries: Tunnel repair is mechanically and histologically inferior to surface repair in a canine model. J Orthop Res. 2006;24(5):990–1000. doi: 10.1002/jor.20084. [DOI] [PubMed] [Google Scholar]
- 9.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. Journal of Bone & Joint Surgery -American Volume. 1993;75(12):1795–803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219–24. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Corry IS, Webb JM, Clingeleffer AJ, Pinczewski LA. Arthroscopic reconstruction of the anterior cruciate ligament. The American Journal of Sports Medicine. 1999;27(4):444–54. doi: 10.1177/03635465990270040701. [DOI] [PubMed] [Google Scholar]
- 12.Altman G, Horan R, Martin I, Farhadi J, Stark P, Volloch V, Vunjak-Novakovic G, Richmond J, Kaplan DL. Cell differentiation by mechanical stress. FASEB J. 2002;16(2):270–2. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 13.Yang PJ, Temenoff JS. Engineering orthopedic tissue interfaces. Tissue Engineering Part B: Reviews. 2009;15(2):127–41. doi: 10.1089/ten.teb.2008.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spalazzi JP, Doty SB, Moffat KL, Levine WN, Lu HH. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Engineering. 2006;12(12):3497–508. doi: 10.1089/ten.2006.12.3497. [DOI] [PubMed] [Google Scholar]
- 15.Spalazzi JP, Vyner MC, Jacobs MT, Moffat KL, Lu H. Mechanoactive scaffold induces tendon remodeling and expression of fibrocartilage. Clinical Orthopedics Related Research. 2008;466:1938–48. doi: 10.1007/s11999-008-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li KW, Lindsey DP, Wagner DR, Giori NJ, Schurman DJ, Goodman SB, Smith RL, Carter DR, Beaupre GS. Gene regulation ex vivo within a wrap-around tendon. Tissue Engineering. 2006;12(9):2611–8. doi: 10.1089/ten.2006.12.2611. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Xie J, Lipner J, Yuan X, Thomopoulos S, Xia Y. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Letters. 2009;9(7):2763–8. doi: 10.1021/nl901582f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons--tendon ‘entheses’. Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology. 2002;133(4):931–45. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 19.Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. Journal of Anatomy. 1986;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw HM, Benjamin M. Structure–function relationships of entheses in relation to mechanical load and exercise. Scandinavian Journal of Medicine & Science in Sports. 2007;17(4):303–15. doi: 10.1111/j.1600-0838.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 21.Kumagai J, Sarkar K, Uhthoff HK, Okawara Y, Ooshima A. Immunohistochemical distribution of type I, II and III collagens in the rabbit supraspinatus tendon insertion. Journal of Anatomy. 1994;182(2):279–84. [PMC free article] [PubMed] [Google Scholar]
- 22.Galatz L, Rothermich S, VanderPloeg K, Petersen B, Sandell L, Thomopoulos S. Development of the supraspinatus tendon-to-bone insertion: localized expression of extracellular matrix and growth factor genes. Journal of Orthopaedic Research. 2007;25(12):1621–8. doi: 10.1002/jor.20441. [DOI] [PubMed] [Google Scholar]
- 23.Liu YX, Thomopoulos S, Birman V, Lee JS, Genin GM. Bi-material attachment through a soft tissue interfacial system. Mechanics of Materials. 2011 doi: 10.1016/j.mechmat.2011.08.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. Journal of Orthopaedic Research. 2003;21(3):413–9. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 25.Stouffer DC, Butler DL, Hosny D. The relationship between crimp pattern and mechanical response of human patellar tendon-bone units. Journal of Biomechanical Engineering. 1985;107(2):158–65. doi: 10.1115/1.3138536. [DOI] [PubMed] [Google Scholar]
- 26.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. Journal of Biomechanical Engineering. 2003;125(1):106–13. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 27.Soslowsky LJ, Carpenter JE, Bucchieri JS, Flatow EL. Biomechanics of the rotator cuff. Orthopedic Clinics of North America. 1997;28(1):17–30. doi: 10.1016/s0030-5898(05)70261-3. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88(8):1699–704. doi: 10.2106/JBJS.E.00835. [DOI] [PubMed] [Google Scholar]
- 29.Lehman C, Cuomo F, Kummer FJ, Zuckerman JD. The incidence of full thickness rotator cuff tears in a large cadaveric population. Bull Hosp Jt Dis. 1995;54(1):30–1. [PubMed] [Google Scholar]
- 30.Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181–7. doi: 10.1016/j.jse.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Harryman DT, Mack LA, Wang KY, Jackins SE, Rischardson ML, FAM Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. Journal of Bone and Joint Surgery. 1991;73(7):982–9. [PubMed] [Google Scholar]
- 32.Neri BR, Chan KW, Kwon YW. Management of massive and irreparable rotator cuff tears. Journal of Shoulder and Elbow Surgery. 18(5):808–18. doi: 10.1016/j.jse.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Brown JCH, Carson EW. Revision anterior cruciate ligament surgery. Clinics in Sports Medicine. 1999;18(1):109–71. doi: 10.1016/s0278-5919(05)70133-2. [DOI] [PubMed] [Google Scholar]
- 34.Keays SL, Bullock-Saxton JE, Keays AC, Newcombe PA, Bullock MI. A 6-year follow-up of the effect of graft site on strength, stability, range of motion, function, and joint degeneration after anterior cruciate ligament reconstruction. The American Journal of Sports Medicine. 2007;35(5):729–39. doi: 10.1177/0363546506298277. [DOI] [PubMed] [Google Scholar]
- 35.Weiler A, Peine R, Pashmineh-Azar A, Abel C, Südkamp NP, Hoffmann RFG. Tendon healing in a bone tunnel. Part I. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2002;18(2):113–23. doi: 10.1053/jars.2002.30656. [DOI] [PubMed] [Google Scholar]
- 36.Zantop T, Weimann A, Schmidtko R, Herbort M, Raschke MJ, Petersen W. Graft laceration and pullout strength of soft-tissue anterior cruciate ligament reconstruction: in vitro study comparing titanium, poly-d,l-lactide, and poly-d,l-lactide-tricalcium phosphate screws. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2006;22(11):1204–10. doi: 10.1016/j.arthro.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Monaco E, Labianca L, Speranza A, Agrò A, Camillieri G, D’Arrigo C, Ferretti A. Biomechanical evaluation of different anterior cruciate ligament fixation techniques for hamstring graft. Journal of Orthopaedic Science. 2010;15(1):125–31. doi: 10.1007/s00776-009-1417-9. [DOI] [PubMed] [Google Scholar]
- 38.Smith C, Young I, Kearney J. Mechanical properties of tendons: changes with sterilization and preservation. Journal of Biomechanical Engineering. 1996;118(1):56–61. doi: 10.1115/1.2795946. [DOI] [PubMed] [Google Scholar]
- 39.Cordrey LJ, McCorkle H, Hilton E. A comparative study of fresh autogenous and preserved homogenous tendon grafts in rabbits. J Bone Joint Surg Br. 1963;45-B(1):182–95. doi: 10.1302/0301-620X.45B1.182. [DOI] [PubMed] [Google Scholar]
- 40.Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. Journal of Biomedical Materials Research Part A. 2008;86A(1):1–12. doi: 10.1002/jbm.a.32073. [DOI] [PubMed] [Google Scholar]
- 41.Jinjin M, Kristen G, Michael S, Tatiana K, Lisa L, Ellen MA. Morphological and functional characteristics of three-dimensional engineered bone-ligament-bone constructs following implantation. Journal of Biomechanical Engineering. 2009;131(10):101017. doi: 10.1115/1.4000151. [DOI] [PubMed] [Google Scholar]
- 42.Wang INE, Shan J, Choi R, Oh S, Kepler CK, Chen FH, Lu HH. Role of osteoblast–fibroblast interactions in the formation of the ligament-to-bone interface. Journal of Orthopaedic Research. 2007;25(12):1609–20. doi: 10.1002/jor.20475. [DOI] [PubMed] [Google Scholar]
- 43.Dormer NH, Berkland CJ, Detamore MS. Emerging techniques in stratified designs and continuous gradients for tissue engineering of interfaces. Ann Biomed Eng. 2010;38(6):2121–41. doi: 10.1007/s10439-010-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ. Design of a multiphase osteochondral scaffold. II. Fabrication of a mineralized collagen-glycosaminoglycan scaffold. Journal of biomedical materials research Part A. 2010;92(3):1066–77. doi: 10.1002/jbm.a.32361. [DOI] [PubMed] [Google Scholar]
- 45.Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ. Design of a multiphase osteochondral scaffold III: Fabrication of layered scaffolds with continuous interfaces. Journal of biomedical materials research Part A. 2010;92(3):1078–93. doi: 10.1002/jbm.a.32387. [DOI] [PubMed] [Google Scholar]
- 46.Lynn AK, Best SM, Cameron RE, Harley BA, Yannas IV, Gibson LJ, Bonfield W. Design of a multiphase osteochondral scaffold. I. Control of chemical composition. Journal of biomedical materials research Part A. 2010;92(3):1057–65. doi: 10.1002/jbm.a.32415. [DOI] [PubMed] [Google Scholar]
- 47.Phillips JE, Burns KL, Le Doux JM, Guldberg RE, Garcia AJ. Engineering graded tissue interfaces. Proceedings of the National Academy of Sciences. 2008;105(34):12170–5. doi: 10.1073/pnas.0801988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi J, Wang L, Zhang F, Li H, Lei L, Liu L, Chen Y. Incorporating protein gradient into electrospun nanofibers as scaffolds for tissue engineering. ACS Applied Materials & Interfaces. 2010;2(4):1025–30. doi: 10.1021/am9007962. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Wenk E, Zhang X, Meinel L, Vunjack-Novakovic G, Kaplan D. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Controlled Release. 2009;134:81–90. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guilak F, Awad H, Fermor B, Leddy H, Gimble J. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology. 2004;41(3-4/2004):389–99. [PubMed] [Google Scholar]
- 52.Kern S, Eichler H, Steove J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 53.Spalazzi JP, Doty SB, Moffat KL, Levine WN, Lu HH. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 2006;12(12):3497–508. doi: 10.1089/ten.2006.12.3497. [DOI] [PubMed] [Google Scholar]
- 54.Lima EG, Mauck RL, Han SH, Park S, Ng KW, Ateshian GA, Hung CT. Functional tissue engineering of chondral and osteochondral constructs. Biorheology. 2004;41(3-4/2004):577–90. [PubMed] [Google Scholar]
- 55.Hung CT, Lima EG, Mauck RL, Taki E, LeRoux MA, Lu HH, Stark RG, Guo XE, Ateshian GA. Anatomically shaped osteochondral constructs for articular cartilage repair. J Biomech. 2003;36(12):1853–64. doi: 10.1016/s0021-9290(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 56.Jiang J, Nicoll SB, Lu HH. Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochem Biophys Res Commun. 2005;338(2):762–70. doi: 10.1016/j.bbrc.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 57.Suo Z, Hutchinson JW. Interface crack between two elastic layers. International Journal of Fracture. 1990;43(1):1–18. [Google Scholar]
- 58.Beuth JL, Narayan SH. Residual stress-driven delamination in deposited multi-layers. International Journal of Solids and Structures. 1996;33(1):65–78. [Google Scholar]
- 59.Kuo CK, Petersen BC, Tuan RS. Spatiotemporal protein distribution of TGF-βs, their receptors, and extracellular matrix molecules during embryonic tendon development. Developmental Dynamics. 2008;237(5):1477–89. doi: 10.1002/dvdy.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments - an adaptation to compressive load. Journal of Anatomy. 1998;193:481–94. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Molecular Cellular Biology. 2005;25(2):699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shukunamai C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Developmental Biology. 2006;298:237–47. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 63.McCullen SD, Zhu Y, Bernacki SH, Narayan RJ, Pourdeyhimi B, Gorga RE, Loboa EG. Electrospun composite poly(L-lactic acid)/tricalcium phosphate scaffolds induce proliferation and osteogenic differentiation of human adipose-derived stem cells. Biomedical Materials. 2009;4(3):1971–81. doi: 10.1088/1748-6041/4/3/035002. [DOI] [PubMed] [Google Scholar]
- 64.Marino G, Rosso F, Cafiero G, Tortora C, Moraci M, Barbarisi M, Barbarisi A. β-Tricalcium phosphate 3D scaffold promote alone osteogenic differentiation of human adipose stem cells: in vitro study. Journal of Materials Science: Materials in Medicine. 2009;21(1):353–63. doi: 10.1007/s10856-009-3840-z. [DOI] [PubMed] [Google Scholar]
- 65.Martin TA, Caliari SR, Williford PD, Harley BA, Bailey RC. The generation of biomolecular patterns in highly porous collagen-GAG scaffolds using direct photolithography. Biomaterials. 2011;32(16):3949–57. doi: 10.1016/j.biomaterials.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matyas JR, Anton MG, Shrive NG, Frank CB. Stress governs tissue phenotype at the femoral insertion of the rabbit MCL. Journal of Biomechanics. 1995;28(2):147–57. doi: 10.1016/0021-9290(94)00058-c. [DOI] [PubMed] [Google Scholar]
- 67.Koob TJ, Vogel K. Proteoglycan synthesis in organ cultures from regions of bovine tendon subjected to different mechanical forces. Biochem J. 1987;246:589–98. doi: 10.1042/bj2460589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Evanko SP, Vogel KG. Proteoglycan synthesis in fetal tendon is differentially regulated by cyclic compression in vitro. Archives of Biochemistry and Biophysics. 1993;307(1):153–64. doi: 10.1006/abbi.1993.1574. [DOI] [PubMed] [Google Scholar]
- 69.Woo S, An K, Frank C, Livesay G, Ma C, Zeminski J, Wayne J, Myers B. Anatomy, biology, and biomechanics of tendon and ligament. Orthopaedic Basic Science. 2000:581–616. [Google Scholar]
- 70.Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Engineering Part A. 2008;14(10):1615–27. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- 71.Juncosa-Melvin N, Maitlin KS, Holdcraft RW, Nirmalanandhan V, Butler DL. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Engineering. 2007;13(6):1219–26. doi: 10.1089/ten.2006.0339. [DOI] [PubMed] [Google Scholar]
- 72.Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Engineering Part A. 2009;15(5):1041–52. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang C-YC, Hagar KL, Frost LE, Sun Y, Cheung HS. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells. 2004;22(3):313–23. doi: 10.1634/stemcells.22-3-313. [DOI] [PubMed] [Google Scholar]
- 74.Peter A, Detlef S, Martin A, Bernd K, Carsten E, Reiner H, Bernd F, Michael N, Carsten N, Richard K. Cyclic, mechanical compression enhances chondrogenesis of mesenchymal progenitor cells in tissue engineering scaffolds. Biorheology. 2004;41(3):335–46. [PubMed] [Google Scholar]
- 75.Mauck RL, Byers BA, Yuan X, Tuan RS. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomechan Model Mechanobiol. 2007;6:113–25. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 76.Terraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, Seliktar D, Elisseeff J. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells. 2007;25(11):2730–8. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- 77.Li Z, Yao S-J, Alini M, Stoddart MJ. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue Engineering Part A. 2009;16(2):575–84. doi: 10.1089/ten.TEA.2009.0262. [DOI] [PubMed] [Google Scholar]
- 78.Thomopoulos S, Das R, Birman V, Smith L, Ku K, Elson EL, Pryse KM, Marquez JP, Genin GM. Fibrocartilage Tissue Engineering: The Role of the Stress Environment on Cell Morphology and Matrix Expression. Tissue Engineering Part A. 2010;17(7–8):1039–53. doi: 10.1089/ten.tea.2009.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thorpe S, Buckley C, Vinardell T, O’Brien F, Campbell V, Kelly D. The response of bone marrow-derived mesenchymal stem cells to dynamic compression following TGF-β3 induced chondrogenic differentiation. Annals of Biomedical Engineering. 2009;38(9):2896–909. doi: 10.1007/s10439-010-0059-6. [DOI] [PubMed] [Google Scholar]
- 80.Connelly JT, Vanderploeg EJ, Mouw JK, Wilson CG, Levenston ME. Tensile loading modulates bone marrow stromal cell differentiation and the development of engineered fibrocartilage constructs. Tissue engineering Part A. 2010;16(6):1913–23. doi: 10.1089/ten.tea.2009.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Janmey PA, Weitz DA. Dealing with mechanics: mechanisms of force transduction in cells. Trends Biochem Sci. 2004;29(7):364–70. doi: 10.1016/j.tibs.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 82.Lee SE, Kamm RD, Mofrad MR. Force-induced activation of talin and its possible role in focal adhesion mechanotransduction. Journal of Biomechanics. 2007;40(9):2096–106. doi: 10.1016/j.jbiomech.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 83.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323(5914):638–41. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nekouzadeh A, Pryse KM, Elson EL, Genin GM. Stretch-activated force shedding, force recovery, and cytoskeletal remodeling in contractile fibroblasts. Journal of Biomechanics. 2008;41(14):2964–71. doi: 10.1016/j.jbiomech.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(3):849–54. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuo CK, Tuan RS. Tissue engineering with mesenchymal stem cells. IEEE Eng Med Biol Mag. 2003;22(5):51–6. doi: 10.1109/memb.2003.1256272. [DOI] [PubMed] [Google Scholar]
- 87.Gimble JM. Adipose tissue-derived therapeutics. Expert Opinion on Biological Therapy. 2003;3(5):705–13. doi: 10.1517/14712598.3.5.705. [DOI] [PubMed] [Google Scholar]
- 88.Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. Journal of Nippon Medical School. 2009;76(2):10. doi: 10.1272/jnms.76.56. [DOI] [PubMed] [Google Scholar]
- 89.Niemeyer P, Kornacker M, Mehlhorn A, Seckinger A, Vohrer J, Schmal H, Kasten P, Eckstein V, Südkamp NP, Krause U. Comparison of Immunological Properties of Bone Marrow Stromal Cells and Adipose Tissue Derived Stem Cells Before and After Osteogenic Differentiation In Vitro. Tissue Engineering. 2007;13(1):111–21. doi: 10.1089/ten.2006.0114. [DOI] [PubMed] [Google Scholar]
- 90.Grimes BR, Steiner CM, Merfeld-Clauss S, Traktuev DO, Smith D, Reese A, Breman AM, Thurston VC, Vance GH, Johnstone BH, Slee RB, March KL. Interphase FISH demonstrates that human adipose stromal cells maintain a high level of genomic stability in long-term culture. Stem cells and development. 2009;18(5):717–24. doi: 10.1089/scd.2008.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.James R, Kumbar SG, Laurencin CT, Balian G, Chhabra AB. Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomed Mater. 2011;6(2):025011. doi: 10.1088/1748-6041/6/2/025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park A, Hogan MV, Kesturu GS, James R, Balian G, Chhabra AB. Adipose-Derived Mesenchymal Stem Cells Treated with Growth Differentiation Factor-5 Express Tendon-Specific Markers. Tissue Eng Part A. 2010;16(9):2941–51. doi: 10.1089/ten.tea.2009.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25(16):3211–22. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 94.Erisken C, Kalyon DM, Wang H, Ornek-Ballanco C, Xu J. Osteochondral tissue formation through adipose-derived stromal cell differentiation on biomimetic polycaprolactone nanofibrous scaffolds with graded insulin and Beta-glycerophosphate concentrations. Tissue engineering Part A. 2011;17(9–10):1239–52. doi: 10.1089/ten.TEA.2009.0693. [DOI] [PubMed] [Google Scholar]