Abstract
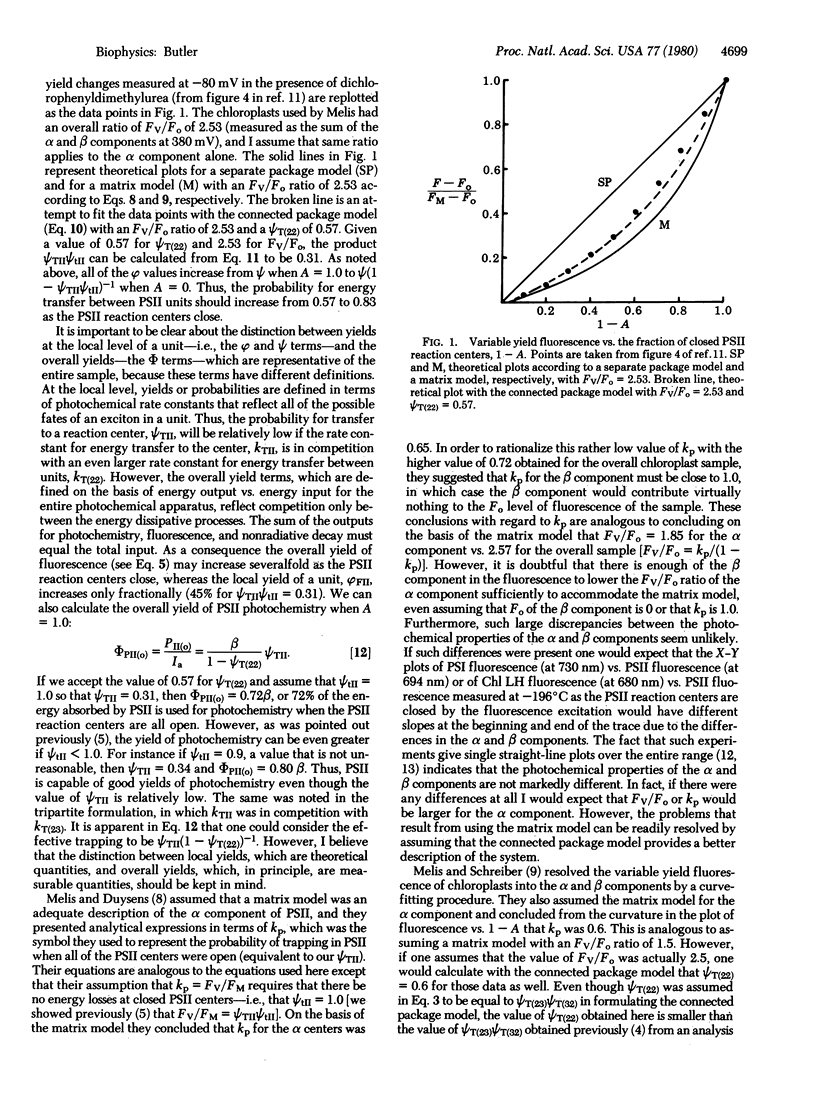
A model of the photochemical apparatus of photosynthesis, presented previously in a tripartite format, is used in a bipartite format to analyze energy transfer between photosystem II units. The model is used to develop analytical expressions for the photochemical properties of chloroplasts that include a term for the probability for energy transfer between photosystem II units. In particular, a normalized plot of the fluorescence of variable yield as a function of the closure of photosystem II reaction centers permits the evaluation of the energy transfer parameter. Data from a study by Melis [Melis, A. (1978) FEBS Lett. 95, 202-206] are used to estimate that the probability for energy transfer between photosystem II units in the α component of photosystem II was approximately 0.57 when the photosystem II reaction centers were all open and 0.83 when the centers were closed. This connected package model appears to provide a better description of the system than the matrix model that was used previously.
Keywords: photochemical model
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler W. L., Kitajima M. Energy transfer between photosystem II and photosystem I in chloroplasts. Biochim Biophys Acta. 1975 Jul 8;396(1):72–85. doi: 10.1016/0005-2728(75)90190-5. [DOI] [PubMed] [Google Scholar]
- Butler W. L., Kitajima M. Fluorescence quenching in photosystem II of chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):116–125. doi: 10.1016/0005-2728(75)90210-8. [DOI] [PubMed] [Google Scholar]
- Butler W. L., Strasser R. J. Does the rate of cooling affect fluorescence properties of chloroplasts at -196 degrees C? Biochim Biophys Acta. 1977 Nov 17;462(2):283–289. doi: 10.1016/0005-2728(77)90126-8. [DOI] [PubMed] [Google Scholar]
- Butler W. L., Strasser R. J. Tripartite model for the photochemical apparatus of green plant photosynthesis. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3382–3385. doi: 10.1073/pnas.74.8.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOLIOT A., JOLIOT P. ETUDE CIN'ETIQUE DE LA R'EACTION PHOTOCHIMIQUE LIB'ERANT L'OXYG'ENE AU COURS DE LA PHOTOSYNTH'ESE. C R Hebd Seances Acad Sci. 1964 May 4;258:4622–4625. [PubMed] [Google Scholar]
- Kitajima M., Butler W. L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta. 1975 Jan 31;376(1):105–115. doi: 10.1016/0005-2728(75)90209-1. [DOI] [PubMed] [Google Scholar]
- Melis A., Homann P. H. Heterogeneity of the photochemical centers in system II of chloroplasts. Photochem Photobiol. 1976 May;23(5):343–350. doi: 10.1111/j.1751-1097.1976.tb07259.x. [DOI] [PubMed] [Google Scholar]
- Melis A. Oxidation-reduction potential dependence of the two kinetic components in chloroplast system II primary photochemistry. FEBS Lett. 1978 Nov 15;95(2):202–206. doi: 10.1016/0014-5793(78)80993-4. [DOI] [PubMed] [Google Scholar]
- Melis A., Schreiber U. The kinetic relationship between the C-550 absorbance change, the reduction of Q(delta A320) and the variable fluorescence yield change in chloroplasts at room temperature. Biochim Biophys Acta. 1979 Jul 10;547(1):47–57. doi: 10.1016/0005-2728(79)90094-x. [DOI] [PubMed] [Google Scholar]



