Abstract
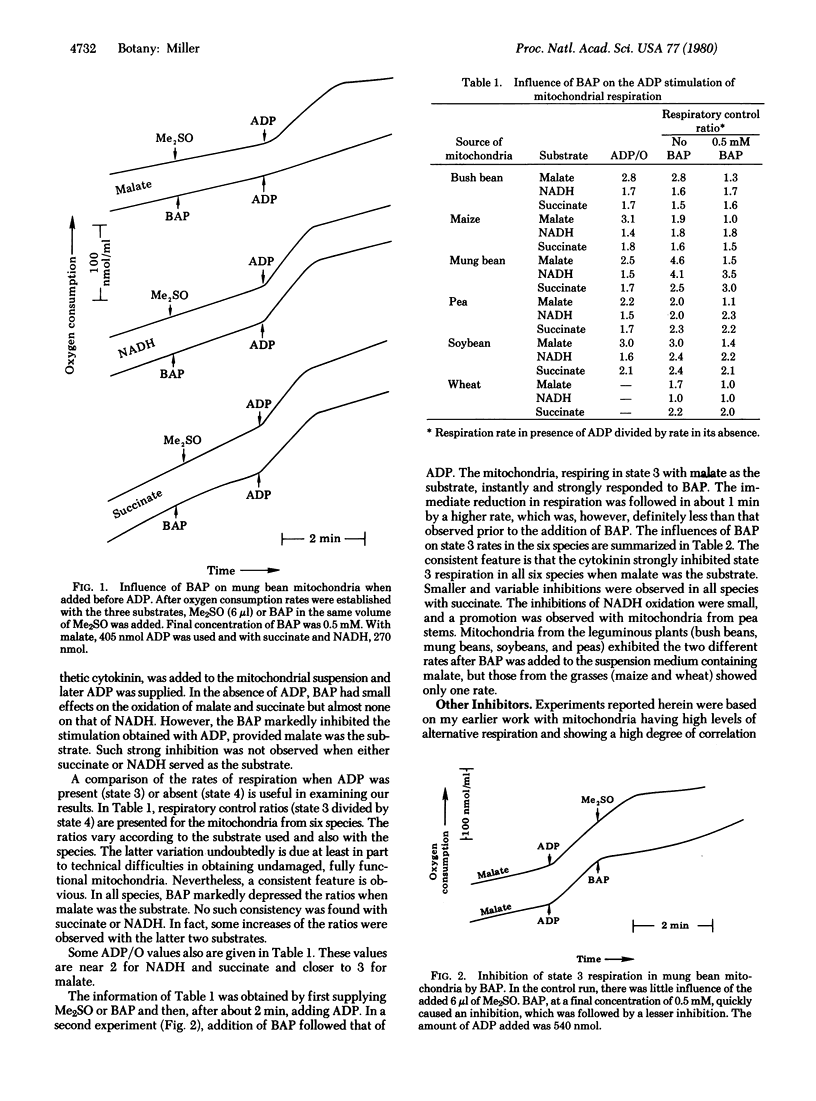
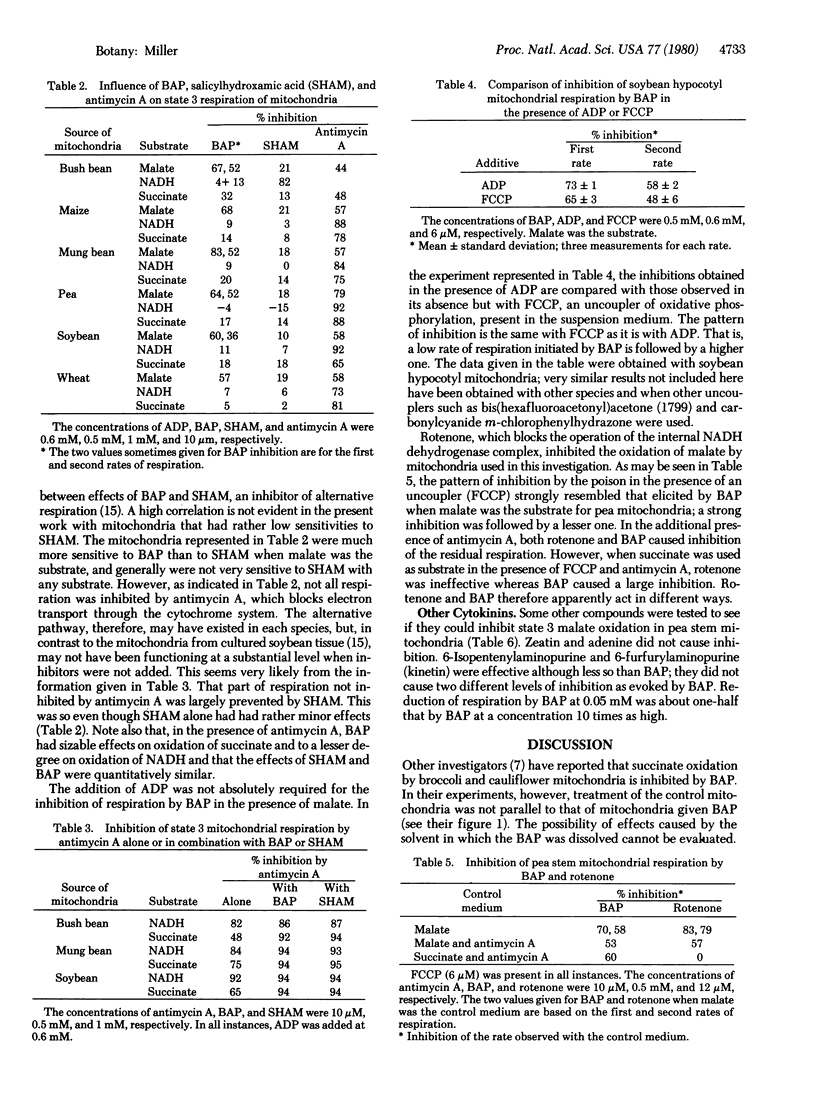
The influence of 6-benzylaminopurine (BAP) on the respiration by mitochondria from bush bean (Phaseolus vulgaris L.), mung bean (P. aureus Roxburgh), soybean [Glycine max (L.) Merrill], maize (Zea mays L.), pea (Pisum sativum L.), and wheat (Triticum aestivum L.) was examined. BAP, a synthetic cytokinin, consistently inhibited oxygen uptake by mitochondria from all species when malate was used as the substrate. The decrease in respiration was especially evident in the presence of ADP or an uncoupler of oxidative phosphorylation. 6-Isopentenylaminopurine and 6-furfurylaminopurine also inhibited malate oxidation, but zeatin and adenine did not. In certain instances, BAP reduced succinate and NADH oxidation. With succinate as the substrate and with antimycin A present, inhibition by BAP paralleled that caused by salicylhydroxamic acid, an inhibitor of alternative respiration. A suggested scheme features a cytokinin-inhibited point located between NADH dehydrogenase and cytochrome b of the electron transport system. Electrons from the NADH generated by malate oxidation are assumed to flow through this point, with electrons from externally supplied or cytosolic NADH and succinate doing so only under certain conditions such as when alternative respiration is occurring. Cytokinin effects on respiration and perhaps on other phenomena may be mediated by this mechanism.
Keywords: 6-benzylaminopurine, ADP stimulation, alternative respiration, malate
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CANNON C., COWAN F. F., KOPPANYI T., MAENGWYN-DAVIES G. D. Explanation of cocaine desensitization of blood pressure responses to ephedrine. Science. 1961 Oct 13;134(3485):1075–1077. doi: 10.1126/science.134.3485.1075-a. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- FOX J. E., CHEN C. M., GILLAM I. GLUCOSE CATABOLISM IN NORMAL AND AUTONOMOUS TOBACCO TISSUE CULTURES. Plant Physiol. 1965 May;40:529–534. doi: 10.1104/pp.40.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy A. H., Dilley D. R., Wittwer S. H. Senescence inhibition and respiration induced by growth retardants and N-benzyladenine. Plant Physiol. 1966 Sep;41(7):1085–1089. doi: 10.1104/pp.41.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. III. Effects of Respiratory Inhibitors. Plant Physiol. 1967 Nov;42(11):1535–1544. doi: 10.1104/pp.42.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUSTINEC J., PETRU E., POKORNA V. Changes in the distribution of glucose-14C in alternative catabolic pathways induced by kinetin-analogue in the callus tissue of carrot (Daucus carota L.). Experientia. 1962 Apr 15;18:187–187. doi: 10.1007/BF02151722. [DOI] [PubMed] [Google Scholar]
- Moore T. S., Miller C. O. Effects of cytokinins on the respiration of soybean callus tissue. Plant Physiol. 1972 Nov;50(5):594–598. doi: 10.1104/pp.50.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. M. The influence of growth regulating substances on the development of enhanced metabolic rates in thin slices of beetroot storage tissue. Plant Physiol. 1966 Sep;41(7):1173–1178. doi: 10.1104/pp.41.7.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich P. R., Bonner W. D. The sites of superoxide anion generation in higher plant mitochondria. Arch Biochem Biophys. 1978 May;188(1):206–213. doi: 10.1016/0003-9861(78)90373-9. [DOI] [PubMed] [Google Scholar]
- Tetley R. M., Thimann K. V. The Metabolism of Oat Leaves during Senescence: I. Respiration, Carbohydrate Metabolism, and the Action of Cytokinins. Plant Physiol. 1974 Sep;54(3):294–303. doi: 10.1104/pp.54.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]


