Abstract
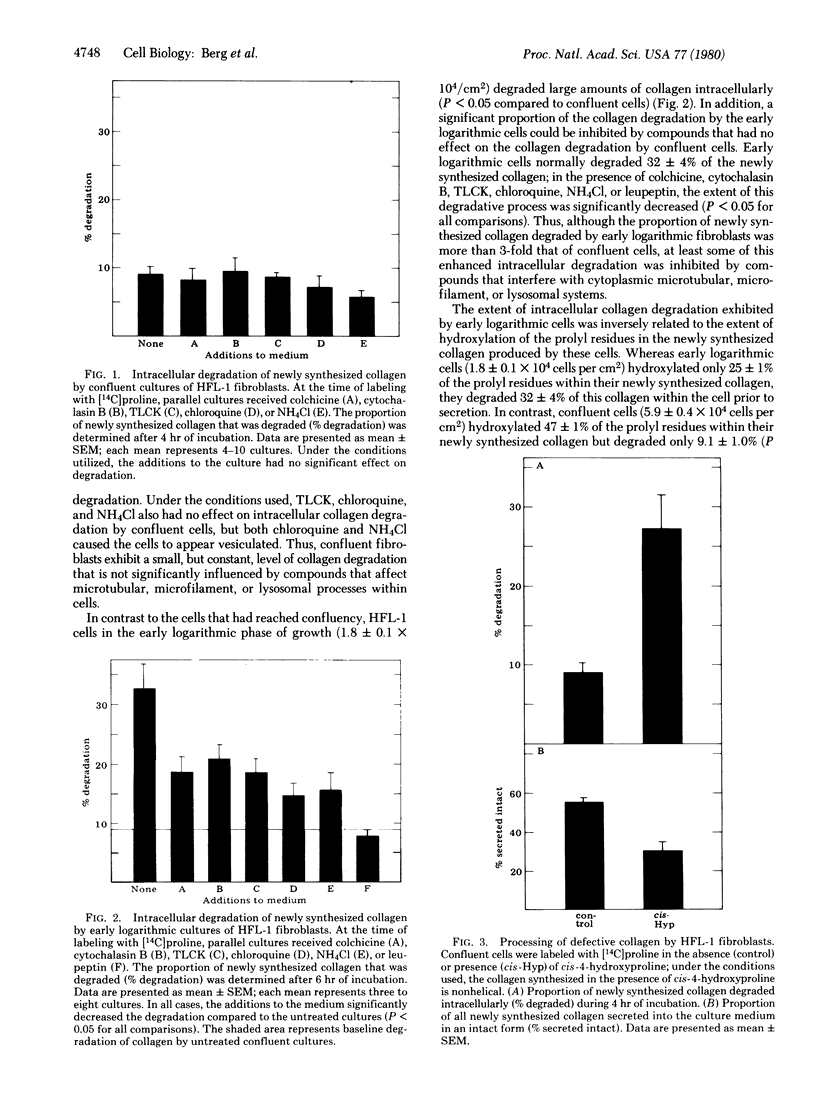
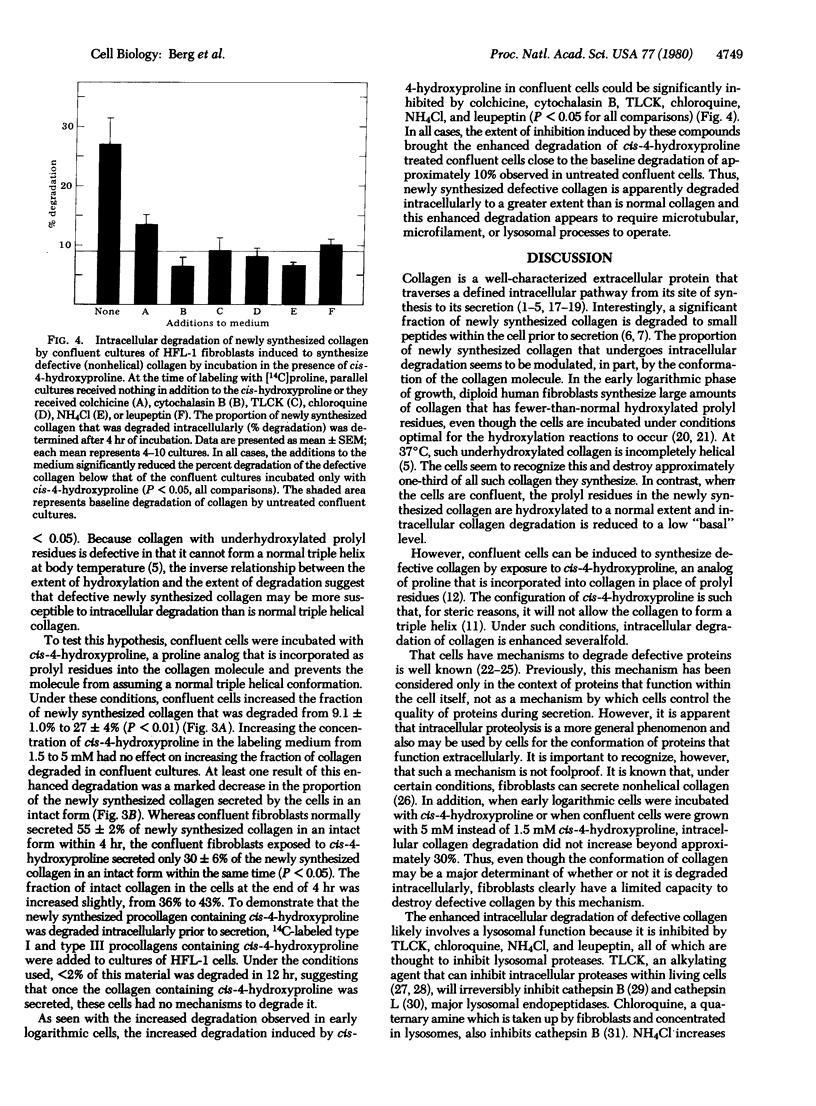
Confluent cultures of human fetal lung fibroblasts degrade approximately 10% of their newly synthesized collagen within the cell prior to secretion. This basal level of intracellular degradation could not be inhibited by colchicine or cytochalasin B, inhibitors of microtubular and microfilament function, respectively, or by Nα-p-tosyl-L-lysine chloromethyl ketone, chloroquine, or NH4Cl, inhibitors of lysosomal enzymes. In contrast, cells in early logarithmic growth degrade approximately 30% of their newly synthesized collagen. This enhanced degradation of collagen in rapidly growing cells could be suppressed by inhibitors of lysosomal proteases and partially inhibited by disrupters of microtubular and microfilament function. A significant proportion of the collagen synthesized by these cultures contained prolyl residues that were incompletely hydroxylated. Because such collagen is “defective” (i.e., not capable of assuming a triple helical conformation), the results suggest that enhanced intracellular degradation may be a mechanism by which cells control the quality of collagen they produce. To test this hypothesis, confluent cells were incubated with the proline analog cis-4-hydroxyproline; such cells demonstrated enhanced collagen degradation that could be inhibited by agents that interfere with lysosomal, microtubular, or microfilament function. Because collagen containing cis-4-hydroxyproline cannot form a perfect triple helix, the data are consistent with the concept that defective collagen is recognized by cells and degraded prior to secretion. Thus, the proportion of newly synthesized collagen that undergoes intracellular degradation seems to be modulated, in part, by the conformation of the collagen molecule. Intracellular proteolysis may represent a means by which collagen-producing cells regulate the quality and quantity of collagen available for extracellular function. Although the exact mechanism of intracellular collagen degradation is unknown, the data presented here are consistent with a role for lysosomal proteases in this process.
Keywords: hydroxyproline, microtubules, microfilaments, proteases, lysosomes
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amenta J. S., Hlivko T. J., McBee A. G., Shinozuka H., Brocher S. Specific inhibition by NH4CL of autophagy-associated proteloysis in cultured fibroblasts. Exp Cell Res. 1978 Sep;115(2):357–366. doi: 10.1016/0014-4827(78)90289-6. [DOI] [PubMed] [Google Scholar]
- Amenta J. S., Sargus M. J., Baccino F. M. Effect of microtubular or translational inhibitors on general cell protein degradation. Evidence for a dual catabolic pathway. Biochem J. 1977 Nov 15;168(2):223–227. doi: 10.1042/bj1680223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B. J., Moss J., Breul S. D., Berg R. A., Crystal R. G. Effect of cyclic AMP on the intracellular degradation of newly synthesized collagen. J Biol Chem. 1980 Apr 10;255(7):2843–2847. [PubMed] [Google Scholar]
- Bienkowski R. S., Baum B. J., Crystal R. G. Fibroblasts degrade newly synthesised collagen within the cell before secretion. Nature. 1978 Nov 23;276(5686):413–416. doi: 10.1038/276413a0. [DOI] [PubMed] [Google Scholar]
- Bienkowski R. S., Cowan M. J., McDonald J. A., Crystal R. G. Degradation of newly synthesized collagen. J Biol Chem. 1978 Jun 25;253(12):4356–4363. [PubMed] [Google Scholar]
- Chandler C. S., Ballard F. J. Accelerated breakdown of reticulocyte protein formed under conditions of amino acid depletion. Biochem J. 1978 Oct 15;176(1):151–158. doi: 10.1042/bj1760151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T., Riley P. A. The degradation of normal and analogue-containing proteins in MRC-5 fibroblasts. Biochim Biophys Acta. 1978 Mar 1;539(2):230–237. doi: 10.1016/0304-4165(78)90009-0. [DOI] [PubMed] [Google Scholar]
- Ehrlich H. P., Ross R., Bornstein P. Effects of antimicrotubular agents on the secretion of collagen. A biochemical and morphological study. J Cell Biol. 1974 Aug;62(2):390–405. doi: 10.1083/jcb.62.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Gribble T. J., Comstock J. P., Udenfriend S. Collagen chain formation and peptidyl proline hydroxylation in monolayer tissue cultures of L-929 fibroblasts. Arch Biochem Biophys. 1969 Jan;129(1):308–316. doi: 10.1016/0003-9861(69)90180-5. [DOI] [PubMed] [Google Scholar]
- Hershko A., Tomkins G. M. Studies on the degradation of tyrosine aminotransferase in hepatoma cells in culture. Influence of the composition of the medium and adenosine triphosphate dependence. J Biol Chem. 1971 Feb 10;246(3):710–714. [PubMed] [Google Scholar]
- Huisman W., Lanting L., Doddema H. J., Bouma J. M., Gruber M. Role of individual cathepsins in lysosomal protein digestion as tested by specific inhibitors. Biochim Biophys Acta. 1974 Nov 25;370(1):297–307. doi: 10.1016/0005-2744(74)90054-0. [DOI] [PubMed] [Google Scholar]
- Inouye K., Sakakibara S., Prockop D. J. Effects of the stereo-configuration of the hydroxyl group in 4-hydroxyproline on the triple-helical structures formed by homogenous peptides resembling collagen. Biochim Biophys Acta. 1976 Jan 20;420(1):133–141. doi: 10.1016/0005-2795(76)90352-4. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Berg R. A., Prockop D. J. Kinetics for the secretion of procollagen by freshly isolated tendon cells. J Biol Chem. 1977 Dec 10;252(23):8391–8397. [PubMed] [Google Scholar]
- Kao W. W., Prockop D. J., Berg R. A. Kinetics for the secretion of nonhelical procollagen by freshly isolated tendon cells. J Biol Chem. 1979 Apr 10;254(7):2234–2243. [PubMed] [Google Scholar]
- Karim A., Cournil I., Leblond C. P. Immunohistochemical localization of procollagens. II. Electron microscopic distribution of procollagen I antigenicity in the odontoblasts and predentin of rat incisor teeth by a direct method using peroxidase linked antibodies. J Histochem Cytochem. 1979 Jul;27(7):1070–1083. doi: 10.1177/27.7.89154. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Langner J., Wiederanders B., Ansorge S., Bohley P. Cathepsin L. A new proteinase from rat-liver lysosomes. Eur J Biochem. 1977 Apr 1;74(2):293–301. doi: 10.1111/j.1432-1033.1977.tb11393.x. [DOI] [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Goldberg A. L. Leupeptin, a protease inhibitor, decreases protein degradation in normal and diseased muscles. Science. 1978 Feb 3;199(4328):534–536. doi: 10.1126/science.622552. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (second of two parts). N Engl J Med. 1979 Jul 12;301(2):77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- STEIN W. H., MOORE S. Chromatographic determination of the amino acid composition of proteins. Cold Spring Harb Symp Quant Biol. 1950;14:179–190. doi: 10.1101/sqb.1950.014.01.022. [DOI] [PubMed] [Google Scholar]
- Shaw E. Selective chemical modification of proteins. Physiol Rev. 1970 Apr;50(2):244–296. doi: 10.1152/physrev.1970.50.2.244. [DOI] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Incorporation of proline analogs into procollagen. Assay for replacement of imino acids by cis-4-hydroxy-L-proline and cis-4-fluoro-L-proline. Arch Biochem Biophys. 1977 May;181(1):293–299. doi: 10.1016/0003-9861(77)90507-0. [DOI] [PubMed] [Google Scholar]
- Warburton M. J., Poole B. Effect of medium composition on protein degradation and DNA synthesis in rat embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2427–2431. doi: 10.1073/pnas.74.6.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M., Leblond C. P. Synthesis, migration, and release of precursor collagen by odontoblasts as visualized by radioautography after (3H)proline administration. J Cell Biol. 1974 Jan;60(1):92–127. doi: 10.1083/jcb.60.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Wibo M., Poole B. Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J Cell Biol. 1974 Nov;63(2 Pt 1):430–440. doi: 10.1083/jcb.63.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]