Abstract
Green tea polyphenols (GTPs) reactivate epigenetically silenced genes in cancer cells and trigger cell cycle arrest and apoptosis; however, the mechanisms whereby these effects occur are not well understood. We investigated the molecular mechanisms underlying the antiproliferative effects of GTP, which may be similar to those of histone deacetylase (HDAC) inhibitors. Exposure of human prostate cancer LNCaP cells (harboring wild-type p53) and PC-3 cells (lacking p53) with 10–80 μg/ml of GTP for 24 h resulted in dose-dependent inhibition of class I HDAC enzyme activity and its protein expression. GTP treatment causes an accumulation of acetylated histone H3 in total cellular chromatin, resulting in increased accessibility to bind with the promoter sequences of p21/waf1 and Bax, consistent with the effects elicited by an HDAC inhibitor, trichostatin A. GTP treatment also resulted in increased expression of p21/waf1 and Bax at the protein and message levels in these cells. Furthermore, treatment of cells with proteasome inhibitor, MG132 together with GTP prevented degradation of class I HDACs, compared with cells treated with GTP alone, indicating increased proteasomal degradation of class I HDACs by GTP. These alterations were consistent with G0–G1 phase cell cycle arrest and induction of apoptosis in both cell lines. Our findings provide new insight into the mechanisms of GTP action in human prostate cancer cells irrespective of their p53 status and suggest a novel approach to prevention and/or therapy of prostate cancer achieved via HDAC inhibition.
Introduction
Posttranslational modifications in core histone proteins are critical in the regulation of gene expression (1). In particular, acetylation of core histones by histone acetyltransferases is linked to chromatin opening and transcriptional gene activation; in contrast, histone deacetylases (HDACs) remove the acetyl group from histones and repress gene transcription (2). HDACs have been shown to regulate many important biological processes, including cell cycle progression, differentiation and development (3). HDACs are broadly classified into four classes based on their sequence homology as follows: class I (HDACs 1–3 and 8), class II (HDACs 4–7 and 9–10), class III (Sirt1–Sirt7) and class IV (HDAC 11). Class I HDACs contain a deacetylase domain and are the homologs of yeast RPD3, whereas class II HDACs are homologs of yeast Hda1 (4). Class III (Sirt1–Sirt7) HDACs are homologs of yeast Sir2 (silent mating type information regulation 2) and form a structurally distinct class of nicotinamide adenine dinucleotide-dependent enzymes (5), and class IV HDACs (HDAC11) have properties of both class I and class II HDACs (6). Class I HDACs are frequently overexpressed in various human cancers including prostate cancer and their differential expression often correlates with drug resistance and poor prognosis, which makes them an attractive target in cancer therapeutics (7–9).
In recent years, HDAC inhibitors such as vorinostat [suberoylanilide hydroxamic acid (SAHA)] and trichostatin A (TSA) have emerged as a promising class of therapeutic drugs. Crystallographic analysis has shown that SAHA and TSA interact directly with the catalytic site of HDAC-like protein and inhibit its enzymatic activity (10). Inhibition of HDAC activity with SAHA and related agents alters gene expression, causing cell cycle arrest and apoptosis in cancer cells primarily by the induction of cell cycle kinase inhibitor p21/waf1 and Bax, a proapoptotic protein (11). Although HDACs exhibit selective toxicity against tumor cells at nanomolar concentration, their prolonged use in patients results in severe immune suppression, fatigue, gastrointestinal side effects and transient cytopenias (12). It would be advantageous to identify HDAC inhibitors that are effective but minimally toxic. Plant-derived polyphenols such as garlic organosulfur compounds, sulforaphane and metabolites of glucobrassicin, 3, 3-diindolylmethane have structural features compatible with HDAC inhibition (13,14).
Dietary polyphenols from green tea and its major constituent, (−) epigallocatechin-3-gallate, have been demonstrated to possess cancer preventive and therapeutic activity (15). These effects are attributed to the alterations in various genes involved in the regulation of cell cycle, apoptosis, invasion, metastasis and angiogenesis (16). Although we have previously demonstrated that green tea polyphenols (GTPs) differentially cause cell cycle arrest and induction of apoptosis in prostate cancer cells by the upregulation of p21/waf1 and Bax proteins (17), the mechanisms underlying these effects remain unclear. More recently, we and others have demonstrated that GTPs have the ability to alter gene expression by epigenetic modifications, including DNA methylation and chromatin remodeling, resulting in reexpression of some tumor suppressor genes (18–20). Since class I HDACs are overexpressed in human prostate cancer specimens compared with high-grade prostatic intraepithelial neoplasia and adjacent normal prostate tissue and in several human prostate cancer cell lines, in the present study, we investigated whether GTPs have the ability to suppress HDAC activity and expression and studied the molecular mechanisms affecting cell cycle arrest and apoptosis. Our results demonstrate that GTPs can downregulate class I HDACs by enhancing their proteasomal degradation and increased acetylation of histone H3, causing accessibility with the promoter region of the p21/waf1 and Bax gene in prostate cancer cells. Downregulation of HDACs represents a novel mechanism underlying the ability of GTPs to induce cell cycle arrest and apoptosis.
Materials and methods
Cell culture and reagents
Androgen-responsive human prostate cancer LNCaP and androgen-refractory PC-3 cells were obtained from American Type Culture Collection (Manassas, VA). Cells were grown and maintained in RPMI 1640 (Hyclone) supplemented with 1% penicillin–streptomycin and 10% fetal bovine serum at 50–70% confluency. Cells received the following treatments: 20 and 80 ng/ml TSA (Sigma, St Louis, MO), 10 μg/ml with MG132 (Calbiochem, Gibbstown, NJ) dissolved in dimethyl sulfoxide, where the final concentration of dimethyl sulfoxide did not exceed 0.1% and is considered toxic to cells; 10–80 μg/ml Polyphenon E® (Mitsui Norin, Japan) hereafter referred as GTPs for indicated times. Concentrations of 10 μg/ml Polyphenon E correspond to 14.0 μM of EGCG. The constituents present in Polyphenon E® are discussed in our previous publication (18).
HDAC enzyme activity
HDAC enzyme activity was determined using HDAC Assay Kit (colorimetric) obtained from (Active Motif, Carlsbad, CA). HDAC assay was performed following the manufacturer's instructions measuring the color intensity at 405 nm.
Quantitative real-time reverse transcription–polymerase chain reaction
Human prostate cancer PC-3 and LNCaP cells were treated with GTP (10–80 μg/ml) for 24 h. RNA was extracted from cells by using Trizol reagent. The complementary DNA was prepared from RNA using a high capacity complementary DNA Reverse Transcription Kit (Applied Biosystems). Expression of p21/waf1 and Bax genes were assayed with the Taq-man(R) gene expression assay systems (p21-Hs00355782_m1, Bax- Hs00180269_m1 and GAPDH-4333764T), using the ABI Prism 7500 real-time polymerase chain reaction (PCR). All reactions were performed in triplicate and standard deviation calculated using the comparative Ct method (ΔΔCt Method, ABI PRISM 7500 Real-Time PCR Software). The mean expression levels are represented as the ratio between the p21/GAPDH and Bax/GAPDH expression.
Extraction and expression of acetylated histone proteins
Human prostate cancer PC-3 and LNCaP cells were treated with GTP (10–80 μg/ml) for 24 h and were harvested and washed twice with ice-cold phosphate-buffered saline (PBS) supplemented with 5 mM sodium butyrate. After wash, cells were resuspended in Triton extraction buffer [PBS containing 0.5% Triton X-100 (vol/vol), 2 mM phenylmethylsulfonyl fluoride, 0.02%(wt/vol) NAN3] and lysed on ice for 10 min with gentle stirring, centrifuged at 2000 r.p.m. for 10 min at 4°C. Pallet was washed in Triton extraction buffer and then resuspended in 0.2 N HCl. Histones were acid extracted overnight at 4°C and centrifuged at 2000 r.p.m. for 10 min at 4°C. Samples were processed for the analysis of histones using immunoblotting.
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay buffer (1% NP40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate in PBS). Complete protease inhibitor cocktail (Roche) was added to lysis buffer before use. Protein concentration was determined by detergent-compatible protein assay (Bio-Rad). Protein samples were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. The membrane was blocked in 5% non-fat milk in TBS for 1 h and incubated with primary antibody overnight and subsequently with appropriate horseradish peroxidase-conjugated secondary antibody. Signals were developed with enhanced chemiluminescence reagents (GE Healthcare) and exposure to Hyblot CL autoradiography film (Denville Scientific). Anti-Ac-histone H3 (07–593) and anti-histone H3 (05–928) were procured from Upstate Biotechnology (Temecula, CA). Anti-p53 (SC-126) anti-p21/waf1 (SC-397), anti-bax (SC-493) anti-HDAC1 (SC-7872), anti-HDAC2 (SC-6296), anti-HDAC3 (SC-11417), anti-HDAC8 (SC-11405) and anti-β-actin (SC-47778) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Chromatin immunoprecipitation assay
Human prostate cancer PC-3 and LNCaP cells treated with various doses of GTP and respective controls were fixed in 1% formaldehyde for 15 min at room temperature. Cross-linking was stopped by adding glycine at a final concentration of 0.125 M. The chromatin was digested with monococcal nuclease enzyme and incubated with anti-acetylated histone H3 (Cat#07-593; Upstate Biotechnology) antibody overnight at 4°C. Cross-linking was reversed by incubating the samples overnight at 65°C. DNA was purified using phenol–chloroform–isoamyl solution with extraction followed by ethanol precipitation. DNA was then resuspended in nuclease-free water. Primers used for p21/waf1 gene promoter were as follows: forward primers 5′-GTGGCTCTGATTGGCTTTCTG-3′, reverse primers 5′-GTGAAAACAGGCAGCCCAAG-3′ and for Bax gene promoter, forward primers 5′-TAATCCCAGCGCTTTGGAA-3′ and reverse primers 5′-TGCAGAGACCTGGATCTAGCAA-3′, respectively. Immunoprecipitated DNAs, beads or input controls were subjected to PCR amplification for 30 cycles of the following cycling conditions: Stage-1—95°C for 2 min (1 cycle), Stage-2—95°C for 30 s, 60°C for 30 s, 72°C for 1 min (30 cycles), Stage-3—72°C for 3 min (1 cycle). PCR products were subjected to electrophoresis using 2% agarose gel.
Cell cycle analysis
A flow cytometric assay was performed to assess effects of GTP on cell cycle. Human prostate cancer PC-3 and LNCaP cells were treated with GTP and cells were washed with PBS and harvested by trypsinization at 24 h posttreatment. Approximately, 1 × 106 cells were fixed in 70% cold methanol and left on ice for at least 30 min and stored at −20°C for at least 48 h. After fixation, cells were washed, pelleted and resuspended in 0.04 μg/ml propidium iodide and 100 μg/ml RNase in PBS. The samples were incubated at room temperature for 30 min and flow cytometry was performed on EPICS-XL MCL flow cytometer and analyzed using Cell Quest Analysis software Modifit to generate histograms to determine number of cells in each phase of the cell cycle.
Detection of apoptosis
The assay for determination of apoptosis in control and treated PC-3 and LNCaP cells by staining with Annexin V-FITC using the protocol provided by the manufacturer and was analyzed on EPICS-XL MCL flow cytometer.
Statistical analysis
The images were digitized and quantification was performed using a software program with Kodak 2000 imaging system. One-way analysis of variance was performed to assess the differences between groups. Differences in means among treatments were tested by Dunnett’s test, and the level of significance was designated as follows: *P < 0.05, **P < 0.001.
Results
GTPs decrease class I HDAC activity and expression in human prostate cancer cells
We recently identified GTP as an HDAC inhibitor in human prostate cancer cells (18), and the present study provides additional confirmation that GTP acts as an inhibitor of class I HDACs in human prostate cancer cells. The HDAC activity was compared with TSA, an HDAC inhibitor. Exposure of PC-3 cells to 20 and 80 ng/ml TSA resulted in 26 and 44% decrease in HDAC enzyme activity. Similar results were obtained with LNCaP cells, where TSA exposure caused a decrease in HDAC enzyme activity to 10 and 35%, respectively. Exposure of PC-3 cells to 10–80 μg/ml GTP resulted in 19–50% decrease in HDAC activity and 16–35% in HDAC activity in LNCaP cells. The validation of the test was ascertained by using HeLa cells with and without TSA, as a positive control (Figure 1A).
Fig. 1.
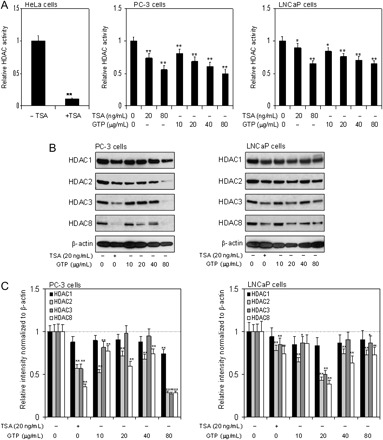
Effect of GTPs and HDAC inhibitor TSA on class I HDAC activity and protein expression in human prostate cancer cells. (A) HDAC activity, panel 1, HDAC activity in nuclear extract of HeLa cells with and without HDAC inhibitor; TSA, panel 2, dose-dependent inhibition of HDAC activity in PC-3 cells exposed to various concentrations of GTP (10–80 μg/ml) and TSA (20 and 80 ng/ml), panel 3, dose-dependent inhibition on HDAC activity in LNCaP cells after treatment with various concentrations of GTP (10–80 μg/ml) and TSA (20 and 80 ng/ml) for 24 h. Data represent the mean ± SD of three different assays. *P < 0.05 and **P < 0.001 versus control. (B) Class I HDACs protein expression, dose-dependent inhibition of class I HDAC proteins at indicated doses of treatment with GTP and HDAC inhibitor TSA in human prostate cancer PC-3 cells lacking p53, panel 1, and in human prostate cancer LNCaP cells harboring wild-type p53, panel 2. (C) Relative intensities of HDAC protein bands normalized to β-actin in PC-3 cells, panel 1 and LNCaP cells, panel 2. Densitometry represents the mean ± SD of three different assays. *P < 0.05 and **P < 0.001 versus control. The details are provided in Materials and methods.
GTPs decrease protein expression of class I HDAC in human prostate cancer cells
To determine if GTP exposure affects the protein expression of class I HDACs, we performed western blot analysis on total cell lysate of PC-3 and LNCaP cells treated with various concentrations of GTP for 24 h. Cells treated with 20 ng/ml TSA were used as positive control. Exposure of cells to GTP decreased the levels of all class I HDACs (HDAC 1, 2, 3 and 8) in a dose-dependent manner in both these cell lines. The effect of HDAC inhibition was prominent in PC-3 cells compared with LNCaP cells. The decrease in HDAC2, HDAC3 and HDAC8 levels were markedly different as compared with HDAC1 (Figure 1B). Compared with untreated cells, a decrease in 12–26% in HDAC1 protein expression, 44–72% in HDAC2, 43–78% in HDAC3 and 65–72% in HDAC8 was observed in PC-3 cells after GTP treatment. In LNCaP cells, 8–26% in HDAC1, 22–54% in HDAC2, 15–49% in HDAC3 and 26–62% in HDAC8 protein expression was observed after GTP treatment (Figure 1C).
GTPs cause proteasome-mediated protein degradation of HDACs in human prostate cancer cells
Next, we determined if proteasome-mediated degradation was involved in the downregulation of HDACs after GTP treatment in cancer cells. For these studies, PC-3 and LNCaP cells were treated with various doses of GTP for 16 h in duplicate followed by addition of 10 μM MG132 to one group and incubated for additional 8 h. Compared with cells treated with GTP alone, MG132 caused a significant increase in the protein expression of all the HDACs in both cell lines (Figure 2A and B). These results demonstrate that proteasome degradation of HDACs may be a mechanism of its downregulation by GTPs.
Fig. 2.
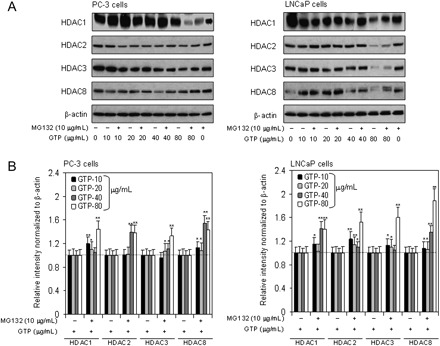
Effect of GTPs in the induction of proteasome-mediated degradation of class I HDACs in human prostate cancer cells. (A) PC-3 (panel 1) and LNCaP (panel 2) cells were treated in duplicates with GTP (10–80 μg/ml) for 16 h, and later one group was cotreated with 10 μM dose of MG132 for additional 8 h. Western blotting was performed for class I HDAC proteins. (B) Relative intensities of HDAC protein bands normalized to β-actin in PC-3 cells, panel 1 and LNCaP cells, panel 2. Densitometry represents the mean ± SD of three different assays. *P < 0.05 and **P < 0.001 versus control. The details are provided in Materials and methods.
GTPs cause increased histone acetylation in human prostate cancer cells
Next, we determined whether decrease in HDAC activity and expression affects histone acetylation in human prostate cancer cells. As shown in Figure 3A, western blotting of acid-extracted proteins from PC-3 and LNCaP cells treated with various doses of GTP exhibited a significant increase in the acetylation of histone H3 as compared with untreated control. An increase in 22–33% histone H3 acetylation was observed in PC-3 cells and 16–30% acetylation in LNCaP cells. Indeed, increase acetylation of histone relaxes chromatin, which could result in increased transcriptional activation (Figure 3B).
Fig. 3.
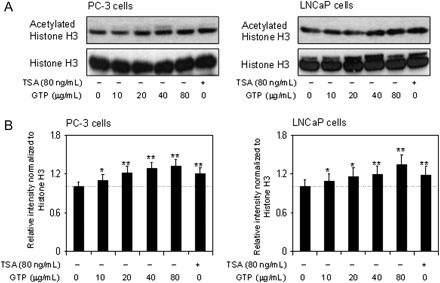
Effect of GTPs on histone H3 acetylation in human prostate cancer cells. (A) Treatment of PC-3 cells (panel 1) and LNCaP cells (panel 2) with various concentrations of GTP (10–80 μg/ml) and TSA (80 ng/ml) for 24 h. GTP treatment increases levels of acetylated histone H3 in both cell lines. (B) Relative intensities of HDAC protein bands normalized to histone H3 in PC-3 cells, panel 1 and LNCaP cells, panel 2. Densitometry represents the mean ± SD of three different assays. *P < 0.05 and **P < 0.001 versus control. The details are provided in Materials and methods.
GTPs activate expression of p21/waf1 and Bax at the protein and message levels in human prostate cancer cells
Class I HDACs have been linked to the transcriptional repression of the cyclin-dependent kinase inhibitor p21/waf1 and proapoptotic gene Bax in various human cancer cell lines (21). HDAC inhibitors have been shown to induce cell cycle arrest by activating the expression of p21/waf1 and induction of apoptosis by increasing Bax expression. Next, we determined whether GTP-induced degradation of class I HDAC and increased acetylation of histone H3 leads to the induction of p21/waf1 and Bax in human prostate cancer cells. As shown in Figure 4A, exposure of PC-3 and LNCaP cells to GTP resulted in significant increases in p21/waf1 and Bax protein expression in both cell lines. Furthermore, GTP treatment resulted in significant increases in messenger RNA expression of p21/waf1 and Bax in both cell lines in a dose-dependent manner. A 17–120% increase in the p21/waf1 and 35–122% increase in the Bax message level were observed in PC-3 cells; and 20–98% increase in the p21/waf1 and 36–88% increase in the Bax message level were observed in LNCaP cells after GTP treatment (Figure 4B).
Fig. 4.
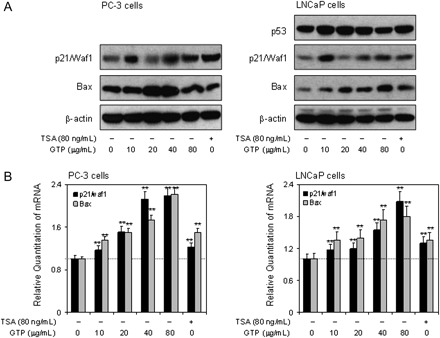
Effect of GTPs on the expression of p21/waf1 and Bax at protein and message level in human prostate cancer cells. (A) Treatment of PC-3 cells (panel 1) and LNCaP cells (panel 2) with various concentrations of GTP (10–80 μg/ml) and TSA (80 ng/ml) for 24 h. Dose-dependent increase in the protein expression levels of p21/waf1 and Bax in PC-3 cell (panel 1) and p53, p21/waf1 and Bax expression in LNCaP cells (panel 2). (B) Dose-dependent increase in the messenger RNA (mRNA) levels of p21/waf1 and Bax in PC-3 cells (panel 1) and p21/waf1 and Bax in LNCaP cells (panel 2) as determined by quantitative real-time PCR. Data represent the mean ± SD of three different assays. *P < 0.05 and **P < 0.001 versus control. The details are provided in Materials and methods.
GTPs cause increase binding of acetylated H3 to the promoters of p21/waf1 and Bax genes
Next, we sought changes in the acetylation status of histone H3 associated with the promoter region of the p21/waf1 gene. Using anti-acetylated histone H3 antibody followed by PCR with the primers specific for p21/waf1 promoter, chromatin immunoprecipitation assay was performed. As shown in Figure 5A, GTP treatment resulted in a dose-dependent increase in the amount of acetylated histone H3 associated with the p21/waf1 promoter. Expression of p21/waf1 increased to 15–54% by GTP in PC-3 cells and 61–84% in LNCaP cells compared with untreated controls, which is a well-known target of HDAC inhibitors (22,23). We also established the association between histone acetylation status and expression of specific apoptosis-related gene. For these studies, we performed chromatin immunoprecipitation using primers specific for the Bax gene promoter. A marked increase of acetylated histone H3 associated with the Bax promoter was observed after treatment of prostate cancer cells with GTP. An increase of 41–52% was observed by GTP in PC-3 cells, 77–93% in LNCaP cells compared with untreated controls (Figure 5B).
Fig. 5.
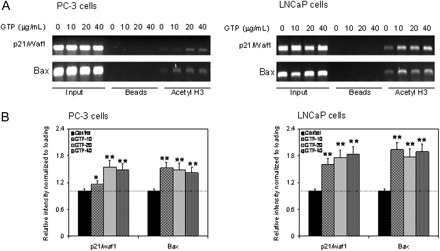
Effect of GTPs in the binding of acetylated histone H3 to the promoters of p21/waf1 and Bax genes. (A) Treatment of PC-3 cells (panel 1) and LNCaP cells (panel 2) with various concentrations of GTP (10–80 μg/ml) and TSA (80 ng/ml) for 24 h. Chromatin immunoprecipitation assay was performed for association of acetylated histone H3 with the promoters of p21/waf1 and Bax in PC-3 (panel 1) and LNCaP cells (panel 2). GTP treatment to PC-3 and LNCaP cells caused increased association of acetylated histone H3 to the promoters of p21/waf1 and Bax. (B) Relative intensities of bands normalized to loading input in PC-3 cells, panel 1 and LNCaP cells, panel 2. Densitometry represents the mean ± SD of three different assays. *P < 0.05 and **P < 0.001 versus control. The details are provided in Materials and methods.
GTPs cause cell cycle arrest and induces apoptosis in human prostate cancer cells
Finally, we examined the effect of GTP on inhibition of cell growth and induction of apoptosis in human prostate cancer cells. Approximately 3.6–12.0% reduction in cell density of PC-3 and 10.9–22.3% reduction in cell density of LNCaP cells were observed after 24 h of 10–80 μg/ml GTP treatment (data not shown). Analysis of DNA content by flow cytometry showed that GTP caused a marked increase in the percentage of cells in the G0–G1 phase of the cell cycle. Compared with the vehicle-treated controls, GTP treatment resulted in an appreciable arrest of PC-3 and LNCaP cells in G0/G1 phase of cell cycle after 24 h of the treatment. The treatment caused an arrest of 39.2% cells in G0/G1 phase of the cell cycle at 10 μg/ml, which further increased to 40.6% at 20 μg/ml, 53.8% at 40 μg/ml and 60.9% at the highest dose of 80 μg/ml, compared with control (35%) in PC-3 cells. Essentially, similar observations were recorded with LNCaP cells where following GTP treatment of cells at 10, 20, 40 and 80 μg/ml doses resulted in 67.6, 69.2, 70.5 and 77.6% arrest compared with 56% in vehicle-treated control, respectively. This increase in G0/G1 cell population was accompanied with a concomitant decrease of cell number in S phase and G2–M phase in both prostate cancer cell lines (Figure 6A). Furthermore, the percentage of cells in G0–G1 phase arrest undergoing apoptosis was determined by measuring with Annexin V-FITC staining. A significant increase in apoptosis ranging from 6.7 to 85.2% was observed in PC-3 cells and in 6.0–83.5% of LNCaP cells after 10–80 μg/ml GTP treatments (Figure 6B). These observations correlate very well with increases in p21/waf1 and Bax levels in both cell lines after GTP treatment.
Fig. 6.
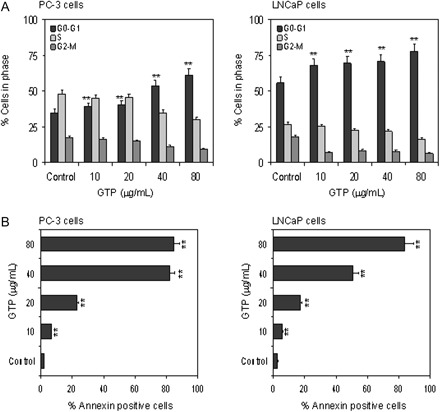
Effect of GTPs on cell cycle arrest and induction of apoptosis in human prostate cancer cells. (A) PC-3 and LNCaP cells were treated with GTP (10–80 μg/ml) for 24 h and distribution of cells was recorded in different stages of cell cycle analyzed using fluorescence activated cell sorting analysis. (B) PC-3 and LNCaP cells were treated with GTP (10–80 μg/ml) for 72 h and the number of cells undergoing apoptosis were determined using Annexin V staining. Data represent the mean ± SD of three different assays. *P < 0.05, and **P < 0.001 versus control. The details are provided in Materials and methods.
Discussion
HDAC inhibitors such as SAHA and TSA are potent inducers of differentiation and/or apoptosis in neoplastic cells in culture and in addition, they inhibit in vivo tumor growth (21–28). In the present study, we demonstrate that GTPs induce the transcription of p21/waf1 and Bax, enhance proteasomal degradation of class I HDACs and increase acetylation of histone H3, effects that lead to cell cycle arrest and apoptosis of prostate cancer cells.
We recently provided the first evidence that GTPs function as HDAC inhibitors in human prostate cancer (LNCaP) cells (18). The present investigation has confirmed that GTP exerts its effects on both prostate cancer cells that harbor wild-type p53 (LNCaP cells) as well as those that lack p53 (PC-3 cells). We observed a differential response of GTP-mediated cell death that was higher in PC-3 cells, compared with LNCaP cells, probably due to significant inhibition of class I HDACs at the protein and message levels. Our studies provide additional evidence that GTPs inhibit class I HDACs by selectively inducing proteasomal degradation of HDCAs, altered the acetylated histone status and enhancing expression of proapoptotic proteins, effects that are accompanied by corresponding increases in cell cycle arrest and apoptosis in these cells. Previous studies have reported cell cycle arrest and induction of apoptosis in various human prostate cancer cells exposed to GTP (29,30) but not in the context of HDAC inhibition. To our knowledge, this is the first study demonstrating a clear-cut relationship between HDAC inhibition and cell cycle perturbations in human prostate cancer cells.
Class I HDACs have been reported to be overexpressed in human prostate cancer specimens and in prostate cancer cell lines (31,32). HDAC1 and HDAC3 are highly expressed in prostate cancer and HDAC2 has been shown to be associated with shorter prostate-specific antigen relapse time (9). Overexpression of HDAC1 in prostate cancer cells causes increased cell proliferation and reduction in cell differentiation markers and might be recognized as a putative therapeutic target (33,34). HDACs work in multi-subunit transcriptional corepressor complexes that are recruited by sequence-specific transcription factors to promoter regions (35). Reports suggest that there are several corepressor complexes for distinct promoters, which recruit specific HDAC isoforms for silencing target genes. Class I HDACs are specifically responsible for deacetylation of catalytic core for different corepressor complexes, resulting in transcriptional repressions. For example, HDAC1 and HDAC2 are present in the CoREST, mi2/NURD and Sin3 complexes, and HDAC3 is responsible for catalytic activity of the N-CoR and SRMT corepressor complexes (35). It is important to note that GTPs affect all class I HDACs and thereby have profound effects on cancer cells, but the specific mechanisms underlying these effects need clarification specifically in understanding the differential response of GTPs in prostate cancer cells varying p53 expression.
Non-histone deacetylation-based gene repression involves deacetylation of various transcription factors by class I HDAC (35,36). Class I HDAC-mediated deacetylation of sequence-specific transcription factors has been shown to decrease their DNA-binding activity, with repression of transcription. For example, covalent modifications of several transcription factors by class I HDACs, including E2F, sp1/sp3, p53, GATA1 and TFIIF have been reported (36,37). HDAC inhibitors reduce HDAC activity, altering the dynamic balance between HDAC and histone acetyltransferase activity and resulting in increase acetylation of non-histone proteins, including p53. Reports suggest that deacetylation of p53 by HDAC1 and HDAC2 results in the degradation of deacetylated p53 and HDAC inhibitors have been shown to reverse this process (38,39). In our studies, inhibition of HDACs might result in the acetylation of wild-type p53 increasing its halftime and binding to the p21/waf1 and Bax promoter, the downstream target which is in direct regulation of p53 (38). Further work is needed to determine the effect of GTP on the acetylation of p53 in human prostate cancer cells. As with other HDAC inhibitors, such as TSA, SAHA and sodium butyrate, GTP increased p21/waf1 protein expression in prostate cancer cells, and chromatin immunoprecipitation assays confirmed an increase in the expression of acetylated histone H3 associated with the p21 promoter independent of p53 status of the cells. Recent studies have shown that p21/waf1 activates Nrf2 by competing with Keap1 for Nrf2 binding, compromising ubiquitination of Nrf2 and protecting cells from oxidative damage (40). In future studies, it may be informative to utilize small interfering RNA to knockdown Nrf2, or Nrf2-deficient cell lines, to examine the inter-relationships between Nrf2 signaling and HDAC inhibition by GTPs.
The levels of HDAC activity within cells can be altered via direct inhibition of the HDAC enzyme and changes in HDAC protein levels. In our previous studies, we reported that GTPs reduced class I HDAC protein expression in human prostate cancer LNCaP cells (18). In this report, we show that GTP can also decrease HDAC enzyme activity and may prove to be very effective in inhibiting cancer cell growth. The degrees of HDAC inhibition and alterations in downstream gene expression induced by GTPs were similar to those of well-known pharmacological inhibitors of HDACs (36,37).
Studies by our group have demonstrated that GTPs are effective cancer chemopreventive agents, capable of inhibiting prostate carcinogenesis in transgenic adenocarcinoma of the mouse prostate (TRAMP) mice and completely abrogating distant site metastasis (41). Studies have shown that OSU-HDAC42, a novel HDAC inhibitor, inhibits prostate tumor progression in TRAMP mice (42). OSU-HDAC42 treatment of TRAMP mice decreased the severity of prostatic intraepithelial neoplasia, completely prevented its progression to poorly differentiated carcinoma and shifted tumorigenesis to a more differentiated phenotype, which was associated with modulation of intraprostatic biomarkers, including those indicative of HDAC inhibition, increased apoptosis and differentiation and decreased proliferation. Similar effects—increased apoptosis and decrease proliferation in the dorso-lateral prostate—have been observed in TRAMP mice exposed to GTPs (41). Whether GTP-mediated suppression of prostate cancer in TRAMP mice is due to HDAC inhibition needs further investigation.
Based on the results of the present study, we conclude that the anticancer effects of GTP may be due, in part, to HDAC inhibition. Our results provide the first evidence that GTPs are inhibitors of the activity and expression of class I HDACs in human prostate cancer cells. We have also demonstrated that this inhibition of class I HDAC by GTPs is due to increased proteasomal degradation. The decrease in HDAC activity coincides with increased global acetylation of histone proteins as well as local hyperacetylation of histone H3 on the p21/waf1 and Bax promoters. Induction of p21/waf1 was rapid and sustained with increase in Bax expression, and these events were associated with dose-dependent increases in G0–G1 arrest and induction of apoptosis by GTP in prostate cancer cells. It will be interesting to investigate whether GTPs and other reported dietary HDAC inhibitors influence other molecular pathways in prostate cancer cells.
Funding
United States Public Health Service (RO1 CA115491, R21 CA109424) to S.G.
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- GTP
green tea polyphenol
- HDAC
histone deacetylase
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- SAHA
suberoylanilide hydroxamic acid
- TRAMP
transgenic adenocarcinoma of the mouse prostate
- TSA
trichostatin A
References
- 1.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Strahl BD, et al. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 3.Gallinari P, et al. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res. 2007;17:195–211. doi: 10.1038/sj.cr.7310149. [DOI] [PubMed] [Google Scholar]
- 4.Haberland M, et al. The many roles of histone deacetylases in development and physiology: implication for disease and therapy. Nat. Rev. Genet. 2009;10:32–34. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blander G, et al. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 6.Gao L, et al. Cloning and functional characterization of HDAC11, a novel member of the histone deacetylase family. J. Biol. Chem. 2002;277:25748–25755. doi: 10.1074/jbc.M111871200. [DOI] [PubMed] [Google Scholar]
- 7.Weichert W, et al. Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin. Cancer Res. 2008;14:1669–1677. doi: 10.1158/1078-0432.CCR-07-0990. [DOI] [PubMed] [Google Scholar]
- 8.Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009;280:168–176. doi: 10.1016/j.canlet.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 9.Weichert W, et al. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br. J. Cancer. 2008;98:604–610. doi: 10.1038/sj.bjc.6604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finnin MS, et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- 11.Myzak MC, et al. Histone deacetylases as targets for dietary cancer preventive agents: lessons learned with butyrate, diallyl disulfide, and sulforaphane. Curr. Drug Targets. 2006;7:443–445. doi: 10.2174/138945006776359467. [DOI] [PubMed] [Google Scholar]
- 12.Prince HM, et al. Clinical studies of histone deacetylase inhibitors. Clin. Cancer Res. 2009;15:3958–3969. doi: 10.1158/1078-0432.CCR-08-2785. [DOI] [PubMed] [Google Scholar]
- 13.Myzak MC, et al. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. FASEB J. 2006;20:506–508. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, et al. Chemopreventive agent 3,3′-diindolylmethane selectively induces proteasomal degradation of class I histone deacetylases. Cancer Res. 2010;70:646–654. doi: 10.1158/0008-5472.CAN-09-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D, et al. Tea polyphenols and their roles in cancer prevention and chemotherapy. Int. J. Mol. Sci. 2008;9:1196–1206. doi: 10.3390/ijms9071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan N, et al. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008;269:269–280. doi: 10.1016/j.canlet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastak K, et al. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 18.Pandey M, et al. Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int. J. Cancer. 2010;126:2520–2533. doi: 10.1002/ijc.24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Link A, et al. Cancer chemoprevention by dietary polyphenols: promising role for epigenetics. Biochem. Pharmacol. 2010;80:1771–1792. doi: 10.1016/j.bcp.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meeran SM, et al. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin. Epigenetics. 2010;1:101–116. doi: 10.1007/s13148-010-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richon VM, et al. Second generation hybrid polar compounds are potent inducers of transformed cell differentiation. Proc. Natl Acad. Sci. USA. 1996;93:5705–5708. doi: 10.1073/pnas.93.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao H, et al. Both Sp1 and Sp3 are responsible for p21waf1 promoter activity induced by histone deacetylase inhibitor in NIH3T3 cells. J. Cell. Biochem. 1999;73:291–302. [PubMed] [Google Scholar]
- 23.DiGiuseppe JA, et al. Phenylbutyrate-induced G1 arrest and apoptosis in myeloid leukemia cells: structure-function analysis. Leukemia. 1999;13:1243–1253. doi: 10.1038/sj.leu.2401471. [DOI] [PubMed] [Google Scholar]
- 24.Roy S, et al. Histone deacetylase inhibitors differentially stabilize acetylated p53 and induce cell cycle arrest or apoptosis in prostate cancer cells. Cell Death Differ. 2005;12:482–491. doi: 10.1038/sj.cdd.4401581. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida M, et al. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 26.Butler LM, et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;60:5165–5170. [PubMed] [Google Scholar]
- 27.Drummond DC, et al. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu. Rev. Pharmacol. Toxicol. 2005;45:495–528. doi: 10.1146/annurev.pharmtox.45.120403.095825. [DOI] [PubMed] [Google Scholar]
- 28.Kelly WK, et al. Drug insight: histone deacetylase inhibitors—development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nat. Clin. Pract. Oncol. 2005;2:150–157. doi: 10.1038/ncponc0106. [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, et al. Molecular pathway for (-)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch. Biochem. Biophys. 2003;410:177–185. doi: 10.1016/s0003-9861(02)00668-9. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqui IA, et al. Beneficial effects of tea and its polyphenols against prostate cancer. Mol. Nutr. Food Res. 2006;50:130–143. doi: 10.1002/mnfr.200500113. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, et al. Increased expression of histone deacetylaces (HDACs) and inhibition of prostate cancer growth and invasion by HDAC inhibitor SAHA. Am. J. Transl. Res. 2009;1:62–71. [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa M, et al. Expression profile of class I histone deacetylases in human cancer tissues. Oncol. Rep. 2007;18:769–774. [PubMed] [Google Scholar]
- 33.Patra SK, et al. Histone deacetylase and DNA methyltransferase in human prostate cancer. Biochem. Biophys. Res. Commun. 2001;287:705–713. doi: 10.1006/bbrc.2001.5639. [DOI] [PubMed] [Google Scholar]
- 34.Halkidou K, et al. Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. Prostate. 2004;59:177–189. doi: 10.1002/pros.20022. [DOI] [PubMed] [Google Scholar]
- 35.Yang XJ, et al. Collaborative spirit of histone deacetylases in regulating chromatin structure and gene expression. Curr. Opin. Genet. Dev. 2003;13:143–153. doi: 10.1016/s0959-437x(03)00015-7. [DOI] [PubMed] [Google Scholar]
- 36.Kim DH, et al. Histone deacetylase in carcinogenesis and its inhibitors as anti-cancer agents. J. Biochem. Mol. Biol. 2003;36:110–119. doi: 10.5483/bmbrep.2003.36.1.110. [DOI] [PubMed] [Google Scholar]
- 37.Buchwald M, et al. HDACi—targets beyond chromatin. Cancer Lett. 2009;280:160–167. doi: 10.1016/j.canlet.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 38.Mellert HS, et al. Deacetylation of the DNA-binding domain regulates p53-mediated apoptosis. J. Biol. Chem. 2011;286:4264–4270. doi: 10.1074/jbc.M110.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh ET, et al. Novel histone deacetylase inhibitor CG200745 induces clonogenic cell death by modulating acetylation of p53 in cancer cells. Invest. New Drugs. 2010 doi: 10.1007/s10637-010-9568-2. [Epub ahead of print] PMID:20978925. [DOI] [PubMed] [Google Scholar]
- 40.Chen W, et al. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol. Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta S, et al. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc. Natl Acad. Sci. USA. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sargeant AM, et al. OSU-HDAC42, a histone deacetylase inhibitor, blocks prostate tumor progression in the transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008;68:3999–4009. doi: 10.1158/0008-5472.CAN-08-0203. [DOI] [PubMed] [Google Scholar]


