Abstract
In developing countries where diarrheal disease is a leading cause of morbidity and mortality in children under 5 years of age, enteric coinfection is common. There is little understanding, however, of the biologic interaction between coinfecting pathogens. The authors investigated the potential for synergistic interaction between coinfecting pathogens on diarrhea pathogenesis using an epidemiologic framework. They conducted community-based, case-control studies in 22 communities in northwestern Ecuador between 2003 and 2008. Risk ratios of diarrhea associated with single infections and coinfections were estimated. Interaction between coinfecting pathogens was assessed through departure from risk ratio additivity and multiplicativity after adjustment for age. On the additive scale, the authors found departure from the null value of 0 for rotavirus-Giardia coinfections (interaction contrast ratio = 8.0, 95% confidence interval: 3.1, 18.9) and for rotavirus-Escherichia coli coinfections (interaction contrast ratio = 9.9, 95% confidence interval: 2.6, 28.4). On the multiplicative scale, they found departure from the value of 1 for rotavirus-Giardia coinfections (multiplicative interaction = 3.6, 95% confidence interval: 1.3, 8.7). This research provides epidemiologic evidence for synergism between rotavirus and other enteric pathogens. During coinfection, the pathogenic potential of each organism appears to be enhanced. The potential for pathogenesis to be more severe in the presence of a rotavirus coinfection amplifies the need for rotavirus vaccination.
Keywords: case-control studies, diarrhea, Escherichia coli, Giardia, interaction, prevalence, rotavirus, synergism
Editor's note: An invited commentary on this article is published on page 396, and the authors' response is published on page 400.
Diarrheal disease is the fifth leading cause of death in low- and middle-income countries (1). Although mortality rates have declined in the past several decades (2), diarrhea still causes up to 1.9 million childhood deaths each year (3). Many enteric viruses, bacterial pathogens, and parasites likely contribute to this disease burden both individually and together (2). Together, coinfecting pathogens may cause more severe diarrhea than infection with either pathogen alone (4). Specific coinfecting pathogens may also act synergistically, resulting in even greater pathogenesis and a larger contribution to the overall diarrheal disease burden.
Mixed infections are commonly detected in case-control studies, making it difficult to establish causal links between a pathogen and diarrhea. Coinfecting pathogens may confound these relations and, if ignored, potentially lead to false inferences. Previous case-control studies (5–12) have found mixed infections in 10%–40% of cases and 0%–15% of controls and as many as 5 coinfecting pathogens (7, 12). Although mixed infections were discussed in all 8 studies, only 3 reported associations between single infections and diarrhea (6, 10, 11). By consistently distinguishing between single and mixed infections, we may be able to improve our understanding of the pathogenic potential of enteric infections.
Mixed infections have been shown to exacerbate diarrheal illness. Earlier studies support increased morbidity and mortality from rotavirus-Escherichia coli coinfections in animals (13–17). Clinic-based studies of diarrhea cases have also suggested greater severity of diarrhea in the presence of a rotavirus-E. coli coinfection (4, 6, 10, 18, 19). In contrast, Unicomb et al. (20) have shown no greater severity than that of single infections with rotavirus or E. coli. Uhnoo et al. (21) have also demonstrated no increase in severity from mixed viral-bacterial infections. Yet, the authors did report prolonged diarrhea associated with coinfections compared with single infections with rotavirus. The pathogenicity of enteric coinfections has been the focus of very few community-based studies but was examined in the report by Bilenko et al. (22). However, much of the clinical research to date supports enhanced pathogenesis from the combined independent actions of 2 pathogens.
Little is known about the potential for pathogens to act synergistically to cause diarrhea. In vitro models have provided us with some insight on the biologic mechanisms behind synergism. These studies (23–25) have shown that intestinal cell lines incubated with rotavirus may predispose cells to increased adhesion, invasion, and multiplication by invasive bacteria. Superti et al. (23) also reported increased levels of viral replication in coinfected cells, demonstrating a synergistic interaction. The relations were time dependent; higher levels of adhesion, invasion, and multiplication were noted as the rotavirus-incubation period lengthened. Furthermore, the inability of noninvasive bacterial strains to traverse the host-cell membrane points to a specific transport mechanism and not just a general increase in permeability. While these in vitro models support a synergistic interaction between rotavirus and coinfecting pathogens, evidence from community-based studies will be important to confirm this action in vivo.
In this study, we use community-based, case-control data to estimate the prevalence and pathogenicity of Giardia, rotavirus, and E. coli (including Shigellae) across all ages in northern coastal Ecuador between 2003 and 2008. In our assessment of pathogenicity, we distinguish between single infections and coinfections. We also examine the evidence for biologic synergism between coinfecting pathogens by estimating their interaction on both additive and multiplicative scales.
MATERIALS AND METHODS
Study region
The study region is located in the northwestern coastal area of Ecuador in the Cantón Eloy Alfaro. This area has experienced rapid development since the introduction of a paved road in 2001. The new road links the region to the coast on the west and the Andes to the east, facilitating the movement of products, people, and pathogens in and out of the region. We sampled 21 villages in the study area, each of which is located along 1 of 3 rivers that drain into Borbón, the urban center of the region also included in the study. These 22 communities generally rely on river water, although some have access to well or piped water. Sanitation facilities range from pit latrines to flush toilets.
Study design
Between August 2003 and February 2008, we conducted up to seven 15-day case-control studies in each of the 22 communities. Before the case-control period began, we conducted a census of the community. During the case-control period, we visited each house daily to identify all cases of diarrhea in the 21 villages (ranging in size from 5 to 200 households) or in a random sample of 200 households in Borbón, which has a population of approximately 1,000 households. For every case, we randomly selected 1 household control and 2 community controls at the time of case identification. Cases were defined as anyone with 3 or more loose stools in a 24-hour period. Controls were eligible if they were free of diarrhea in the previous 6 days (no other inclusion or exclusion criteria were applied). Stool specimens were collected from all cases and controls. We obtained oral consent from each village and household in the study. Approximately 99% of houses consented to participate in the study, and 93% of cases submitted stool specimens. Institutional review board committees at the University of Michigan, Trinity College, and Universidad San Francisco de Quito approved all protocols.
Pathogen detection
Stool samples were tested for rotavirus, pathogenic E. coli, E. coli Shigellae, and Giardia. Rotavirus was detected in the field by using a commercial immunochromatographic test (RIDA QUICK rotavirus; R-Biopharm AG, Darmstadt, Germany). Fecal samples were plated directly onto xylose lysine deoxycholate (XLD) or MacConkey agar. All lactose-negative isolates, identified as either E. coli or Shigellae by an API-20E test kit (bioMerieux, Inc., Hazelwood, Missouri), and a random sample of 5 lactose-positive isolates were analyzed by polymerase chain reaction. Pathotype-specific primers were used to identify enterotoxigenic E. coli (ETEC) (heat-labile toxin gene (eltB) and heat-stable toxin gene (estA)), enteropathogenic E. coli (EPEC) (bundle-forming pilus gene (bfp)), and enteroinvasive E. coli (EIEC) or Shigellae (invasion plasmid antigen gene (ipahH)). Additionally, an aliquot of fecal material was frozen in liquid nitrogen and transported to a laboratory in Quito, where Giardia was detected by using an enzyme-linked immunosorbent assay kit (RIDASCREEN Giardia; R-Biopharm). Further details can be found (26).
Statistical analysis
We pooled 152 case-control studies and analyzed these together using R, version 2.11.1 (R Foundation for Statistical Computing, Vienna, Austria). Estimates of diarrhea prevalence were based on the number of cases identified, the number of household residents, and the community population during each case-control visit. To estimate the prevalence of enteric infection, we assigned inverse probability sampling weights to all cases and controls. Probability sampling weights were adjusted to the age distribution of the community population for age-specific estimates only. Using these weights and the standard Horvitz-Thompson theory (27), we achieved unbiased estimation of the 15-day prevalence. The pathogenicity of each organism was quantified by the risk ratio between the presence of the pathogen and diarrheal symptoms. These risk ratios were calculated directly from the 2 × 2 table whose entries were filled in with the weighted proportions. To investigate pathogenic effects both within and across age groups, we estimated the strata-specific, crude, and Mantel-Haenszel pooled risk ratio for diarrhea associated with single and multiple infections (28). Biologic interaction between 2 coinfecting pathogens was assessed on the additive scale by the interaction contrast ratio (ICR) (28) and age-standardized risk ratios (RRs):
 |
and, on the multiplicative scale, by estimating departure from multiplicativity of the age-standardized risk ratios (28), which we refer to as “multiplicative interaction”:
 |
Use of age-standardized risk ratios allowed us to account for potential confounding by age. To make statistical inferences while accounting for correlated data, we characterized the sampling distribution of the interaction contrast ratio, multiplicative interaction, and all risk ratios by bootstrapping the data. We sampled with replacement from the original data set a number of observations equal to the original sample size. By use of this new data set, estimates of the interaction contrast ratio, multiplicative interaction, and all risk ratios were calculated. This process was repeated 1,000 times to produce estimates of the sample distributions associated with each statistic. The lower 0.025 and upper 0.975 percentiles of the bootstrap distribution of these statistics are presented as 95% confidence intervals. Prevalence and pathogenicity estimates for Plesiomonas shigelloides are reported elsewhere (29). Because EIEC and E. coli Shigellae share similar molecular mechanisms and phylogeny, these organisms were grouped together and are noted as EIEC/Shigella in the subsequent text.
RESULTS
Case-control sample
Between August 2003 and February 2008, a total of 3,314 stool samples were collected from 883 cases and 2,431 controls in the study region. We isolated pathogens in 499 cases (56.5%) and in 780 controls (32.1%) (Table 1). Mixed infections were detected in 21.2% of cases and in 4.2% of controls. The most common infections were either single or mixed infections with Giardia, found in 31.5% of cases and 20.4% of controls. Rotavirus was detected in 22.2% of cases and 2.6% of controls, and E. coli or Shigella was found in 17.1% of cases and 6.8% of controls. Multiple E. coli pathotypes were isolated in 21 individuals (Table 2). Our sample size, which was originally reduced from 3,326 to 3,314 observations because of missing information on case-control status, was further reduced to 3,107 because of missing individual census data. For all subsequent analyses, we used a sample size of 3,107. The median ages of cases and controls were 3 years (range: 0–81) and 16 years (range: 0–99), respectively.
Table 1.
Infection Patterns Identified in Cases and Controls From 22 Communities in Northwestern Ecuador, 2003–2008
| Mutually Exclusive Infection Categories | Cases (n = 883) |
Controls (n = 2,431) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Giardia | 137 | 15.5 | 408 | 16.8 |
| Rotavirus | 85 | 9.6 | 47 | 1.9 |
| Rotavirus + Giardia | 52 | 5.9 | 9 | 0.4 |
| Escherichia colia | 52 | 5.9 | 107 | 4.4 |
| Giardia + E. colia | 46 | 5.2 | 42 | 1.7 |
| Plesiomonas shigelloides | 38 | 4.3 | 116 | 4.8 |
| Rotavirus + E. colia | 23 | 2.6 | 4 | 0.2 |
| Giardia + P. shigelloides | 17 | 1.9 | 32 | 1.3 |
| Rotavirus + P. shigelloides | 12 | 1.4 | 2 | 0.1 |
| E. colia + P. shigelloides | 9 | 1.0 | 8 | 0.3 |
| Rotavirus + Giardia + E. colia | 12 | 1.4 | 0 | 0.0 |
| Rotavirus + Giardia + P. shigelloides | 7 | 0.8 | 1 | 0.0 |
| Giardia + E. colia + P. shigelloides | 4 | 0.5 | 3 | 0.1 |
| Rotavirus + Giardia + E. colia + P. shigelloides | 3 | 0.3 | 0 | 0.0 |
| Rotavirus + E. colia + P. shigelloides | 2 | 0.2 | 1 | 0.0 |
| Total | 499 | 56.5 | 780 | 32.1 |
a Includes Shigella.
Table 2.
Escherichia coli and Shigellae Infection Patterns Identified in Cases and Controls Exclusively Infected With E. coli and/or Shigellae in 22 Communities in Northwestern Ecuador, 2003–2008
| Mutually Exclusive Infection Categories | Cases (n = 883) |
Controls (n = 2,431) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Enteroinvasive Escherichia coli | 11 | 1.2 | 48 | 2.0 |
| Shigellae | 6 | 0.7 | 16 | 0.7 |
| Enterotoxigenic E. coli | 22 | 2.5 | 23 | 0.9 |
| Enteropathogenic E. coli | 4 | 0.5 | 8 | 0.3 |
| Enteroinvasive E. coli + Shigellae | 5 | 0.6 | 9 | 0.4 |
| Enteroinvasive E. coli + enterotoxigenic E. coli | 2 | 0.2 | 1 | 0.0 |
| Shigellae + enterotoxigenic E. coli | 1 | 0.1 | 0 | 0.0 |
| Shigellae + enteropathogenic E. coli | 1 | 0.1 | 1 | 0.0 |
| Enteroinvasive E. coli + Shigellae + enterotoxigenic E. coli | 0 | 0.0 | 1 | 0.0 |
| Total | 52 | 5.9 | 107 | 4.4 |
Community prevalence
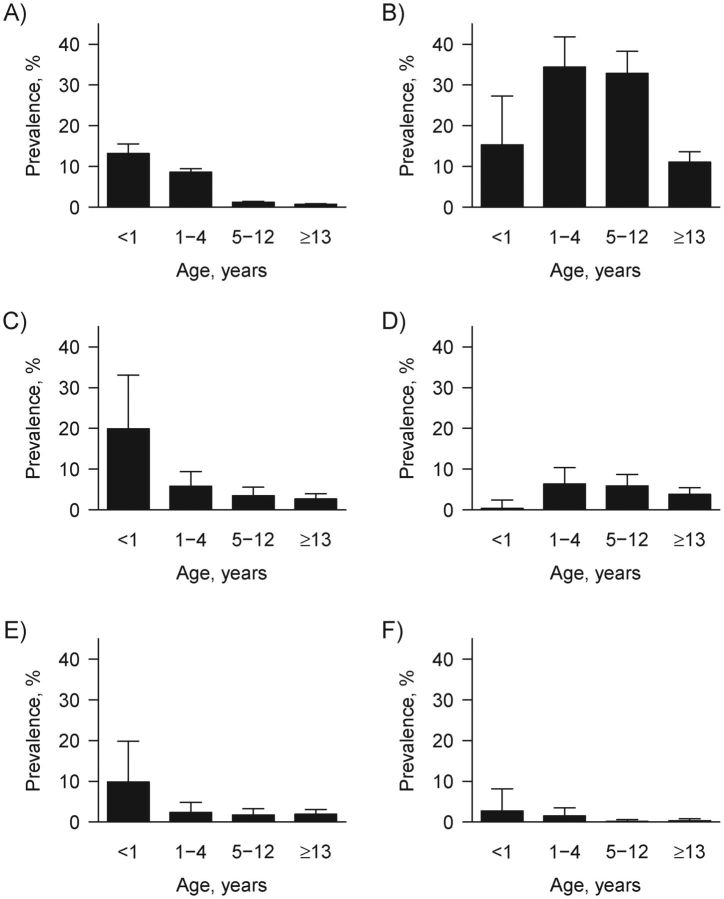
The 15-day-period prevalence of diarrhea was 2.3% (95% confidence interval (CI): 2.1, 2.5) (Table 3). Although there were cases of diarrhea in older children and adults, diarrhea was most prevalent in children younger than 5 years (Figure 1). The prevalences of Giardia and rotavirus were 20.3% (95% CI: 18.1, 22.5) and 3.2% (95% CI: 2.2, 4.2), respectively. Giardia and rotavirus infections were prevalent across all age categories, although the latter were most prevalent in children under 1 year of age. Of the E. coli pathotypes, EIEC/Shigellae was the most prevalent at 4.5% (95% CI: 3.3, 5.7) and was evenly distributed across the age groups with the exception of infants; only 1 EIEC/Shigellae infection was identified in infants younger than 1 year.
Table 3.
Weighted Community Prevalence and 95% Confidence Intervals Estimated by Using the Horvitz-Thompson Theory, 2003–2008
| Prevalence (%)a | 95% Confidence Interval | |
|---|---|---|
| All-cause diarrhea | 2.3 | 2.1, 2.5 |
| Giardia | 20.3 | 18.1, 22.5 |
| Rotavirus | 3.2 | 2.2, 4.2 |
| Enteroinvasive Escherichia coli/Shigellae | 4.5 | 3.3, 5.7 |
| Enterotoxigenic E. coli | 2.0 | 1.2, 2.7 |
| Enteropathogenic E. coli | 0.6 | 0.1, 1.0 |
a Weighted prevalence estimates were based on analyses of stool samples from cases and controls.
Figure 1.
Weighted community prevalence and upper confidence limit of all-cause diarrhea (A), Giardia (B), rotavirus (C), enteroinvasive Escherichia coli or Shigellae (D), enterotoxigenic E. coli (E), and enteropathogenic E. coli (F) across 4 age categories in 22 communities in northwestern Ecuador, 2003–2008.
Pathogenicity
A single infection with rotavirus was significantly associated with diarrhea in children from 1 to 4 years of age (RR = 2.4, 95% CI: 1.1, 6.2) and in those older than 13 years (RR = 6.6, 95% CI: 3.2, 15.1) (Table 4). In comparison, a single infection with ETEC increased the risk of diarrhea in children aged 1 through 12 years but not in adults. Single infections with rotavirus were significantly associated with diarrhea across all age groups (Mantel-Haenszel RR = 2.3, 95% CI: 1.3, 4.8). Age appeared to confound the associations between diarrhea and each of Giardia, rotavirus, ETEC, and EPEC. In all instances, crude estimates of the risk ratios were higher than Mantel-Haenszel risk ratios pooled across age groups. In addition, where we had enough data to report estimates across infection categories, Mantel-Haenszel risk ratios associated with a coinfection and any infection were greater than the risk ratios associated with single infections by the same pathogen.
Table 4.
Age-specific, Mantel-Haenszel Pooled and Crude Risk Ratios for Diarrhea and Bootstrap 95% Confidence Intervals in Northwestern Ecuador, 2003–2008
| Infection Category | <1 Yeara–d |
1–4 Yearsa–d |
5–12 Yearsa–d |
≥13 Yearsa–d |
Mantel-Haenszel Pooleda–d |
Crudea–c |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk Ratio | 95% CI | Risk Ratio | 95% CI | Risk Ratio | 95% CI | Risk Ratio | 95% CI | Risk Ratio | 95% CI | Risk Ratio | 95% CI | |
| Giardia | ||||||||||||
| Any infection | 2.3 | 1.2, 4.5 | 1.8 | 1.2, 2.7 | 1.1 | 0.6, 1.8 | 1.6 | 0.9, 2.7 | 1.7 | 1.3, 2.3 | 2.6 | 2.1, 3.2 |
| Single infection | 1.4 | 0.5, 3.8 | 1.2 | 0.8, 1.9 | 0.6 | 0.3, 1.2 | 0.6 | 0.1, 1.3 | 1.1 | 0.8, 1.5 | 1.5 | 1.2, 2.0 |
| Any coinfection | 3.9 | 2.4, 7.4 | 3.6 | 1.7, 7.2 | 7.8 | 3.3, 16.7 | 4.1 | 2.9, 6.0 | 7.6 | 5.6, 10.4 | ||
| Rotavirus | ||||||||||||
| Any infection | 2.0 | 0.7, 5.1 | 4.2 | 2.3, 8.0 | 7.0 | 3.2, 20.6 | 14.1 | 7.8, 27.2 | 4.3 | 2.8, 7.1 | 10.7 | 7.9, 15.1 |
| Single infection | 1.4 | 0.4, 5.2 | 2.4 | 1.1, 6.2 | 2.5 | 0.6, 10.9 | 6.6 | 3.2, 15.1 | 2.3 | 1.3, 4.8 | 5.8 | 3.8, 9.4 |
| Any coinfection | 8.7 | 6.2, 12.1 | 20.2 | 8.5, 58.3 | 59.0 | 30.6, 122.1 | 9.4 | 6.9, 12.7 | 25.5 | 17.3, 39.6 | ||
| Enteroinvasive Escherichia coli and Shigellae | ||||||||||||
| Any infection | 2.4 | 1.2, 5.0 | 3.9 | 1.8, 7.5 | 4.1 | 2.0, 7.9 | 2.9 | 1.8, 4.8 | 3.6 | 2.4, 5.0 | ||
| Single infection | 1.2 | 0.4, 5.7 | 2.6 | 0.8, 5.6 | 1.5 | 0.7, 3.2 | 1.6 | 0.9, 2.7 | ||||
| Any coinfection | 3.4 | 1.7, 7.9 | 7.4 | 3.5, 15.7 | 10.3 | 3.4, 33.1 | 4.6 | 2.7, 8.4 | 6.7 | 4.1, 10.5 | ||
| Enterotoxigenic E. coli | ||||||||||||
| Any infection | 1.5 | 0.4, 4.9 | 5.4 | 3.1, 9.8 | 8.8 | 3.4, 21.4 | 3.3 | 0.9, 12.2 | 3.7 | 2.2, 6.5 | 7.1 | 4.6, 12.1 |
| Single infection | 4.1 | 1.5, 10.7 | 8.2 | 1.8, 24.5 | 1.8 | 0, 9.8 | 2.2 | 1.0, 6.3 | 3.8 | 2.0, 8.2 | ||
| Any coinfection | 6.5 | 3.4, 11.1 | 9.4 | 2.0, 41.0 | 16.2 | 0, 158.5 | 6.0 | 3.8, 8.8 | 13.9 | 7.5, 28.7 | ||
| Enteropathogenic E. coli | ||||||||||||
| Any infection | 2.7 | 0.9, 8.1 | 1.7 | 0.7, 4.8 | 5.7 | 2.0, 14.3 | ||||||
| Single infection | ||||||||||||
| Any coinfection | 3.5 | 1.1, 10.8 | 3.4 | 1.2, 8.7 | 11.6 | 4.2, 44.9 | ||||||
Abbreviation: CI, confidence interval.
a Risk ratios compare the risk of diarrhea in those exposed to risk with those unexposed to Giardia, rotavirus, pathogenic E. coli, Shigellae, and Plesiomonas shigelloides.
b Risk ratios based on cell counts of <5 were excluded.
c All risk ratios were weighted by inverse sampling probabilities.
d Strata-specific and pooled estimates are weighted by inverse age-specific sampling probabilities.
Coinfections
We found evidence for greater than additive and greater than multiplicative effects of rotavirus coinfections on the risk of having diarrhea. Under the null hypothesis of no interaction on the additive and multiplicative scales, we would expect the interaction contrast ratio to equal 0 and the multiplicative interaction to equal 1, respectively. The interaction contrast ratio specific to coinfection with rotavirus and Giardia was 7.96 (95% CI: 3.13, 18.92), and the multiplicative interaction was 3.61 (95% CI: 1.33, 8.71) (Table 5). Coinfections with rotavirus and Giardia were found in all age categories. Excluding 2 coinfected individuals with missing birth dates, 31 (63%) rotavirus-Giardia coinfections occurred in children under the age of 5 years. The interaction contrast ratio related to coinfection with rotavirus and E. coli was 9.93 (95% CI: 2.61, 28.41), while the multiplicative interaction was 3.06 (95% CI: 0.75, 7.27). Of these 24 coinfections, 1 was missing a birth date, 10 (43%) were found in children under the age of 5, and 16 were specific to EIEC/Shigellae. We found no interaction effects associated with a Giardia and E. coli coinfection on diarrhea.
Table 5.
Assessment of the Biologic Interaction Between Coinfecting Pathogens Associated With Diarrhea on Additive and Multiplicative Scales by Using Age-standardized Risk Ratios and Bootstrap 95% Confidence Intervals in Northwestern Ecuador, 2003–2008
| Age-standardized Estimates |
|||||
|---|---|---|---|---|---|
| Infection Category | RR | Additive Model |
Multiplicative Model |
||
| Interaction Contrast Ratioa | 95% CI | Multiplicative Interactionb | 95% CI | ||
| Rotavirus (single infection) | 2.63 | ||||
| Giardia (single infection) | 1.13 | ||||
| Rotavirus and Giardia (coinfection) | 10.72 | ||||
| 7.96 | 3.13, 18.92 | 3.61 | 1.33, 8.71 | ||
| Rotavirus (single infection) | 2.63 | ||||
| Escherichia coli/Shigellae (single infection) | 1.64 | ||||
| Rotavirus and E. coli/Shigellae (coinfection) | 13.20 | ||||
| 9.93 | 2.61, 28.41 | 3.06 | 0.75, 7.27 | ||
| Giardia (single infection) | 1.13 | ||||
| E. coli/Shigellae (single infection) | 1.64 | ||||
| Giardia and E. coli/Shigellae (coinfection) | 3.02 | ||||
| 1.25 | −1.48, 3.13 | 1.63 | 0.47, 3.06 | ||
Abbreviations: CI, confidence interval; RR, risk ratio.
a Interaction contrast ratio = RRcoinfection − RRsingle infection 1 − RRsingle infection 2 + 1.
b Multiplicative interaction = RRcoinfection/(RRsingle infection 1 × RRsingle infection 2).
DISCUSSION
Using community-level data, we provide evidence that coinfecting pathogens act synergistically with rotavirus to cause diarrhea. Possible mechanisms for these synergistic effects may be specific, involving attachment and invasion of the intestinal epithelia by pathogens, or nonspecific resulting from inflammation. We also found evidence that a single infection with rotavirus is pathogenic in young children and adults. The potential for rotavirus to cause disease in these age groups and the potential for pathogenesis to be more severe in the presence of a rotavirus coinfection may warrant targeting rotavirus prevention efforts to both young children and adults.
Synergism of coinfecting pathogens
Simultaneous infection with rotavirus and Giardia or rotavirus and E. coli (including Shigellae) resulted in a greater risk of having diarrhea than would be expected if the coinfecting organisms acted independently of one another. The idea that rotavirus and Giardia act synergistically contradicts findings by Bilenko et al. (22), who compared the severity scores of single and mixed infections in Bedouin infants. However, their inferences were based on a small sample size with only 12 single rotavirus infections and 3 rotavirus-Giardia coinfections. Regarding coinfection with rotavirus and E. coli, our findings disagree with those of Uhnoo et al. (21), which could be the result of differences between developed and developing country settings. Unicomb et al. (20) also reported findings dissimilar to ours, but the E. coli pathotypes detected were different. Approximately two-thirds of the rotavirus-E. coli coinfections found in their study were specific to diffuse-adherent E. coli and enteroaggregative E. coli while, in our study, a similar proportion involved EIEC/Shigellae. On the other hand, the ability of these coinfecting pathogens to have at least additive effects is supported by other studies in children (4, 6, 10, 18, 19). Our community-based study provides evidence for superadditive effects of coinfecting pathogens. Unlike these studies, ours includes older age groups and uses a different outcome measure. Rather than severity of diarrhea, we have considered the ability of coinfecting pathogens to cause diarrhea, defined as 3 or more loose stools passed in a 24-hour period. Because our definition likely includes diarrhea ranging in severity, our findings that coinfecting pathogens act synergistically to cause either mild or severe diarrhea complement these previous works.
The evidence for synergistic interaction between rotavirus and other coinfecting pathogens is important to the global burden of diarrhea given that developing and rural regions may experience a high prevalence of enteric infections. Giardia, for example, was estimated to affect one-fifth of the population in our study region, creating the potential for high levels of coinfection. Furthermore, the lack of improved water and sanitation in developing regions may facilitate simultaneous transmission of enteric pathogens. Synergistic interaction between rotavirus and coinfecting pathogens calls for targeted rotavirus prevention, as well as more general water, sanitation, and hygiene improvements, to curb transmission of coinfecting pathogens.
Mechanisms for synergistic interaction
In vitro models of pathogenesis indicate that synergism between rotavirus and invasive bacteria involves specific biologic pathways (23–25). These pathways may involve the attachment of, or the invasion by, coinfecting pathogens through an up-regulation of specific receptors. It is interesting that we found no evidence for pathogenic effects of EIEC/Shigellae alone, yet when infection occurred in the presence of rotavirus the risk of diarrhea was enhanced. It should be noted that rotavirus, ETEC, EPEC, and Giardia predominately affect the small bowel, while EIEC and Shigellae colonize the large bowel (30). Pathogenesis studies in the rhesus monkey (31) and rabbits (32) have suggested that Shigella passage through the jejunum of the small intestine may alter secretion of sodium and water, potentially contributing to watery diarrhea. The heightened pathogenicity of Giardia in the presence of rotavirus may be related to a more successful attachment of the trophozoite ventral disk to the infected epithelium (33).
Alternatively, the biologic mechanisms behind synergistic interaction may be less specific than in vitro models predict. The inflammatory response induced by rotavirus likely damages the epithelium and alters the mucosal structure facilitating the attachment and invasion of coinfecting pathogens. Inflammation is also characterized by the release of fluid, mucin, and cellular debris, which potentially contain high-energy nutrients for pathogens (34). Furthermore, the secretion of antimicrobials during inflammation could alter the composition of the gut microbiota, allowing pathogens to occupy the commensal niche (35). More research is needed to elucidate the pathogenesis of diarrhea during rotavirus coinfection.
Prevalence and pathogenicity
Regarding the pathogenicity of single infections, our findings that rotavirus is associated with diarrhea when pooled across age groups are consistent with those of other community- and clinic-based studies (7, 36, 37). However, the lack of an association between rotavirus and diarrhea in infants younger than 1 year is inconsistent with the literature (6, 7, 38) and may correspond to the presence of maternal antibodies (39), our small sample size in this age group, or misclassification of diarrhea owing to looser stools in infants at baseline. Despite the probable exposure to early infection with rotavirus, the ability of rotavirus to cause diarrhea in adolescents and adults was observed. The presence of rotavirus-induced diarrhea in older age groups may be accounted for by frequent transmission between infants and their caregivers, potential inclusion of immunocompromised and malnourished individuals in our sample, inclusion of mild diarrhea events, waning of rotavirus immunity over time, and exposure to a variety of circulating genotypes (40). A previous study from this region found high rates of the emerging G9 genotype (41), while another reported subsequent replacement of the G9 genotype by the G1 and G2 genotypes (42). In developing countries, rotavirus-specific interventions may also need to be targeted to older children and adults.
Overall, we estimated that 2.3% of the population, as well as 9.3% of children under the age of 5, had diarrhea during any given 15-day period. Although diarrhea prevalence in the total population may have been low, we found high prevalence of infection. High prevalence of asymptomatic infection may reflect early exposure to enteric pathogens and naturally acquired immunity. High prevalence of Giardia in our region may be further explained by frequent exposure, rapid recolonization rates, and long-term shedding (43). The overall isolation rates of Giardia, rotavirus, EIEC/Shigellae, ETEC, and EPEC from cases and controls were comparable to those found in other studies of children under 5 years of age (5, 8, 9, 36). Our detection of pathogens in 56.5% of cases was similar to isolation rates from other community-based studies (5, 44, 45) and, as expected, was lower than those from hospital-based studies in children, which capture more severe diarrhea (7, 10, 46).
Although cases and controls were not age matched while sampling, all age categories had a sufficient number of cases and controls for analyses. Our age-specific estimates of pathogenicity were limited solely by the prevalence of pathogen infections within each age category. Confounding by age was addressed in our analyses. Additional confounders of the association between enteric infection and diarrhea, such as environmental or social variables, potentially act through other enteric pathogens to cause diarrhea. Therefore, adjustment for other enteric pathogens addresses confounding, which in our analyses was done through the exclusion of other measured pathogens from the risk ratio estimates. There are unmeasured pathogens that may have resulted in confounding. However, unpublished data from our region indicate that the majority of the helminthes in circulation are nonpathogenic and that there are low rates of other potentially pathogenic organisms, such as Vibrio cholerae, Cryptosporidium, Salmonella spp., and Aeromonas spp. Published data from other regions suggest that the predominant pathogens are those captured in our study (11, 36, 37).
Conclusions
Given the high prevalence of enteric pathogen coinfection found in our study and those of others (7, 9, 10, 12, 46), a true understanding of the pathogenesis of diarrheal disease is incomplete without a thorough understanding of the biologic interaction of these pathogens. Furthermore, reducing the diarrheal disease burden is dependent upon our knowledge of the pathogenesis of diarrhea. This study is one of the few to consider pathogenicity of both single and mixed infections in all age groups, with a particular focus on the synergistic interaction between coinfecting pathogens. Our literature search suggests that this is the first community-based study of diarrhea to examine synergistic effects using theory rooted in foundational epidemiology. Further research is needed to address the specific biologic mechanisms of enteric pathogens resulting in their synergistic interaction.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan (Darlene Bhavnani, Jason E. Goldstick, Joseph N. S. Eisenberg); Centro de Biomedicina, Universidad Central del Ecuador, Quito, Ecuador (William Cevallos); and Department of Microbiology, Universidad San Francisco de Quito, Quito, Ecuador (Gabriel Trueba).
This work was supported by the National Institutes of Health (R01-AI050038) and the University of Michigan Interdisciplinary Training Program in Infectious Diseases funded by the National Institute of Allergy and Infectious Diseases (T32AI 049816).
The authors would like to thank Dr. Carl F. Marrs and Dr. Alan J. Hogg for their helpful comments and the Ecología, Desarrollo, Salud y Sociedad (EcoDESS) project field team for their invaluable contributions to data collection.
Conflict of interest: none declared.
REFERENCES
- 1.Global Health Observatory. Causes of death in 2008. Geneva, Switzerland: World Health Organization; 2012. (www.who.int/gho/mortality_burden_disease/causes_death_2008/en/index.html. ). (Accessed October 28, 2011) [Google Scholar]
- 2.O'Ryan M, Prado V, Pickering LK. A millennium update on pediatric diarrheal illness in the developing world. Semin Pediatr Infect Dis. 2005;16(2):125–136. doi: 10.1053/j.spid.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Boschi-Pinto C, Velebit L, Shibuya K. Estimating child mortality due to diarrhoea in developing countries. Bull World Health Organ. 2008;86(9):710–717. doi: 10.2471/BLT.07.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimprel E, Rodrigo C, Desselberger U. Rotavirus disease: impact of coinfections. Pediatr Infect Dis J. 2008;27(1 suppl):S3–S10. doi: 10.1097/01.inf.0000197563.03895.91. [DOI] [PubMed] [Google Scholar]
- 5.Hien BT, Trang do T, Scheutz F, et al. Diarrhoeagenic Escherichia coli and other causes of childhood diarrhoea: a case-control study in children living in a wastewater-use area in Hanoi, Vietnam. J Med Microbiol. 2007;56(Pt 8):1086–1096. doi: 10.1099/jmm.0.47093-0. [DOI] [PubMed] [Google Scholar]
- 6.Black RE, Lopez de Romana G, Brown KH, et al. Incidence and etiology of infantile diarrhea and major routes of transmission in Huascar, Peru. Am J Epidemiol. 1989;129(4):785–799. doi: 10.1093/oxfordjournals.aje.a115193. [DOI] [PubMed] [Google Scholar]
- 7.Albert MJ, Faruque AS, Faruque SM, et al. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J Clin Microbiol. 1999;37(11):3458–3464. doi: 10.1128/jcm.37.11.3458-3464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orlandi PP, Silva T, Magalhaes GF, et al. Enteropathogens associated with diarrheal disease in infants of poor urban areas of Porto Velho, Rondonia: a preliminary study. Mem Inst Oswaldo Cruz. 2001;96(5):621–625. doi: 10.1590/s0074-02762001000500005. [DOI] [PubMed] [Google Scholar]
- 9.Bodhidatta L, McDaniel P, Sornsakrin S, et al. Case-control study of diarrheal disease etiology in a remote rural area in western Thailand. Am J Trop Med Hyg. 2010;83(5):1106–1109. doi: 10.4269/ajtmh.2010.10-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souza EC, Martinez MB, Taddei CR, et al. Etiologic profile of acute diarrhea in children in Sao Paulo. (In Portuguese) J Pediatr (Rio J) 2002;78(1):31–38. [PubMed] [Google Scholar]
- 11.Ochoa TJ, Ecker L, Barletta F, et al. Age-related susceptibility to infection with diarrheagenic Escherichia coli among infants from periurban areas in Lima, Peru. Clin Infect Dis. 2009;49(11):1694–1702. doi: 10.1086/648069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres ME, Pirez MC, Schelotto F, et al. Etiology of children's diarrhea in Montevideo, Uruguay: associated pathogens and unusual isolates. J Clin Microbiol. 2001;39(6):2134–2139. doi: 10.1128/JCM.39.6.2134-2139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzipori SR, Makin TJ, Smith ML, et al. Clinical manifestations of diarrhea in calves infected with rotavirus and enterotoxigenic Escherichia coli. J Clin Microbiol. 1981;13(6):1011–1016. doi: 10.1128/jcm.13.6.1011-1016.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newsome PM, Coney KA. Synergistic rotavirus and Escherichia coli diarrheal infection of mice. Infect Immun. 1985;47(2):573–574. doi: 10.1128/iai.47.2.573-574.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzipori S, Chandler D, Makin T, et al. Escherichia coli and rotavirus infections in four-week-old gnotobiotic piglets fed milk or dry food. Aust Vet J. 1980;56(6):279–284. doi: 10.1111/j.1751-0813.1980.tb05724.x. [DOI] [PubMed] [Google Scholar]
- 16.Thouless ME, DiGiacomo RF, Deeb BJ. The effect of combined rotavirus and Escherichia coli infections in rabbits. Lab Anim Sci. 1996;46(4):381–385. [PubMed] [Google Scholar]
- 17.Wray C, Dawson M, Afshar A, et al. Experimental Escherichia coli and rotavirus infection in lambs. Res Vet Sci. 1981;30(3):379–381. [PubMed] [Google Scholar]
- 18.Katouli M, Shokouhi F, Farhoudi-Moghaddam AA, et al. Occurrence of colonization factor antigens I & II in enterotoxigenic Escherichia coli associated diarrhea in Iran & correlation with severity of disease. Indian J Med Res. 1992;95:115–120. [PubMed] [Google Scholar]
- 19.Hori H, Akpedonu P, Armah G, et al. Enteric pathogens in severe forms of acute gastroenteritis in Ghanaian children. Acta Paediatr Jpn. 1996;38(6):672–676. doi: 10.1111/j.1442-200x.1996.tb03729.x. [DOI] [PubMed] [Google Scholar]
- 20.Unicomb LE, Faruque SM, Malek MA, et al. Demonstration of a lack of synergistic effect of rotavirus with other diarrheal pathogens on severity of diarrhea in children. J Clin Microbiol. 1996;34(5):1340–1342. doi: 10.1128/jcm.34.5.1340-1342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uhnoo I, Olding-Stenkvist E, Kreuger A. Clinical features of acute gastroenteritis associated with rotavirus, enteric adenoviruses, and bacteria. Arch Dis Child. 1986;61(8):732–738. doi: 10.1136/adc.61.8.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilenko N, Levy A, Dagan R, et al. Does co-infection with Giardia lamblia modulate the clinical characteristics of enteric infections in young children? Eur J Epidemiol. 2004;19(9):877–883. doi: 10.1023/b:ejep.0000040533.75646.9c. [DOI] [PubMed] [Google Scholar]
- 23.Superti F, Petrone G, Pisani S, et al. Superinfection by Listeria monocytogenes of cultured human enterocyte-like cells infected with poliovirus or rotavirus. Med Microbiol Immunol. 1996;185(3):131–137. doi: 10.1007/s004300050022. [DOI] [PubMed] [Google Scholar]
- 24.Di Biase AM, Petrone G, Conte MP, et al. Infection of human enterocyte-like cells with rotavirus enhances invasiveness of Yersinia enterocolitica and Y. pseudotuberculosis. J Med Microbiol. 2000;49(10):897–904. doi: 10.1099/0022-1317-49-10-897. [DOI] [PubMed] [Google Scholar]
- 25.Bukholm G. Human rotavirus infection enhances invasiveness of enterobacteria in MA-104 cells. APMIS. 1988;96(12):1118–1124. doi: 10.1111/j.1699-0463.1988.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 26.Eisenberg JN, Cevallos W, Ponce K, et al. Environmental change and infectious disease: how new roads affect the transmission of diarrheal pathogens in rural Ecuador. Proc Natl Acad Sci U S A. 2006;103(51):19460–19465. doi: 10.1073/pnas.0609431104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horvitz DG, Thompson DJ. A generalization of sampling without replacement from a finite universe. J Am Stat Assoc. 1952;47(260):663–685. [Google Scholar]
- 28.Rothman KJ, Greenland S. Modern Epidemiology. 2nd. Philadelphia, PA: Lippincott-Raven Publishers; 1998. [Google Scholar]
- 29.Escobar JC, Bhavnani D, Trueba G, et al. Plesiomonas shigelloides infection, Ecuador, 2004–2008. Emerg Infect Dis. 2012;18(2):322–324. doi: 10.3201/eid1802.110562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2(2):123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 31.Kinsey MD, Formal SB, Dammin GJ, et al. Fluid and electrolyte transport in rhesus monkeys challenged intracecally with Shigella flexneri 2a. Infect Immun. 1976;14(2):368–371. doi: 10.1128/iai.14.2.368-371.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kandel G, Donohue-Rolfe A, Donowitz M, et al. Pathogenesis of Shigella diarrhea. XVI. Selective targetting of Shiga toxin to villus cells of rabbit jejunum explains the effect of the toxin on intestinal electrolyte transport. J Clin Invest. 1989;84(5):1509–1517. doi: 10.1172/JCI114327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller N, von Allmen N. Recent insights into the mucosal reactions associated with Giardia lamblia infections. Int J Parasitol. 2005;35(13):1339–1347. doi: 10.1016/j.ijpara.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Stecher B, Hardt WD. The role of microbiota in infectious disease. Trends Microbiol. 2008;16(3):107–114. doi: 10.1016/j.tim.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Raffatellu M, Baumler AJ. Salmonella's iron armor for battling the host and its microbiota. Gut Microbes. 2010;1(1):70–72. doi: 10.4161/gmic.1.1.10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasan KZ, Pathela P, Alam K, et al. Aetiology of diarrhoea in a birth cohort of children aged 0–2 year(s) in rural Mirzapur, Bangladesh. J Health Popul Nutr. 2006;24(1):25–35. [PubMed] [Google Scholar]
- 37.Zaki AM, DuPont HL, el Alamy MA, et al. The detection of enteropathogens in acute diarrhea in a family cohort population in rural Egypt. Am J Trop Med Hyg. 1986;35(5):1013–1022. doi: 10.4269/ajtmh.1986.35.1013. [DOI] [PubMed] [Google Scholar]
- 38.Velazquez FR, Matson DO, Guerrero ML, et al. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis. 2000;182(6):1602–1609. doi: 10.1086/317619. [DOI] [PubMed] [Google Scholar]
- 39.Brussow H, Sidoti J, Barclay D, et al. Prevalence and serotype specificity of rotavirus antibodies in different age groups of Ecuadorian infants. J Infect Dis. 1990;162(3):615–620. doi: 10.1093/infdis/162.3.615. [DOI] [PubMed] [Google Scholar]
- 40.Anderson EJ, Weber SG. Rotavirus infection in adults. Lancet Infect Dis. 2004;4(2):91–99. doi: 10.1016/S1473-3099(04)00928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Endara P, Trueba G, Solberg OD, et al. Symptomatic and subclinical infection with rotavirus P[8]G9, rural Ecuador. Emerg Infect Dis. 2007;13(4):574–580. doi: 10.3201/eid1304.061285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasing ME, Trueba G, Baquero MI, et al. Rapid changes in rotaviral genotypes in Ecuador. J Med Virol. 2009;81(12):2109–2113. doi: 10.1002/jmv.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilman RH, Marquis GS, Miranda E, et al. Rapid reinfection by Giardia lamblia after treatment in a hyperendemic Third World community. Lancet. 1988;1(8581):343–345. doi: 10.1016/s0140-6736(88)91131-2. [DOI] [PubMed] [Google Scholar]
- 44.Molbak K, Wested N, Hojlyng N, et al. The etiology of early childhood diarrhea: a community study from Guinea-Bissau. J Infect Dis. 1994;169(3):581–587. doi: 10.1093/infdis/169.3.581. [DOI] [PubMed] [Google Scholar]
- 45.Baqui AH, Sack RB, Black RE, et al. Enteropathogens associated with acute and persistent diarrhea in Bangladeshi children less than 5 years of age. J Infect Dis. 1992;166(4):792–796. doi: 10.1093/infdis/166.4.792. [DOI] [PubMed] [Google Scholar]
- 46.Urbina D, Arzuza O, Young G, et al. Rotavirus type A and other enteric pathogens in stool samples from children with acute diarrhea on the Colombian northern coast. Int Microbiol. 2003;6(1):27–32. doi: 10.1007/s10123-003-0104-5. [DOI] [PubMed] [Google Scholar]