Abstract
Silica nanoparticles have been functionalized by click chemistry and atom transfer radical polymerization (ATRP) simultaneously. First, the silanized silica nanoparticles were modified with bromine end group, and then the azide group was grafted onto the surface via covalent coupling. 3-Bromopropyl propiolate was synthesized, and then the synthesized materials were used to react with azide-modified silica nanoparticles via copper-mediated click chemistry and bromine surface-initiated ATRP. Transmission electron microscopy, Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy, and thermogravimetric analysis were performed to characterize the functionalized silica nanoparticles. We investigated the enrichment efficiency of bare silica and poly(ethylene glycol) methacrylate (PEGMA)-functionalized silica nanoparticles in Pb(II) aqueous solution. The results demonstrated that PEGMA-functionalized silica nanoparticles can enrich Pb(II) more quickly than pristine silica nanoparticles within 1 h.
Keywords: Silica nanoparticles; Surface modification; Click, ATRP; Enrichment; Pb(II)
Background
In recent years, silica nanoparticles (SNPs) have received significant attention due to their chemical inertness, nontoxicity, optical transparency, and excellent thermal stability [1-3], which can be widely used in catalysis [4], chemical process industry [5], removal of metal ions [6], and metal ion preconcentration [7-9] through polymer coatings or other functional groups. For many applications, there are several chemical methods for controlling the nanoparticles’ surface functionality, such as chemisorptions [10], sol–gel process, and immobilization of organic molecules by silane coupling reagents [11], which can result in the immobility, mechanical stability, and water insolubility of functionalized SNPs [12]. Polymerization methods involving living free radical nitroxide-mediated polymerization, reversible addition-fragmentation chain transfer (RAFT), and atom transfer radical polymerization (ATRP) were commonly considered as effective techniques to functionalize materials [13-16]. Especially, the ATRP technique has been developed rapidly due to the advantages of being simple, inexpensive, and more general for controlled radical polymerization when it was firstly proposed by Wang and Matyjaszewsi [17]. There are a greater number of researchers using ATRP to functionalize SNPs [18-22].
With the appearance of the click chemistry proposed by Sharpless [23], the copper(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition reaction also becomes a particularly powerful approach to synthesize designed molecules or modify inorganic materials because only mild reaction conditions are required and the extreme selectivity toward molecules bearing azides and alkynes prevents unwanted side product [24-26]. Recently, some literatures reported that gold nanoparticles, silicon oxides, carbon nanotubes, and other materials were functionalized by click chemistry [27-30].
Based on the advantages of ATRP and click chemistry, the combination of these two methods has attracted attention to functionalize nanomaterials. Ranjan and Brittain [31] successfully grafted polymer chains onto SNPs through a combination of RAFT polymerization and click chemistry. Wang and co-workers [32] utilized ATRP and click chemistry to modify particles. Polystyrene brushes were firstly introduced on the surface via ATRP, and then the click reaction was generated on the surface of polymerized SNPs. The combination of surface-initiated ATRP and Huisgen [3 + 2] cycloaddition [33,34] was also developed as a versatile method for the functionalizations of SNPs. Most of the literatures reported the combination of ATRP and click chemistry to modify nanomaterials in tandem.
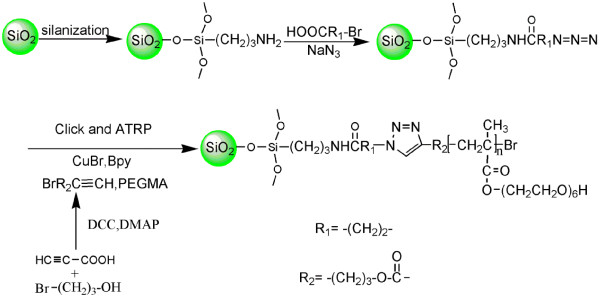
Herein, we described the immobilization of the initiator on the SNP surface via 1,3-dipolar cycloaddition, which can simultaneously initiate surface-induced ATRP of poly(ethylene glycol) methacrylate on the SNP surface. The general steps for the preparation of functionalized SNPs are shown in Figure 1. Based on the special characteristics of the ethylene glycol end group, we investigated the enrichment properties of Pb(II) in aqueous solution.
Figure 1.
The steps of functionalized silica nanoparticles by click chemistry and ATRP.
Methods
Materials
3-Bromopropanoic acid and propargyl acid were obtained from Alfa Aesar (Ward Hill, MA, USA). Poly(ethylene glycol) methacrylate (PEGMA), 1,3-dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), 4-dimethylaminopyridime (DMAP), and sodium azide (NaN3) were obtained from Sigma-Aldrich Corporation (St. Louis, MO, USA). 3-Aminopropyltriethoxysilane (APTES) was purchased from Dow Corning Corporation (Midland, MI, USA). CuBr and 3-bromo-1-propanol were obtained from Shanghai Jingchun Reagent Co., Ltd. (Aladdin; Shanghai, China). Tetraethyl orthosilicate (TEOS) and 2,2′-bypiridine (Bpy) were purchased from Tianjin Chemical Reagent Co. Ltd. (Tianjin, China).
Synthesis of 3-bromopropyl propiolate
3-Bromo-1-propanol (3 ml, 33 mmol), DCC (5 g, 25.5 mmol), and DMAP (0.6 g, 5.04 mmol) were dissolved in 100 ml of dichloromethane (DCM), then propargyl acid (4.5 ml, 42 mmol) was added into the solution slowly. The mixture was stirred at room temperature in a dark room for 24 h, diluted with DCM, filtered off, and washed with DCM until white particles were observed. Then, the liquid was evaporated completely using a rotary evaporator. The products were dried in a clean vacuum at 50°C overnight.
Preparation and modification of silica particles
The spherical silica particles were prepared from TEOS using NH3·H2O as catalyst according to the Stöber method [35]. Then, 65 ml of ethanol, 3 ml of deionized water, and 10 ml of NH3·H2O (25%) were added into a flask with violent stirring at 40°C for 2 h; 5 ml of TEOS was added into the above solution dropwise. The mixture was kept at 40°C with stirring overnight. After the reaction, the mixture was separated by centrifugation and the white particles were washed with water, ethanol, and toluene, respectively. Then, the silica particles were dispersed in 50 ml of toluene by ultrasonication to produce a homogeneous suspension.
The surface of SiO2 has functioned as an amino group by a silanization reaction according to a previous report. Namely, the prepared SiO2 homogeneous suspension was put into a 100-ml round flask, and APTES (5.3 ml, 23 mmol) was added using a syringe. The reaction mixture was refluxed at 95°C in oil bath with stirring for 10 h. The obtained amino group-immobilized silica particles, defined as SiO2-NH2, were collected by centrifugation and washed with ethanol (2 × 50 ml) and N,N-dimethylformamide (DMF; 2 × 50 ml) in turn, then dispersed in 50 ml of DMF to immobilize the azide group.
3-Bromopropionic acid (2.8 g, 18.4 mmol), NHS (2.4 g, 7 mmol), and EDC (4 g, 22.3 mmol) were added to the SiO2-NH2 and DMF solution with stirring and protected from light at 80°C under N2. After 24 h, 3 g of NaN3 was added into the above system for 24 h. Then, particles called SiO2-N3 were collected by centrifugation and washed with water and DMF.
Surface-initiated atom transfer radical polymerization by click chemistry
The reaction involved in click reaction and atom transfer radical polymerization was described as follows: The prepared azide-modified SiO2 and 3-bromopropyl propiolate were dispersed in 50 ml of DMF, then CuBr (43 mg, 0.3 mmol), Bpy ligand (90 mg, 0.6 mmol), and PEGMA (10 ml, 30 mmol) were added quickly; the reaction was stirred at 80°C under N2 for 24 h. After the reaction, the grafted PEGMA silica particles were collected by centrifugation, referred as SiO2-PEGMA; the product was washed with DCM at least three times to remove the excess reactant, then washed with ethanol and deionized water, and dried in a clean vacuum oven at 50°C overnight.
Sensing of Pb2+
Sensing of Pb2+ experiments were carried out by the following steps: a 10 ml portion of the aqueous sample solution, containing 100 mg/l Pb2+, was prepared, and the pH value was adjusted to 6 with aqueous HCl. Then, 50 mg of SiO2 nanoparticles and SiO2-PEGMA were dispersed in eight portions of the above solution with an ultrasonic oscillator, and the solutions were stewed for 0.5, 1, 2, and 3 h, respectively. The particles were separated by centrifugation. The supernatant was detected by atomic absorption spectroscopy (AAS).
Surface characterization
Fourier transform infrared (FT-IR) spectra were obtained using a Spectrum One FT-IR spectrometer (IR Prestige-21, Shimadzu Corporation, Kyoto, Japan) with a resolution of 4 cm−1. To characterize the layers formed on the surface of the nanoparticle, the powder was milled with KBr, and the mixture was pressed into a disk for analysis. The morphology and size of SNPs were studied by transmission electron microscopy (TEM) with a JEOL 200CX (Akishima-shi, Japan). X-ray photoelectron spectroscopy (XPS) was performed on a PHI 5500 electron spectrometer (Physical Electronics, Inc., Chanhassen, MN, USA) using 200-W Mg radiations. The binding energies were referenced to the C 1s line at 284.8 eV from adventitious carbon. Thermogravimetric analysis (TGA) was carried out using a Netzsch STA449 F3 thermogravimetric instrument (Wolverhampton, UK) at a heating rate of 10°C/min under a flow of nitrogen. AAS was carried out using an AA-6800 instrument (Shimadzu Corporation).
Results and discussion
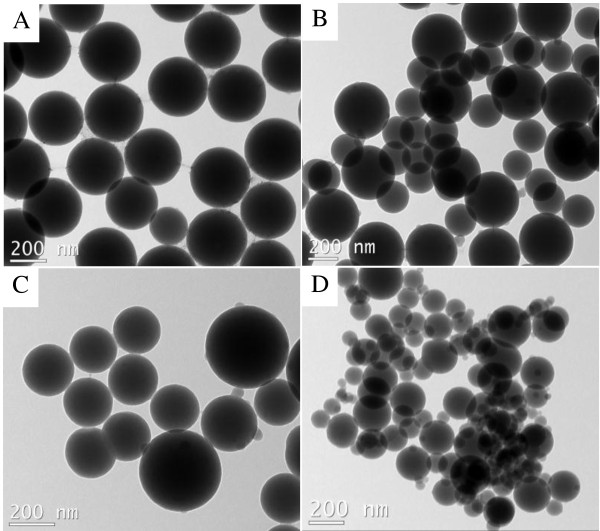
We performed TEM to characterize the morphologies of SiO2 and modified SiO2 nanoparticles (Figure 2). From Figure 2A, we can see clearly that the mean size of the particles is about 300 nm and the shape shows regulated sphericity. The average diameter of amino group-modified SNPs is about 355 nm (Figure 2B). Azide-functionalized SNPs and SNPs modified via click chemistry and ATRP simultaneously are presented in Figure 2C,D, respectively. The shape of the modified SNPs maintained the original spherical morphology.
Figure 2.
TEM images. (A) Silica nanoparticles, (B) silica nanoparticles treated with APTES, (C) azide-functionalized silica nanoparticles, and (D) silica nanoparticles modified via click chemistry and ATRP simultaneously.
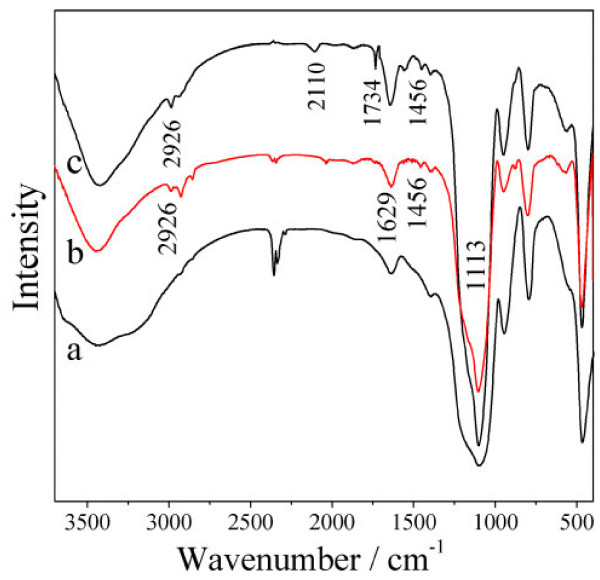
The FT-IR spectra of SiO2, SiO2-NH2, and SiO2-N3 are shown in Figure 3. Strong adsorption peaks at about 1,113 cm−1 were observed for all the samples, indicating the existence of Si-O-Si stretching vibration of silanol groups. The -CH2 groups were confirmed by C-H stretching at 2,926 cm−1 and C-H scissoring vibration at 1,456 cm−1. O-H stretching and bending vibrations could be detected at 1,629 and 3,600 to 3,300 cm−1. In Figure 3b, the adsorption peak at about 3,420 cm−1 was assigned to N-H stretching vibration, suggesting that the amino organic groups were introduced to the surface of SNPs. A peak at 1,734 cm−1 observed in Figure 3c corresponded to the C = O stretching. Furthermore, the azide group-modified SNPs are an essential intermediate for the click chemistry reaction, which was demonstrated by a new peak at 2,110 cm−1 in the spectrum of Figure 3c.
Figure 3.
FT-IR spectra of SiO2(a), SiO2-NH2(b), and SiO2-N3(c) nanoparticles.
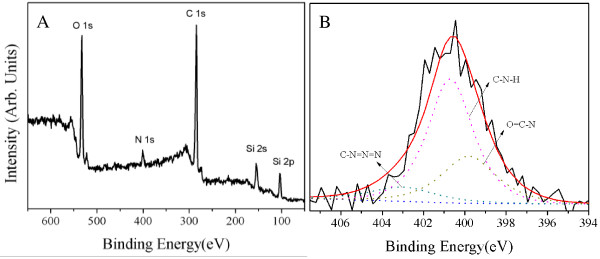
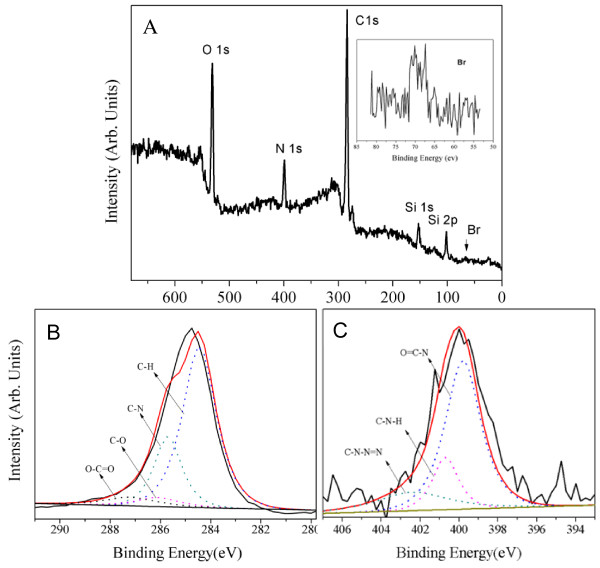
In order to further confirm the SNPs modified via click chemistry and ATRP simultaneously, the products were characterized by XPS analysis. The introduction of the azide group onto SNPs was demonstrated by the XPS spectrum in Figure 4, which presented the wide-scan (A) and N 1s core-level (B) spectra. The wide-scan spectra of the SiO2-N3 surface was dominated clearly by signals attributable to Si 2p, Si 2s, C 1s, N 1s, and O 1s with binding energies at about 101.2, 154, 284.2, 401.2, and 535.2 eV, respectively. The N 1s core-level spectra of azide-modified SNPs can be curve-fitted into three peak components with binding energies at about 399.8, 400.7, and 403 eV, attributable to the O = C-N, C-N-H, and C-N = N = N species, respectively.
Figure 4.
Wide-scan (A) and N 1score-level (B) spectra of azide-functionalized silica nanoparticles.
Figure 5A showed the wide-scan spectrum of the SiO2-PEGMA surface, which was dominated by signals attributable to Si 2p, Si 2s, C 1s, N 1s, O 1s, and Br with binding energies at about 101.8, 153, 284.2, 399.4, 531.4, and 67.4 eV, respectively. The peak of Br was observed at 67.4 eV, which could have originated from 3-bromopropyl propiolate. Figure 5B and C present the XPS spectra of C 1s and N 1s narrow scan on the SiO2-PEGMA nanoparticles. The C 1s core-level spectrum can be curve-fitted with four peak components having binding energies at about 284.4, 285.7, 286.2, and 287.2 eV attributable to the C-H, C-N, C-O, and O = C-O species, respectively. The N 1s core-level spectrum can be curve-fitted into three peak components with binding energies at about 397.9, 399.8, and 400.7 eV which are ascribed to the C-N-N = N, O = C-N, and C-N-H species, respectively (Figure 5C).
Figure 5.
Wide-scan (A) and C 1s(B) and N 1s(C) core-level spectra of modified SNPs. The inset in (A) is Br 3d core-level spectrum.
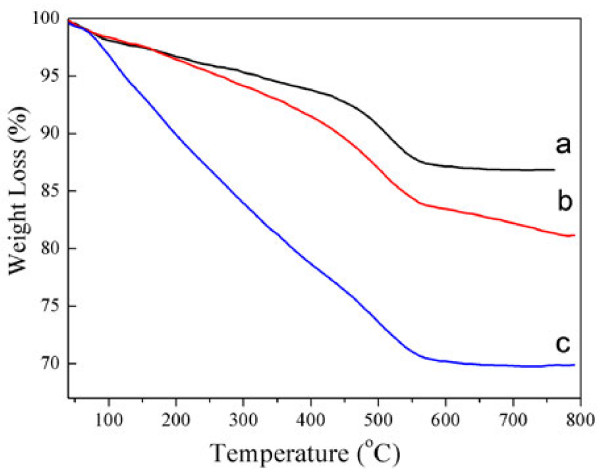
The TGA curves of the functionalized nanoparticles are shown in Figure 6, from which an approximate amount of functional groups on the silica surface could be confirmed. The weight loss of APTES-treated SNPs was about 13%, which is attributed to the loss of the APTES layer. We can see obviously from curve b of Figure 6 that the weight loss of azide-modified nanoparticles is about 18.9% for the whole temperature range. It was calculated that there was about 5.9% mass loss for azide molecule-functionalized SNPs. After click chemistry and ATRP simultaneously, the first stage shows a weight loss of about 21% at 400°C while the second stage accounts for 8.7% of another weight loss (Figure 6c). These two stages of weight loss may indicate the degradation of PEGMA and that 1,2,3-triazole ring derivates molecules on the surface of SNPs, respectively.
Figure 6.
TGA of APTES-treated (a), azide-modified (b), click and ATRP-functionalized (c) silica nanoparticles.
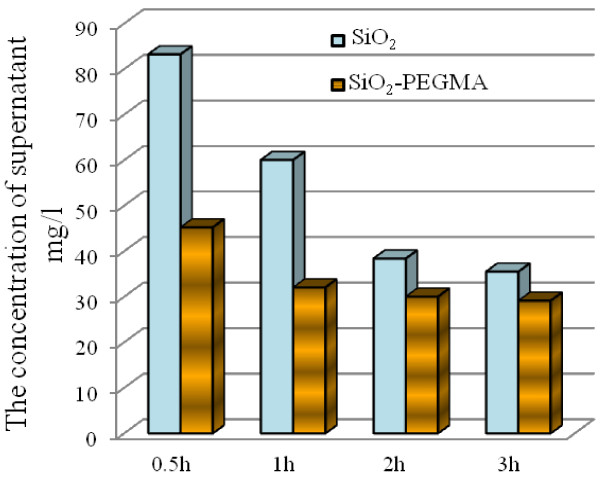
The mechanism of metal ion adsorption from aqueous solution is related with physical and chemical processes, and the chemical binding reactions which happened involve metal ions and surface functional groups [36]; adsorption will be affected as the functional groups increase. After the successful preparation of PEGMA-modified SNPs, functionalized nanoparticles were utilized to absorb lead ions in the aqueous solution. From the adsorption experiment, the concentration of supernatant fluid is presented in Figure 7. We can find that polymerized SNPs were adsorbed faster than uncoated nanoparticles and the adsorption capacity of polymerized SNPs was also higher than that of uncoated nanoparticles, particularly within 1 h. It may be due to the polymerization process which can immobilize more functional groups on the surface of SNPs to chelate metal ions.
Figure 7.
The comparison of supernatants’ Pb2+concentration in SiO2and SiO2-PEGMA solutions. The initial concentration of Pb2+ is 100 mg/l; the volume of Pb2+ is 10 ml; the weight of nanoparticles is 50 mg; the adsorption times are 0.5, 1, 2, and 3 h; and the temperature is 25°C.
Conclusions
After introducing amino groups onto silica surfaces, the ATRP initiator was successfully immobilized via click chemistry while poly(ethylene glycol) methacrylate chain-modified SNPs were obtained. This method takes advantage of click chemistry to optimize ATRP, which we have offered as a versatile pathway to functionalize SNPs. It will develop a series of desired functional groups and also extend this current different functional polymer chains which have potential application in the fabrication of SNP-based nanocomposites. Aside from the applications that require the enrichment of lead, because of the diversity and controllable ability of functional molecules on the surface of SNPs, efforts in our group are being made to extend current work to applications in selective removal of heavy metal ions such as Hg(II), Cu(II), As(III), and Cd(II).
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
WL carried out the preparation and modification of silica nanoparticles. YX participated in the analysis and the testing of the nanocomposite. YZ and WM supervised this work, helped in the analysis and interpretation of data, and, together with SW, worked on the drafting and revisions of the manuscript. WM and YD conceived of the study and participated in its design and coordination. SW participated in the design of the study and provided analysis instruments. All authors read and approved the final manuscript.
Authors’ information
WL and YX are MD students. YZ is an associate professor and WM is a professor and the Dean of the Faculty of Metallurgical and Energy Engineering. SW is a postdoctoral fellow in Kunming University of Science and Technology. YD is an academician of the Chinese Academy of Engineering.
Contributor Information
Wei Li, Email: weili86@sina.com.
Yaohui Xu, Email: xuyaohui88@163.com.
Yang Zhou, Email: zhouyangnano@163.com.
Wenhui Ma, Email: mwhsilicon@163.com.
Shixing Wang, Email: wsxkm@sina.com.
Yongnian Dai, Email: daiyn@kmust.edu.cn.
Acknowledgements
Financial support of this work from NSFC (50903041), Natural Science Foundation of Yunnan Province (2009CD026 and 2010CA019), and Inspection and Quarantine of the People’s Republic of China (2009QK406) were gratefully acknowledged.
References
- Zhao B, Brittain WJ. Polymer brushes: surface-immobilized macromolecules. Prog Polym Sci. 2000;25:677–710. doi: 10.1016/S0079-6700(00)00012-5. [DOI] [Google Scholar]
- Hua D, Tang J, Jiang JL, Gu ZQ, Dai LL, Zhu XL. Controlled grafting modification of silica gel via RAFT polymerization under ultrasonic irradiation. Mater Chem Phys. 2009;114:402–406. doi: 10.1016/j.matchemphys.2008.09.046. [DOI] [Google Scholar]
- Yang S, Ding J, Radosz M, Shen YQ. Reversible catalyst supporting via hydrogen-bonding-mediated self-assembly for atom transfer radical polymerization of MMA. Macromolecules. 2004;37:1728–1734. doi: 10.1021/ma035322c. [DOI] [Google Scholar]
- Wegmann J, Albert K, Purch M, Snader LC. Poly (ethylene-co-acrylic acid) stationary phases for the separation of shape-constrained isomers. Anal Chem. 2001;73:1814–1820. doi: 10.1021/ac000992r. [DOI] [PubMed] [Google Scholar]
- Grynbaum MD, Meyer C, Putzbach K, Rehbein J, Albert K. Application of polymer based stationary phases in high performance liquid chromatography and capillary high performance liquid chromatography hyphenated to microcoil 1H nuclear magnetic resonance spectroscopy. J Chromatogr A. 2007;1156:80–86. doi: 10.1016/j.chroma.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Bagwe RP, Hilliard LR, Tan WH. Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir. 2006;22:4357–4362. doi: 10.1021/la052797j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman MM, Kholeif SA, Al-Maaty NAA, Mahmoud ME. Metal sorption, solid phase extraction and preconcentration properties of two silica gel phases chemically modified with 2-hydroxy-1-naphthaldehyde. Microchim Acta. 2003;143:25–31. doi: 10.1007/s00604-003-0045-x. [DOI] [Google Scholar]
- Shiraishi Y, Nishimura G, Hirai T, Komasawa I. Separation of transition metals using inorganic adsorbents modified with chelating ligands. Ind Eng Chem Res. 2002;41:5065–5070. doi: 10.1021/ie020119b. [DOI] [Google Scholar]
- Jurjen K, Willem D, Klaus LK, Jan RR. Highly selective and efficient recovery of Pd, Pt, and Rh from precious metal-containing industrial effluents with silica-based (poly)amine ion exchangers. Hydrometallurgy. 2002;64:59–68. doi: 10.1016/S0304-386X(02)00010-5. [DOI] [Google Scholar]
- Pyunand J, Matyjaszewski K. Synthesis of nanocomposite organic/inorganic hybrid materials using controlled /“living” radical polymerization. Chem Mater. 2001;13:3436–3448. doi: 10.1021/cm011065j. [DOI] [Google Scholar]
- Jal PK, Patel S, Mishra BK. Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta. 2004;62:1005–1028. doi: 10.1016/j.talanta.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Jal PK, Dutta RK, Sudarshan M, Saha A, Bhattacharyya SN, Chintalapudi SN, Mishra BK. Extraction of metal ions using chemically modified silica gel: a PIXE analysis. Talanta. 2001;55:233–240. doi: 10.1016/S0039-9140(00)00678-0. [DOI] [PubMed] [Google Scholar]
- Hawker CJ, Bosman AW, Harth E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem Rev. 2001;101:3661–3688. doi: 10.1021/cr990119u. [DOI] [PubMed] [Google Scholar]
- Li CH, Han JW, Ryu CY, Benicewicz BC. A versatile method to prepare RAFT agent anchored substrates and the preparation of PMMA grafted nanoparticles. Macromolecules. 2006;39:3175–3183. doi: 10.1021/ma051983t. [DOI] [Google Scholar]
- Harrak AE, Carrot G, Oberdisse J, Eychenne-Baron C, Boue F. Surface atom transfer radical polymerization from silica nanoparticles with controlled colloidal stability. Macromolecules. 2004;37:6376–6384. doi: 10.1021/ma035959w. [DOI] [Google Scholar]
- Matyjaszewski K, Xia JH. Atom transfer radical polymerization. Chem Rev. 2001;101:2921–2990. doi: 10.1021/cr940534g. [DOI] [PubMed] [Google Scholar]
- Wang JS, Matyjaszewsi K. Controlled/“living” radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J Am Chem Soc. 1995;117:5614–5615. doi: 10.1021/ja00125a035. [DOI] [Google Scholar]
- Huang CL, Tassone T, Woodberry K, Sunday D, Green DL. Impact of ATRP initiator spacer length on grafting poly (methyl methacrylate) from silica nanoparticles. Langmuir. 2009;25(23):1335–1336. doi: 10.1021/la901918v. [DOI] [PubMed] [Google Scholar]
- Perruchot C, Khan MA, Kamitsi A, Armes SP. Synthesis of well-defined, polymer-grafted silica particles by aqueous ATRP. Langmuir. 2001;17:4479–4481. doi: 10.1021/la0102758. [DOI] [Google Scholar]
- Morandi G, Pascual S, Montembault V, Legoupy S, Delorme N, Fontaine L. Synthesis of brush copolymers based on a poly (1,4-butadiene) backbone via the “grafting from” approach by ROMP and ATRP. Macromolecules. 2009;42:6927–6931. doi: 10.1021/ma900987m. [DOI] [Google Scholar]
- Rahman MM, Czaun M, Takafuji M, Ihara H. Organogel from silica: a new approach to prepare packing materials for high-performance liquid chromatography. Chem Eur J. 2008;14:1312–1321. doi: 10.1002/chem.200701302. [DOI] [PubMed] [Google Scholar]
- Nagase K, Watanabe M, Kikuchi A, Yamato M, Okano T. Thermo-responsive polymer brushes as intelligent biointerfaces: preparation via ATRP and characterization. Macromol Biosci. 2011;11:400–409. doi: 10.1002/mabi.201000312. [DOI] [PubMed] [Google Scholar]
- Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: copper (I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem. 2002;114:2708–2711. doi: 10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Edit. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Schlossbauer A, Schaffert D, Kecht J, Wagner E, Bein T. Click chemistry for high-density biofunctionalization of mesoporous silica. J Am Chem Soc. 2008;38:12259. doi: 10.1021/ja803018w. [DOI] [PubMed] [Google Scholar]
- Evans RA. The rise of azide–alkyne 1,3-dipolar ‘click’ cycloaddition and its application to polymer science and surface modification. Aust J Chem. 2007;60:384–39. doi: 10.1071/CH06457. [DOI] [Google Scholar]
- Tornoe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1, 2, 3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- Collman JP, Devaraj NK, Chidsey CED. “Clicking” functionality onto electrode surfaces. Langmuir. 2004;20:1051–1053. doi: 10.1021/la0362977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lummerstorfer T, Hoffmann H. Click chemistry on surfaces: 1,3-dipolar cycloaddition reactions of azide-terminated monolayers on silica. J Phys Chem B. 2004;108:3963–3966. doi: 10.1021/jp049601t. [DOI] [Google Scholar]
- Prakash S, Long TM, Selby JC, Moore JS, Shannon MA. “Click” modification of silica surfaces and glass microfluidic channels. Anal Chem. 2007;79:1661–1667. doi: 10.1021/ac061824n. [DOI] [PubMed] [Google Scholar]
- Ranjan R, Brittain WJ. Combination of living radical polymerization and click chemistry for surface modification. Macromolecules. 2007;40:6217–6223. doi: 10.1021/ma0705873. [DOI] [Google Scholar]
- Wang YP, Chen JC, Xiang JM, Li HJ, Shen YQ, Gao XH, Liang Y. Synthesis and characterization of end-functional polymers on silica nanoparticles via a combination of atom transfer radical polymerization and click chemistry. React Funct Polym. 2009;69:393–399. doi: 10.1016/j.reactfunctpolym.2009.03.005. [DOI] [Google Scholar]
- Golas PL, Tsarevsky NV, Sumerlin BS, Matyjaszewski K. Catalyst performance in “click” coupling reactions of polymers prepared by ATRP: ligand and metal effects. Macromolecules. 2006;39:6451–6457. doi: 10.1021/ma061592u. [DOI] [Google Scholar]
- Chen JC, Luo WQ, Wang HD, Xiang JM, Jin HF, Chen F, Cai ZW. A versatile method for the preparation of end-functional polymers onto SiO2nanoparticles by a combination of surface-initiated ATRP and Huisgen [3 + 2] cycloaddition. Appl Surf Sci. 2010;256:2490–2495. doi: 10.1016/j.apsusc.2009.10.093. [DOI] [Google Scholar]
- Stöber W, Fink A, Bohn E. Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci. 1968;26:62–69. doi: 10.1016/0021-9797(68)90272-5. [DOI] [Google Scholar]
- Liang XF, Xu YX, Sun GH, Wang L, Sun W, Qin X. Preparation, characterization of thiol-functionalized silica and application for sorption of Pb2+and Cd2+ Colloid Surf A Physicochem Eng Asp. 2009;349:61–68. doi: 10.1016/j.colsurfa.2009.07.052. [DOI] [Google Scholar]