Abstract
Wingless locust nymphs can form massive migratory groups known as bands, whose coordinated movement results from local interactions. We analysed the spatial distribution of locusts within naturally occurring bands and compared them with computer simulations to infer which interaction rules are used by individuals. We found that the empirical radial distribution of neighbours around a focal individual was isotropic, indicating a tendency for locusts to interact with neighbours all around them, rather than a bias towards pursuing individuals ahead or escaping from the ones following behind. By using maps of neighbour densities and pair correlation functions, we found evidence for a short-range repulsion force, balanced by a clustering force, presumably alignment and/or attraction, at a distance of around 3 cm. These results were similar to those observed when using a ‘zonal’ self-propelled particles model where repulsion/alignment/attraction forces are delimited by concentric circular zones of set radii. However, the profiles obtained either by using different combinations of forces, limiting the number of neighbours involved in interactions, or by varying the range of some zones, all appeared to produce similar results, thereby limiting the ability to more precisely determine the rules underlying locust interactions.
Keywords: collective movement, locusts, self-propelled particles
1. Introduction
Before locusts form their infamous flying swarms as adults, flightless juveniles gather and mass migrate in what is one of the most impressive displays of animal collective movement: locust marching bands. Locust species can be found in two different phases, which differ in their behaviour and, in some species, in their morphology and colour: the solitarious phase is characterized by individuals actively avoiding conspecifics, while the gregarious phase is characterized by individuals actively aggregating [1]. Locust marching bands are groups of gregarious nymphs that start moving in unison when temperatures are sufficiently high, usually in the morning, until they aggregate again during the cooler temperatures of evening and night [2–6]. The shape of these groups varies between species, with some, such as the Australian plague locust Chortoicetes terminifera, characterized by a dense and wide crescent-shaped front followed by much lower densities [5], while others such as the brown locust Locustana pardalina [4] form networks of dense and intertwined streams.
The mechanism responsible for locust cohesive mass movement has long been hypothesized to be a tendency for locusts to align with each other [4], but it has never been quantitatively tested. Laboratory and field experiments in the desert locust, Schistocerca gregaria [7], and C. terminifera [5] have shown that locust collective movement undergoes a rapid density-driven transition from disordered movement to a highly aligned state, reminiscent of phase transitions in physical systems. These studies show that this transition closely matches the predictions of the most common class of collective motion model, the so-called self-propelled particles (SPP) model, in which individuals align with their neighbours in a local radius surrounding them [8,9]. Moreover, in the field, pairs of individuals of C. terminifera found at the back of bands were aligned only within a limited radius of 13.5 cm [5], which confirmed the local scale of interactions compared with the size of the whole group, which can spread over hundreds of metres. However, it has also been found that the onset of collective movement in S. gregaria [10,11] is shaped by cannibalistic interactions that are also present in C. terminifera [12] as well as in migratory band-forming Mormon crickets [13]. Following this idea, an alternative model of collective motion was recently proposed [14], in which individuals pursue those in front of them while escaping from those behind. This model led to a similar pattern of collective alignment as the one observed in locusts.
With widely different interaction mechanisms leading to seemingly indistinguishable patterns of collective motion, there has been a need to more directly quantify individual interactions from empirical data. Inter-individual interactions have recently been analysed in several species, using different techniques. By using the spatial distribution of individuals in the group, one can infer the relative distribution of individuals around a focal individual and pinpoint anisotropy in the radial distribution of neighbours [15] or radial concentrations of neighbours at set distances that likely reflect inter-individual repulsion, alignment and/or attraction [16]. Recent studies in fish schools have allowed automated video tracking of individuals and their neighbours through time, leading to the precise inference that individuals tend to follow their nearest neighbour in mosquito fish [17] and adjust their speed to that of their neighbour(s) in both mosquito fish and golden shiners [17,18]. These results illustrate yet another mechanism leading to collective motion and differing widely from the concentric model of repulsion/alignment/attraction implemented in the most common zonal models (such as SPP models) of collective motion.
Locust collective movement continues to pose a major challenge to automated tracking, as their displacement, in field conditions, combines walking with fast and lengthy hops. On the other hand, field studies of migratory bands containing millions of mobile individuals allow for the collection of large amounts of positional data extracted from recording of the flow of marching locusts. In this paper, we analyse the spatial distribution of locusts in marching bands of C. terminifera, and compare it with the predictions of models using various behavioural rules. We first determine whether the radial distribution of locusts around focal individuals is anisotropic, and then examine the two-dimensional density maps of neighbours to assess whether interaction rules can be inferred from the spatial distribution of individuals.
2. Empirical data acquisition and analysis
Data were collected during field trips described in detail in Buhl et al. [5]. For this analysis, we used footage from five different bands of C. terminifera studied in November 2007 near Katanning in Western Australia; in November 2008 near Ravensthorpe, Western Australia; and in January 2009 near Parkes, New South Wales. All bands comprised a mix of juvenile developmental stages (third to fifth instars). We recorded the flow of locusts under tripod-mounted 1080 p cameras filming vertically overhead (20 recordings; average area recorded: 0.68 m2 ± 0.24 s.d.). One frame from every 30 s of this footage was then displayed in a custom Matlab interface in which the coordinates of the front and back of each locust present on the frame were manually pointed (see [5] for details). For each frame (n = 1824), the density was calculated by dividing the total number of locusts N by the area of the frame. We calculated the direction of the locust as the angle 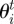 of the vector starting from the coordinates of the abdomen (xt, yt) and ending at the tip of the head (xh, yh) for each of the N locusts. Each locust was then characterized by its position vector ci using coordinates
of the vector starting from the coordinates of the abdomen (xt, yt) and ending at the tip of the head (xh, yh) for each of the N locusts. Each locust was then characterized by its position vector ci using coordinates
and its unit direction vector vi using the angle 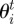 .
.
For each individual, we calculated the position of each neighbour in a coordinate system centred on the focal individual, with the x-axis extending along the orientation of the locust body (longitudinal axis) and the y-axis perpendicularly and to the left side of it (transverse axis). To avoid biases introduced by the bounded field of view, we consider neighbours within only a radius that is inferior or equal to the distance to the nearest edge of the field of view, a procedure referred to as the Hanisch correction [19,20].
The distribution of neighbours was discretized with bins of size Δx = Δy = 1 cm. We calculated the average density of neighbours per focal individual observed in each bin and normalized it by dividing it by the average density of neighbours in the entire observed area. Therefore, a completely homogeneous distribution of neighbours would result in the relative density tending towards a value of 1 in all bins. These distributions of relative neighbour densities can then be plotted as colour-coded two-dimensional maps revealing the likely positions of neighbours relative to a focal individual.
The pair correlation function g(r) [20] is a similar measure but considers the density of neighbours all around the focal individual within an annulus comprised between a radius of r and r + Δr (we used Δr = 1 cm here), and normalized by dividing it by the average density of neighbours in the entire area of observation.
As proposed in Lukeman et al. [16], in the presence of a peak observed in g(r) at a particular distance rp, we calculated the radial distribution of neighbours around the focal individual i at the preferred distance re (within an annulus between radius re − Δr and re + Δr, with Δr = 1 cm here) by calculating the angle φ formed between the longitudinal axis of individual i and the line joining the coordinates of i to those of its neighbour j. We also determined the radial distributions of the nearest neighbour (i.e. using the angle φ between a focal individual and its nearest neighbour only) as calculated in previous studies in starlings [15,21]. Circular statistics were performed using CircStat [22] for Matlab.
3. Collective movement models
All of the model variants used here assume that locusts have a limited range of interaction with their neighbours, which is consistent with our previous observations, namely that pairs of locusts appear aligned within 13.5 cm [5], and the idea that locusts have a limited range of vision. Default parameter values were estimated from laboratory data, field experiments and previous models [5,23]. We divided the models into two main classes inspired from a large number of previous studies. The specific rules and parameters are described in further detail in the electronic supplementary material.
The first class belonged to the family of SPP models [8,9]. We chose the more complex versions introduced by Gregoire et al. [9], with successive concentric zones around the focal individual defining whether the main force exerted by a neighbour is repulsion (short range), alignment (intermediate range) or attraction (long range). As in [9], repulsion or attraction is calculated as a Lennard-Jones force acting within a range rp, where both forces balance each other at a preferred distance re. The alignment force corresponds to the average direction of neighbours located at an intermediate range, overlapping the repulsion/attraction, when the distance rij between the focal individual i and a neighbour j is comprised between the two ranges rc < rij < ra ≤ rp. Because interactions are spatially defined within concentric zones, this type of model is also referred to as ‘metric’ or ‘zonal’ model. Within this class of model, we explored how the presence of the three components of the social force or only some of them (repulsion/alignment) influenced the spatial distribution of individuals. We also implemented a so-called ‘topological’ variant where only a defined number of nearest neighbours was taken into account when calculating social forces applying to an individual.
The second class of model was first described as a ‘pursuit/escape’ model, where individuals are attracted towards neighbours ahead of and moving away from them, while they are repelled by individuals that are behind and moving towards them [14] (see electronic supplementary material for a complete model description). Such model rules have an obvious resemblance to cannibalistic interactions observed in both S. gregaria [10,11] and C. terminifera [12] as well as in Mormon crickets [13]. Within this class, we tested variants where only escape or pursuit forces were present, or where both of them acted together.
As locusts combine walking and hopping when moving in bands, and most often more of the latter than the former in C. terminifera, we also tested the influence of hopping on the spatial distribution of individuals obtained using both model classes.
In all model variants, individuals were initially randomly distributed in an area representing 1 m2 (with periodic boundary conditions), and the parameters we used led to rapid collective alignment. The distribution of all the individuals' coordinates and orientations was then extracted so that the same analysis as that used on the empirical data could be applied to the simulation dataset. The normalized root mean square error (n.r.m.s.e.) was calculated to estimate the goodness of fit (the closer to zero the n.r.m.s.e. value is the better is the fit) between the empirically observed pair correlation function g(r) and that produced by the simulations.
4. Results
Our empirical data comprised 1824 frames, with an average density (across frames) of 30.34 locusts m−2 (ranging from 2.12 to 567.14). In total, there were 31 284 locusts serving as focal individuals (i.e. not all individuals on a frame appeared as focal individuals: some had no neighbours or stood next to the edge of the field of recording).
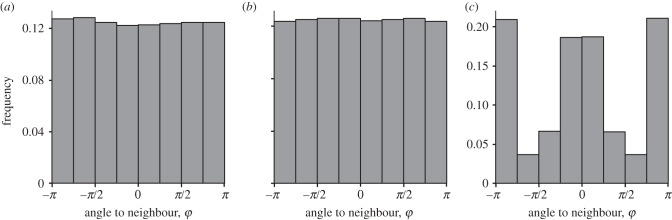
The radial distribution of the nearest neighbours in the empirical data was isotropic (figure 1; Rayleigh's test for uniformity: N = 19 929, Z = 1.375, p = 0.2528; see also the electronic supplementary material for the radial distribution of neighbours at set distances). This was also the case at lower densities (Rayleigh's test on observations under the median density of 15.75 locusts m−2: N = 1948, Z = 1948, p = 0.83). The results were similar for all variants of the SPP model class (figure 1b), which was expected, given the isotropic nature of all interaction rules implemented in the model. In contrast, variants of the pursuit/escape model were characterized by a marked anisotropy of the radial distribution of neighbours, with fewer neighbours on the sides and more of them at the front and/or the back, no matter what density or frequency of hopping was used (see figure 1c and electronic supplementary material).
Figure 1.
(a) Radial distribution of the nearest neighbours around a focal individual in the empirical data. This figure shows the distribution of the angle between the axis formed by the orientation of the focal individual and a line joining its position to its nearest neighbour. (b) The radial distribution of neighbours in an SPP model (here with 100 individuals) is clearly isotropic. (c) The radial distribution of neighbours in a pursuit/escape model (here with 100 individuals) is strongly anisotropic, with nearest neighbours more often observed in front of and behind the focal individual.
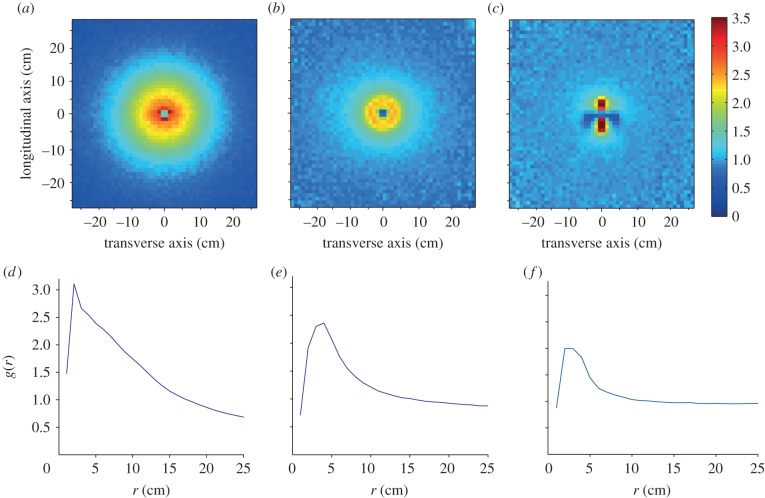
In the empirical data, the two-dimensional maps of neighbour relative densities revealed a gap in the neighbour distribution at a very close range, while a ring of densities greater than one can clearly be observed at a few centimetres (2–3 cm) around the focal individual (figure 2a). Given the isotropic nature of this distribution, the pair correlation function g(r) gives a clearer view of the relationship between relative neighbour density and distance, with a peak clearly visible at a distance of about 3 cm from the focal individual and a progressive decrease back towards the value of 1 at higher distances (figure 2b).
Figure 2.
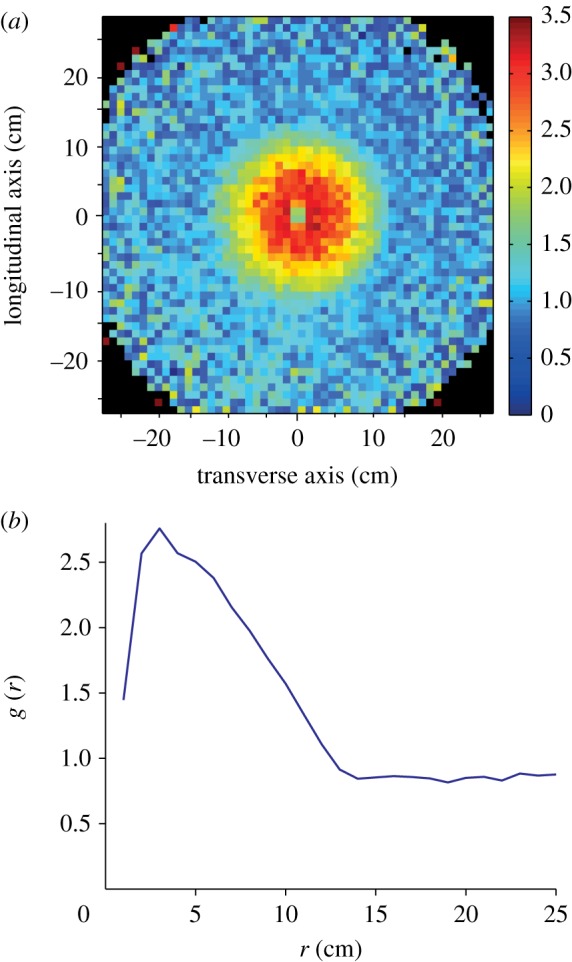
(a) Relative density of neighbours around a focal individual. The longitudinal axis represents the relative densities (colour coded; positions with no data shown in black) of neighbours along the axis defined by the body orientation of the focal individual, with positive values ahead of it and negative ones behind. The transverse axis represents neighbours’ densities along the axis perpendicular to the body of the focal individual, with negative values representing positions to the left of it and positive ones to the right. In the empirical data, a gap with lower densities is observed at very close range (within 1 cm), while a ring of higher densities is observed a few centimetres away from the individual and spread over roughly 10 cm until the values fall back towards 1, indicative of a homogeneous distribution of neighbours. (b) Pair correlation function g(r) in the empirical data. There is a clear peak at a distance of around 3 cm, which further indicates some clustering of neighbours at that distance and repulsion between individuals at distances below it.
In comparison with the empirical data, all SPP model variants appeared similar both in their spatial distributions of neighbours' density and in g(r) functions (see figure 3 and electronic supplementary material). The pursuit/escape model class, instead, exhibited distinctive patterns that did not resemble the empirical data, as expected given the strong anisotropic nature of the radial distribution of nearest neighbours (figure 3c). Within the SPP model class, it was not possible to ascertain which particular model variant (e.g. ‘metric’ or ‘combined metric/topological’), as well as which alignment and attraction ranges best matched the empirical data, as several factors affected the shape of g(r) in similar ways: variations in the interaction ranges (ra, rp), the number of neighbours K and the probability to hop ph provided many combinations leading to a good fit (n.r.m.s.e. < 0.2) between simulated and empirically observed g(r). It was clear, however, that an SPP model rather than a pursuit/escape one best described the empirical data, and that the empirical data best matched a repulsion force, balancing itself with clustering forces (attraction and/or alignment) at a preferred distance of about re = 3 cm, because a peak in g(r) directly reflects the value of re (see electronic supplementary material).
Figure 3.
In a ‘metric’ SPP model where repulsion/alignment/attraction forces are used, (a) the two-dimensional neighbours' density pattern is similar to that observed in the empirical data (figure 2), as evidenced more clearly by (d) the pair correlation function g(r) (n.r.m.s.e. = 0.115). A combined metric/topological SPP model variant where interactions are limited to the three nearest neighbours also produced (b) a similar two-dimensional neighbours' density pattern and (e) pair correlation function g(r) to what was observed in the empirical data (figure 2; n.r.m.s.e. = 0.19). The pursuit/escape model, instead, shows a very different pattern, with (c) the two-dimensional distribution of neighbours markedly concentrated in zones ahead of and behind the focal individual, while (f) the function g(r), which is not suited to pinpoint anisotropic distributions, appears relatively similar to the empirical data (n.r.m.s.e. = 0.28) and other model variants. All simulations presented here used n = 100 individuals.
5. Discussion
By comparing the spatial distribution of locusts recorded in the field while they marched collectively to the results of computer simulation models, we were able to make several inferences regarding the rules of interactions underlying locust mass movement. Neighbours were distributed in a clear isotropic manner around focal individuals. This was evident both by looking at the radial distribution of the nearest neighbours and on the two-dimensional maps of neighbour relative densities. All our variants of the pursuit/escape model class differed markedly from the empirical data by yielding characteristic anisotropic radial distributions of neighbours.
Australian plague locusts hence differ from other collectively moving animals, including fish [17,18,24] and birds [15,16], in which anisotropic distributions of neighbours have been observed. In starling flocks, a blind angle in the visual field of view was invoked as one of the potential explanations for the anisotropy [15], and a blind angle behind individuals is a common assumption in most models of fish schools [25–27]. In locusts, individuals have an uninterrupted field of view that extends all around their body at the horizon level, so that their interactions should not be limited by their visual capabilities [28]. Another explanation invoked for the anisotropic distribution in other animals is the advantageous configurations of individuals with regard to their hydrodynamic or aerodynamic properties [15,24,29], but this is not relevant to locust marching. More generally, a tendency to maintain a particular configuration related to neighbours, both in terms of angles and distances, might be less relevant for individuals that regularly interchange neighbours as a result of long hops.
An isotropic distribution of neighbours need not always be grounds for rejection of a pursuit/escape model: in one recent empirical study in fish [18], the authors found that the distribution of neighbours around a focal individual became isotropic at high densities (consistent with what was obtained with a zonal SPP model), while the inferred forces clearly matched a pursuit/escape (and speed matching) scenario. This was true only at higher densities; at lower densities, the radial distribution reflected the anisotropic nature of the interaction forces. In our empirical data, radial distributions appeared totally isotropic even at lower densities. Moreover, in the pursuit/escape model variant, the anisotropy of the radial distribution of nearest neighbours was always observed for the parameters and range of densities used (which covered the relevant range of densities for our empirical data and locust bands in general). Taken together, these results strongly suggest that the pursuit/escape model is not appropriate to describe the interaction rules used by C. terminifera while actively marching within bands. It should be noted, however, that pursuit/escape interactions might still be important forces at the onset of marching (usually in the morning) and during re-aggregation at the end of the day [2–4], neither of which were recorded and analysed here. Other locust species might differ from C. terminifera in the interaction rules that produce their mass movement. For example, a tendency for individuals to follow those ahead has been proposed to explain the long streams formed by the migratory bands of the brown locust L. parladina [4].
In our study, a zonal, SPP-like model provided the best candidate for the locust interaction rules involved in band collective movement. Our results clearly indicate the presence of short-range repulsion acting up to 3 cm, where it is balanced by a force generating some clustering, which is likely to be alignment and/or attraction. What remains unclear is the range at which these forces operate. Our previous analysis of locust pairs found that alignment between pairs of locusts is observed only up to a range of 13.5 cm [5]. Attraction however could operate at a longer range and the methods used here are not sufficient to determine this precisely. The analysis of the spatial distribution of individuals is also not sufficient to establish clearly the number of neighbours to which a locust pays attention and, more generally, through which process alignment arises. For example, SPP models, specifically designed to study minimal sets of rules, traditionally assume that individuals continuously adjust their orientation according to the average orientation of their neighbours. However, the orientation chosen by an individual might involve memorizing the orientation of other individuals over earlier periods of time, as recently suggested in glass prawns [30]. More generally, the assessment of neighbours' orientation might not happen continuously and instead be limited to discrete events—for example, the periods between each long-range hop in locusts. Detailed automated tracking of individuals over time will be required to further test how alignment arises in locust collective movement.
Studies of collective movement in biological systems have tended to focus on the similarities between species and their universal properties, such as the phase transitions from disorder to order that are observed in a large number of mass-moving animal species and other entities [31]. With recent empirical studies, it is now becoming clear that animal groups differ widely in the interaction rules and locomotion mode they use during collective movement. Hence, although a ‘zonal’ model [16] was the most suitable candidate for locust collective movement in the present study, recent work on birds and fish has suggested that it is not the most suitable in these cases [17,18]. This suggests that it is not simply differences in parameter values within a common overarching model that explain why these groups vary in shapes and fulfil different functions, but rather fundamental differences in interaction rules between species. Among mass-moving animal groups, locusts represent a particularly promising model to further study the evolution of collective motion. Mass migration in both migratory bands and flying swarms has independently evolved multiple times in different, relatively unrelated locust species, all using the same locomotion mode and a similar set of behaviours. With rapidly improving methods to gather empirical data in the field, it will soon be possible through comparative analyses to find out precisely which differences at the individual level or the environment are responsible for the markedly different collective patterns observed across locust species and how these differences relate to the ecological function of these groups.
Acknowledgements
J.B., G.A.S. and S.J.S. were funded by the ARC Linkage and Discovery programmes. J.B. was also funded by an ARC Future Fellowship, and S.J.S. by an ARC Laureate Fellowship.
References
- 1.Pener M. P., Simpson S. J. 2009. Locust phase polyphenism: an update. Adv. Insect Physiol. 36, 1–286 10.1016/S0065-2806(08)36001-9 (doi:10.1016/S0065-2806(08)36001-9) [DOI] [Google Scholar]
- 2.Clark L. R. 1949. Behaviour of swarm hoppers of the Australian plague locust (Chortoicetes terminifera Walk). CSIRO Bull. 245, 1–27 [Google Scholar]
- 3.Ellis P. E., Ashall C. 1957. Field studies on diurnal behaviour, movement and aggregation in the Desert Locust (Schistocerca gregaria Forsk). Anti-Locust Bull. 25, 1–103 [Google Scholar]
- 4.Uvarov B. P. 1977. Grasshoppers and locusts: a handbook of general acridology. Vol. 2. Behaviour, ecology, biogeography, population dynamics. London, UK: Centre for Overseas Pest Research [Google Scholar]
- 5.Buhl J., Sword G. A., Clissold F. J., Simpson S. J. 2011. Group structure in locust migratory bands. Behav. Ecol. Sociobiol. 65, 265–273 10.1007/s00265-010-1041-x (doi:10.1007/s00265-010-1041-x) [DOI] [Google Scholar]
- 6.Lecoq M., Foucart A., Balança G. 1999. Behaviour of Rhammatocerus schistocercoides (Rehn, 1906) hopper bands in Mato Grosso, Brazil (Orthoptera : Acrididae : Gomphocerinae). Ann. Soc. Entomol. Fr. 35, 217–228 [Google Scholar]
- 7.Buhl J., Sumpter D. J. T., Couzin I. D., Hale J., Despland E., Miller E. R., Simpson S. J. 2006. From disorder to order in marching locusts. Science 312, 1402–1406 10.1126/science.1125142 (doi:10.1126/science.1125142) [DOI] [PubMed] [Google Scholar]
- 8.Vicsek T., Czirok A., Ben-Jacob E., Cohen I., Shochet O. 1995. Novel type of phase transition in a system of self-driven particles. Phys. Rev. Lett. 75, 1226–1229 10.1103/PhysRevLett.75.1226 (doi:10.1103/PhysRevLett.75.1226) [DOI] [PubMed] [Google Scholar]
- 9.Gregoire G., Chate H., Tu Y. H. 2003. Moving and staying together without a leader. Physica D 181, 157–170 10.1016/S0167-2789(03)00102-7 (doi:10.1016/S0167-2789(03)00102-7) [DOI] [Google Scholar]
- 10.Bazazi S., Buhl J., Hale J. J., Anstey M. L., Sword G. A., Simpson S. J., Couzin L. D. 2008. Collective motion and cannibalism in locust migratory bands. Curr. Biol. 18, 735–739 10.1016/j.cub.2008.04.035 (doi:10.1016/j.cub.2008.04.035) [DOI] [PubMed] [Google Scholar]
- 11.Bazazi S., Romanczuk P., Thomas S., Shimansky-Geier L., Hale J. J., Miller G. A., Sword G. A., Simposon S. J., Couzin I. D. 2011. Nutritional state and collective motion: from individuals to mass migration. Proc. R. Soc. B 278, 356–363 10.1098/rspb.2010.1447 (doi:10.1098/rspb.2010.1447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen M. J., Buhl J., Bazazi S., Simpson S. J., Sword G. A. 2011. Cannibalism in the lifeboat: collective movement in Australian plague locusts. Behav. Ecol. Sociobiol. 65, 1715–1720 10.1007/s00265-011-1179-1 (doi:10.1007/s00265-011-1179-1) [DOI] [Google Scholar]
- 13.Simpson S. J., Sword G. A., Lorch P. D., Couzin I. D. 2006. Cannibal crickets on a forced march for protein and salt. Proc. Natl Acad. Sci. USA 103, 4152–4156 10.1073/pnas.0508915103 (doi:10.1073/pnas.0508915103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romanczuk P., Couzin I., Shimansky-Geier L. 2009. Collective motion due to individual escape and pursuit response. Phys. Rev. Lett. 102, 010602. 10.1103/PhysRevLett.102.010602 (doi:10.1103/PhysRevLett.102.010602) [DOI] [PubMed] [Google Scholar]
- 15.Ballerini M., et al. 2008. Interaction ruling animal collective behavior depends on topological rather than metric distance: evidence from a field study. Proc. Natl Acad. Sci. USA 105, 1232–1237 10.1073/pnas.0711437105 (doi:10.1073/pnas.0711437105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukeman R., Li Y. X., Edelstein-Keshet L. 2010. Inferring individual rules from collective behavior. Proc. Natl Acad. Sci. USA 107, 12 576–12 580 10.1073/pnas.1001763107 (doi:10.1073/pnas.1001763107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbert-Read J. E., Perna A., Mann R. P., Schaerf T. M., Sumpter D. J. T., Ward A. J. W. 2011. Inferring the rules of interaction of shoaling fish. Proc. Natl Acad. Sci. USA 108, 18 726–18 731 10.1073/pnas.1109355108 (doi:10.1073/pnas.1109355108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz Y., Tunstrom K., Ioannou C. C., Huepe C., Couzin I. D. 2011. Inferring the structure and dynamics of interactions in schooling fish. Proc. Natl Acad. Sci. USA 108, 18 720–18 725 10.1073/pnas.1107583108 (doi:10.1073/pnas.1107583108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanisch K.-H. 1984. Some remarks on estimators of the distribution function of nearest-neighbour distance in stationary spatial point patterns. Statistics 15, 409–412 [Google Scholar]
- 20.Diggle P. 2003. Statistical analysis of spatial point patterns, 2nd edn London, UK: Edward Arnold [Google Scholar]
- 21.Ballerini M., et al. 2008. Empirical investigation of starling flocks: a benchmark study in collective animal behaviour. Anim. Behav. 76, 201–215 10.1016/j.anbehav.2008.02.004 (doi:10.1016/j.anbehav.2008.02.004) [DOI] [Google Scholar]
- 22.Berens P. 2009. CircStat: a MATLAB toolbox for circular statistics. J. Stat. Software 31, 1–21 [Google Scholar]
- 23.Buhl J., Gautrais J., Reeves N., Solé R. V., Valverde S., Kuntz P., Theraulaz G. 2006. Topological patterns in street networks of self-organized urban settlements. Eur. Phys. J. B 49, 512–513 10.1140/epjb/e2006-00085-1 (doi:10.1140/epjb/e2006-00085-1) [DOI] [Google Scholar]
- 24.Partridge B. L., Pitcher T. J., Cullen J. M., Wilson J. 1980. The three-dimensional structure of fish schools. Behav. Ecol. Sociobiol. 6, 277–288 10.1007/BF00292770 (doi:10.1007/BF00292770) [DOI] [Google Scholar]
- 25.Hemelrijk C. K., Kunz H. 2005. Density distribution and size sorting in fish schools: an individual-based model. Behav. Ecol. 16, 178–187 10.1093/beheco/arh149 (doi:10.1093/beheco/arh149) [DOI] [Google Scholar]
- 26.Couzin I. D., Krause J., James R., Ruxton G. D., Franks N. R. 2002. Collective memory and spatial sorting in animal groups. J. Theor. Biol. 218, 1–11 10.1006/jtbi.2002.3065 (doi:10.1006/jtbi.2002.3065) [DOI] [PubMed] [Google Scholar]
- 27.Viscido S., Parrish J., Grunbaum D. 2005. The effect of population size and number of influential neighbors on the emergent properties of fish schools. Ecol. Model. 183, 347–363 10.1016/j.ecolmodel.2004.08.019 (doi:10.1016/j.ecolmodel.2004.08.019) [DOI] [Google Scholar]
- 28.Rogers S. M., Harston G. W. J., Kilburn-Toppin F., Matheson T., Burrows M., Gabbiani F., Krapp H. G. 2010. Spatiotemporal receptive field properties of a looming-sensitive neuron in solitarious and gregarious phases of the desert locust. J. Neurophysiol. 103, 779–792 10.1152/jn.00855.2009 (doi:10.1152/jn.00855.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weihs D. 1973. Hydrodynamics of fish schooling. Nature 241, 290–291 10.1038/241290a0 (doi:10.1038/241290a0) [DOI] [Google Scholar]
- 30.Mann R. P., Perna A., Strombom D., Garnett R., Herbert-Read J. E., Sumpter D. J. T., Ward A. J. W. 2012. Multi-scale inference of interaction rules in animal groups using Bayesian model selection. PLoS Comput. Biol. 8, e1002308. 10.1371/journal.pcbi.1002308 (doi:10.1371/journal.pcbi.1002308) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Vicsek T., Zafeiris A. In press. Collective motion. Phys. Rep. 10.1016/j.physrep.2012.03.004 (doi:10.1016/j.physrep.2012.03.004) [DOI] [Google Scholar]