Abstract
Homologous sense suppression of a gene encoding lignin pathway caffeic acid O-methyltransferase (CAOMT) in the xylem of quaking aspen (Populus tremuloides Michx.) resulted in transgenic plants exhibiting novel phenotypes with either mottled or complete red-brown coloration in their woody stems. These phenotypes appeared in all independent transgenic lines regenerated with a sense CAOMT construct but were absent from all plants produced with antisense CAOMT. The CAOMT sense transgene expression was undetectable, and the endogenous CAOMT transcript levels and enzyme activity were reduced in the xylem of some transgenic lines. In contrast, the sense transgene conferred overexpression of CAOMT and significant CAOMT activity in all of the transgenic plants' leaves and sclerenchyma, where normally the expression of the endogenous CAOMT gene is negligible. Thus, our results support the notion that the occurrence of sense cosuppression depends on the degree of sequence homology and endogene expression. Furthermore, the suppression of CAOMT in the xylem resulted in the incorporation of a higher amount of coniferyl aldehyde residues into the lignin in the wood of the sense plants. Characterization of the lignins isolated from these transgenic plants revealed that a high amount of coniferyl aldehyde is the origin of the red-brown coloration—a phenotype correlated with CAOMT-deficient maize (Zea mays L.) brown-midrib mutants.
Lignin, a complex aromatic polymer, is the major component of wood that must be degraded to extract cellulose fibers for paper making. In angiosperm dicots lignin is composed of G and S monomers that are derived from coniferyl (Fig. 1, 14) and sinapyl (Fig. 1, 16) alcohols (monolignols), respectively. It has been shown that wood-pulping efficiency is greatly influenced by lignin content, as well as its monomeric composition, since G lignin is more difficult to remove than is G-S lignin during chemical pulping (Chang and Sarkanen, 1973; Kondo et al., 1987; Chiang and Funaoka, 1990). Thus, genetic engineering of lignin biosynthesis in pulpwood species has the potential to generate new tree clones with altered lignin content and/or structure to facilitate wood pulping.
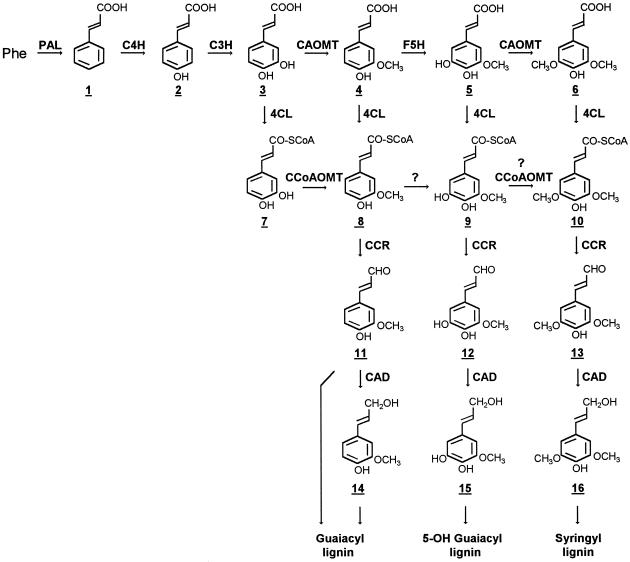
Figure 1.
The biosynthetic pathway of monolignols in angiosperm dicots. The pathway intermediates are indicated with underlined numbers. Question marks indicate not-yet-confirmed steps. PAL, Phe ammonia-lyase; C4H, cinnamate 4hydroxylase; C3H, coumarate 3-hydroxylase; F5H, ferulate 5-hydroxylase; and CCR, cinnamoyl-CoA reductase.
The biosynthetic pathway of monolignols has been extensively studied revealing that at least 10 enzymatic steps are involved in converting Phe into coniferyl and sinapyl alcohols. S-Adenosyl-l-Met-dependent CAOMT (Fig. 1) is an enzyme in this pathway that catalyzes the methylation of phenylpropanoid precursors (Fig. 1, 3 and 5) for the synthesis of both G and S lignins in angiosperm dicots (Sarkanen, 1971; Higuchi, 1985). CAOMT has been suggested to play an important role in mediating the synthesis of S lignin (Higuchi, 1985; Li et al., 1997) and together with CAD (Fig. 1) are the two enzymes found to be responsible for the low-lignin-content-associated maize (Zea mays L.) and sorghum (Sorghum bicolor L.) bm (brown-midrib) mutants that exhibit red coloration in their lignified tissues (for review, see Cherney et al., 1991). Therefore, both CAOMT and CAD have been regarded with considerable interest as targets for genetic engineering of lignin biosynthesis in angiosperm (Dwivedi et al., 1994; Halpin et al., 1994; Higuchi et al., 1994; Ni et al., 1994; Atanassova et al., 1995; Van Doorsselaere et al., 1995; Baucher et al., 1996; Boerjan et al., 1997). All of the CAOMT-related studies so far have revealed that the expression of an antisense CAOMT transgene could influence the lignin structure, resulting in a decreased ratio of S/G in the stem xylem of transgenic plants with, in some cases, a concomitant appearance of abnormal 5-hydroxyguaiacyl (Fig. 1, 15) lignin moieties (Atanassova et al., 1995; Van Doorsselaere et al., 1995). However, none of these studies has yielded unequivocal evidence of red coloration in lignified tissue, as seen in the maize bm3 mutants, which are CAOMT deficient because of mutations in the CAOMT gene (Vignols et al., 1995). Thus, the origin of the red coloration in CAOMT-deficient maize bm3 mutants still remains unclear.
However, red coloration caused by depleted CAD activity in maize and sorghum bm (Bucholtz et al., 1980; Pillonel et al., 1991; Provan et al., 1997) and loblolly pine (Pinus taeda) mutants (MacKay et al., 1997) could be simulated in the stem xylem of transgenic tobacco and poplars by down-regulating CAD gene expression through the antisense approach (Halpin et al., 1994; Higuchi et al., 1994; Baucher et al., 1996). Consistent with the mutants, these CAD-suppressed transgenic plants also showed altered lignin structures containing a higher amount of coniferyl aldehyde (Fig. 1, 11) end groups. The abnormally high amount of coniferyl aldehyde has been suggested to be the cause of the red coloration in the stem xylem of these transgenic plants (Halpin et al., 1994; Higuchi et al., 1994; Baucher et al., 1996), as well as in maize and sorghum bm mutants (Bucholtz et al., 1980; Pillonel et al., 1991; Provan et al., 1997). Higuchi et al. (1994) showed further evidence that coniferyl aldehyde and coniferyl alcohol can be efficiently polymerized in vitro, producing a lignin polymer with a yield of 100%. This in vitro lignin exhibited characteristics that closely resembled the lignins in CAD-deficient transgenic tobacco and forage bm mutants, including dark-reddish-brown coloration. Thus, it was inferred that the coloration associated with CAD-suppressed plants is due to the presence of extensively conjugated coniferyl aldehyde groups in their lignins, as opposed to the not-yet-identified origin of red coloration associated with CAOMT-deficient maize bm mutants.
We report here the occurrence of mottled reddish-brown coloration in woody stems of transgenic aspen (Populus tremuloides Michx.) with down-regulated xylem CAOMT activity via cosuppression by a homologous sense full-length CAOMT transgene. The development of such coloration was random but remarkably efficient, occurring in every transgenic line that harbored the CAOMT transgene and in their vegetative propagules. We demonstrate that a higher amount of coniferyl aldehyde residues was incorporated into the xylem lignin of the transgenic plants. Such structural modification of lignin has not been discovered previously for transgenic plants with altered CAOMT. We also show that an abnormal amount of coniferyl aldehyde residues in lignin gave rise to the reddish-brown coloration in woody stems. Thus, this study provides the first evidence, to our knowledge, that the presence of an abnormal amount of coniferyl aldehyde residues in lignin could be the common cause of the coloration observed in transgenic plants with either inhibited CAOMT or CAD activities and that the transgenic plants with down-regulated CAOMT activity exhibit characteristics that correlate with the phenotype associated with CAOMT-deficient maize bm3 mutants.
MATERIALS AND METHODS
Binary Vector Construction and Agrobacterium tumefaciens-Mediated Transformation and Regeneration of Transgenic Plants
Two oligonucleotides, 5′-TTGTCGACCATGGGTTCAACAGGTGA-3′ (plus strand) and 5′-TTTTCTAGATTAGGCCTTCTTGCGGA-3′ (minus strand), conjugated with NcoI and XbaI restriction sites (underlined), respectively, were designed to amplify the coding region of aspen (Populus tremuloides Michx.) CAOMT cDNA (Bugos et al., 1991) by PCR. The amplified 1.1-kb fragments were digested with NcoI and XbaI and were cloned in a sense orientation with respect to the double 35S promoter/alfalfa mosaic virus RNA4-untranslated leader (Datla et al., 1993) in a pBIN 19 backbone (Bevan, 1984) to generate the binary vector construct pFOMT1. The binary vector was mobilized into A. tumefaciens strain C58/pMP90 (Koncz and Schell, 1986) by a freeze-and-thaw method (Holsters et al., 1978). Quaking aspen was transformed according to a previously established protocol (Tsai et al., 1994). Transformants confirmed by PCR (Tsai et al., 1994) were transferred into soil and grown in a greenhouse for further characterization.
Northern Hybridization and Primer-Extension Analysis
Total RNA was isolated from young leaves, developing sclerenchyma, and xylem tissues of control and transgenic aspen, as described by Bugos et al. (1995). RNA gel electrophoresis and blotting were performed according to the method of Sambrook et al. (1989). Randomly primed 32P-labeled aspen CAOMT cDNA probe was synthesized using the DECAprime II DNA-labeling kit (Ambion, Austin, TX). Northern-blot hybridization was performed as described previously (Church and Gilbert, 1984), except that 0.25 m sodium phosphate buffer (pH 7.4) was used. The blot was washed twice at 65°C with 2× SSC/0.5% SDS and twice with 0.1× SSC/0.1% SDS and autoradiographed on x-ray film at −80°C using an intensifying screen. Primer-extension analysis was carried out according to the method of Tsai et al. (1996) using 10 μg of total RNA extracted from developing xylem. Two [α-32P]dATP cycle-labeled oligonucleotides, 5′-GGAGTCATCTGAGTTTCACCTGTTG, located 5 to 29 nucleotides downstream from the ATG start codon of the aspen CAOMT gene (Tsai et al., 1996), and 5′-TGCGGGCTAAAGATGCAG, located 13 to 30 nucleotides upstream from the ATG start codon of the aspen 4CL gene (Hu et al., 1998), were used to map the transcription start site of CAOMT and 4CL mRNA, respectively. The extension products were analyzed by electrophoresis on an 8% Long Ranger sequencing gel (FMC BioProducts, Rockland, ME).
Enzyme Assays
Crude protein was extracted from young leaves, developing xylem, and sclerenchyma tissues of control and transgenic plants harvested during the growth season using extraction buffer containing 0.1 m Tris-HCl (pH 7.5), 30% (w/v) glycerol, 2.5% (w/v) polyvinylpolypyrrolidone, and 5 mm mercaptoethanol. The extract was filtered through four layers of cheesecloth and centrifuged at 4°C for 20 min. The supernatant was used for enzyme assays and protein concentrations were determined by the method of Bradford (1976) using the Bio-Rad reagent. CAOMT enzyme assays were performed according to the method of Bugos et al. (1992) with approximately 20 μg of crude protein, and the CCoAOMT enzyme assays were carried out with approximately 10 μg of crude protein as described by Li et al. (1997). Assays were performed in duplicate or triplicate. 4CL and CAD activity were determined spectrophotometrically, according to the methods of Ranjeva et al. (1976) and Lüderitz and Grisebach (1981), respectively.
Phloroglucinol Staining and Lignin Characterization
After immersion in the Wiesner reagent (Adler et al., 1948) for 3 s, the fresh free-hand stem cross-section was thoroughly washed with distilled water and photographed. For NaBH4 reduction, the cross-section was treated with 6% NaBH4 in 0.05 n NaOH overnight under N2 and then washed with 0.1 n HCl and distilled water. The NaBH4-reduced cross-section was stained with Wiesner reagent for 15 min and washed with distilled water and photographed. For lignin characterization, air-dried wood meal (80 mesh) from transgenic or control plants was extracted with benzene:ethanol (2:1, v/v) for 48 h. After air drying, the extracted wood meal was used for lignin content determination by the Klason and acetyl bromide methods according to the method of Chiang and Funaoka (1990) and for S/G ratio determination by thioacidolysis as described by Rolando et al. (1992).
Spectrophotometric Analysis of Dioxane Lignins and Phenolic Lignin Model Compounds
Dioxane lignin was isolated from extracted wood meal with 0.2 n HCl in dioxane at room temperature for 3 weeks, according to the method of Stumpf and Freudenberg (1950). Phenolic aldehyde compounds were obtained from Aldrich and an arylglycerol-β-aryl dimeric compound was provided by Prof. Ryuichiro Kondo (Kyushu University, Japan). The dioxane lignin or phenolic compound was solubilized in 96% aqueous dioxane to a final concentration of 0.5 mg mL−1. Neutral and sodium borohydride-reduced spectra were determined based on the method of Kirk and Chang (1975) using 3 mL of the solubilized dioxane lignin or phenolic compound diluted to 25 mL with 50% dioxane. In the case of coniferyl and sinapyl aldehydes, the solutions were further diluted by a factor of 10 for the Wiesner reaction and NaBH4 reduction. The reduction reaction was carried out at room temperature overnight with the addition of 20 mg NaBH4 mg−1 lignin or phenolic compound. Wiesner-reaction (phloroglucinol-HCl) spectra were obtained by mixing 1 mL of the neutral or NaBH4-reduced solutions with 4 mL of the phloroglucinol reagent and reading the A550 after exactly 3 min.
Determination of Coniferyl Aldehyde
Amounts of coniferyl aldehyde present in dioxane lignin were estimated spectrophotometrically based on the Wiesner reaction. The extinction coefficient (50.5 cm2 mg−1) of the coniferyl aldehyde condensation product from the Wiesner reaction was determined empirically and used to calculate the amount of coniferyl aldehyde present in the samples. The coniferyl aldehyde present in dioxane lignin was also determined using the thioacidolysis procedure as described by Rolando et al. (1992).
RESULTS
Production of Transgenic Aspen with Reddish-Brown Wood
Binary vector pFOMT1 was constructed by inserting a 1.1-kb complete coding sequence of CAOMT cDNA (Bugos et al., 1991) in a sense orientation between the double cauliflower mosaic virus 35S promoter/alfalfa mosaic virus RNA4-untranslated leader (Datla et al., 1993) and nopaline synthase terminator. Twenty-five aspen transformants were regenerated through A. tumefaciens-mediated transformation (Tsai et al., 1994). The control plant was also a transgenic plant harboring the binary vector with no CAOMT cDNA insert. Integration of the CAOMT construct in the nuclear genome of 13 randomly selected independent transgenic plants was confirmed by Southern-hybridization analysis, which indicated at least two copies of the transgene in these transgenic plants (data not shown).
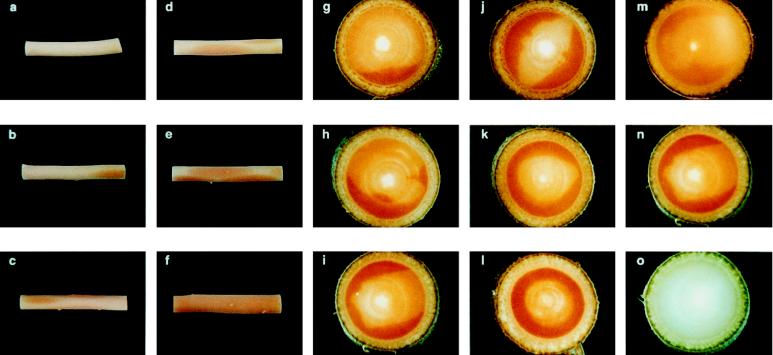
When the transgenic aspens were harvested for further characterization, an unusual red-brown was observed in the woody stem of all of the transgenic lines produced. This wood coloration was not observed in any of the transgenic aspen lines produced with antisense CAOMT. Woody stem segments of a few selected sense transgenic lines are shown in Figure 2, b to f. One of these transgenic lines (S13, Fig. 2f) showed a complete red coloration throughout the entire stem, whereas others were mottled with the pattern and degree of coloration varying from tree to tree (Fig. 2, b–e). To our knowledge, this is the first report of genetically engineered mottled coloration patterns in woody tissue of a transgenic tree species.
Figure 2.
a, Stem segment of control aspen with a typical pale-yellow color; b to e, stem segments of transgenic aspen with mottled reddish-brown coloration; f, stem segment of transgenic plant S13 with a reddish-brown coloration throughout; g to l, stem cross-sections of transgenic plants with irregularly colored areas that were intensified to dark red with Wiesner reagent; m to o, serial stem cross-sections of a transgenic plant showing the red-brown color (m) that was intensified into a dark-red color after the Wiesner reaction (n) and the disappearance of the red-brown color after NaBH4 reduction with a negative response to a prolonged Wiesner reaction (o).
The colored woody tissue was intensified to dark red (Fig. 2, g–l) instantaneously upon contacting Wiesner color reagents (Adler et al., 1948), whereas the woody tissue with normal wood color exhibited a positive response to Wiesner reagents only after a prolonged reaction. This instantaneous response to the Wiesner reaction is indicative of the existence of a high amount of cinnamyl aldehyde derivatives in the lignin of the reddish-brown woody tissue. This was further supported by the observation that the reddish-brown in the cross-section of the transgenic stem (Fig. 2m) disappeared after NaBH4 reduction, and the NaBH4-reduced woody stem responded negatively to a prolonged Wiesner-staining reaction (Fig. 2o).
Expression of the Introduced CAOMT Gene in Transgenic Aspen
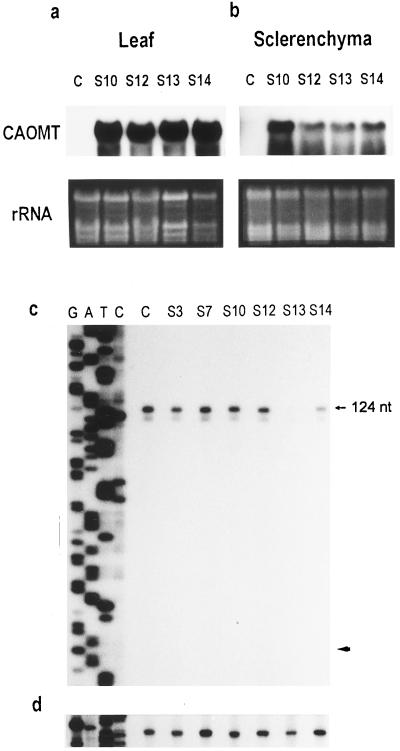
Expression of the introduced sense CAOMT gene in leaf, sclerenchyma, and xylem tissues was tested for the 13 randomly selected transgenic plants. When compared with the negligible, endogenous CAOMT message in leaves and sclerenchyma of the control plant (Fig. 3, a and b, lanes C), the intense hybridizing signals shown on northern blots for the transgenic plants (Fig. 3, a and b, lanes S10, S12, S13, and S14) indicated an ectopic expression of the CAOMT transgene in these tissues. However, because of their similar size, the transgene messages and the abundant endogenous CAOMT transcripts in xylem tissue cannot be differentiated on the northern blot. Therefore, we adopted the primer-extension analysis to simultaneously illustrate the expression of endogenous and introduced CAOMT mRNAs in the xylem of transgenic aspen (Tsai et al., 1996). As shown in Figure 3c, expression of endogenous CAOMT in the xylem is markedly reduced in transgenic line S13, followed by S14 and S3, as indicated by the intensities of the 5′ terminus signals mapped at 124 nucleotides from the primer.
Figure 3.
Ten micrograms of total RNA from young leaves (a) and sclerenchyma tissue (b) of control (lanes C) and transgenic aspen (lanes S10–S14) was probed with 32P-labeled CAOMT cDNA. The rRNA was visualized with UV illumination as a control for the RNA-loading amount. Ten micrograms of total RNA from the xylem of control (lanes C) and various transgenic aspen lines (lanes S3–S14) was subjected to primer-extension analysis using a CAOMT gene-specific oligonucleotide (c) and a 4CL gene-specific oligonucleotide (d) as a control. The extension products of the endogenous CAOMT transcript are located 124 nucleotides (nt) upstream from the primer and the expected size of the extension products of transgene mRNA is indicated by the arrowhead.
To show that approximately equal amounts of total RNA were examined in the analysis shown in Figure 3c, a parallel control primer-extension experiment was conducted using an aspen 4CL (Fig. 1) gene-specific primer (Hu et al., 1998). As shown in Figure 3d, similar intensities of the 5′ end signal of 4CL mRNA were observed in control and transgenic plants, demonstrating that the diminished 5′ end signal of CAOMT mRNA in the xylem of S13 and S14 is not an artifact. Repeated primer-extension analysis revealed that no CAOMT transgene transcription start site signal could be detected in the xylem of any of the transgenic lines tested (Fig. 3c, arrowhead indicates the expected length of 72 nucleotides). This was not an artifact of the primer-extension assay, because intense transcription start site signals of the CAOMT transgene transcripts were readily mapped in leaves of the transgenic plants (Tsai et al., 1996). Taken together, the most likely interpretation of these results is that the reduction of endogenous and introduced CAOMT gene transcripts occurs via homologous sense transgene-mediated suppression (for review, see Depicker and van Montagu, 1997).
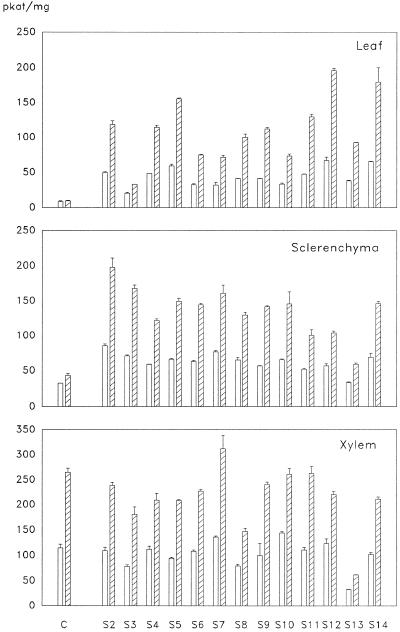
Activities of Lignin Pathway Enzymes, CAOMT, CCoAOMT, 4CL, and CAD in Different Tissues of Transgenic Aspen
Crude protein was extracted from leaf, developing sclerenchyma, and xylem tissues of control and transgenic aspen for assaying CAOMT activities with caffeic (Fig. 1, 3) and 5-hydroxyferulic (Fig. 1, 5) acids. Whereas relatively low levels of endogenous CAOMT activities were found in both leaves and sclerenchyma of the control, significant CAOMT activities were detected in these tissues of transgenic plants. The activities were increased by 5- to 13-fold in leaves and by 1- to 2-fold in sclerenchyma (Fig. 4), consistent with the elevated expression levels of CAOMT in these two tissue types in transgenic plants (Fig. 3, a and b). However, CAOMT activities in developing xylem were inhibited in the majority of the transgenic lines (Fig. 4), which is in agreement with the result that the expression of CAOMT was suppressed in the xylem of transgenic plants (Fig. 3c). Although CAOMT activities with caffeic and 5-hydroxyferulic acids were reduced in the xylem of these transgenic plants, the ratios of activity with 5hydroxyferulic acid to activity with caffeic acid were not significantly altered, as compared with the ratio of 2.3 for the control plant (Fig. 4). These results indicated that the inhibition was specifically toward CAOMT, which uses both caffeic and 5-hydroxyferulic acids as the primary substrates.
Figure 4.
CAOMT activities in various tissue types of control and 13 transgenic aspen lines. Caffeic (open bars) and 5-hydroxyferulic (striped bars) acids were the substrates.
Protein extracts from the xylem of control and various transgenic aspen were also characterized for the activities of several other key lignin pathway enzymes, such as CCoAOMT (Fig. 1), 4CL, and CAD. No difference in these enzyme activities could be detected between the control and various transgenic plants (data not shown). This reveals that down-regulating CAOMT did not influence the functions of these enzymes and that the red-brown coloration described here could not be ascribed to the alteration of 4CL (Kajita et al., 1996) or CAD activities. The unchanged 4CL activity is also in agreement with the unaltered expression of the 4CL gene in these transgenic aspen, as compared with that in the control plant (Fig. 3d).
Content and Chemical Composition of Lignins in Transgenic and Control Aspen
Lignin contents of control and transgenic aspen were determined by Klason and acetyl bromide methods (Chiang and Funaoka, 1990), and the lignin contents obtained by these methods were consistent, as indicated by the average values of 20.78 ± 0.64% and 20.48 ± 0.66% for Klason and acetyl bromide methods, respectively. There was no difference in lignin content between control (average 20.56%) and transgenic plants. However, the structure of lignins in transgenic plants was modified, as revealed by the decreased S/G ratio of transgenic plants, with reduction levels ranging from 17 to 74%, as compared with the S/G ratio of the control plant (Table I). A 74% reduction in S/G ratio and 72 and 77% reduction in CAOMT activities with caffeic and 5-hydroxyferulic acids, respectively, representing the highest reduction levels among the transgenic plants, were observed in the xylem of transgenic line S13, which had a complete red coloration in the woody stem. Overall, the degree of red coloration appears to be inversely related to the S/G ratio and to the level of residual CAOMT activity in the xylem tissue (Table I).
Table I.
Biochemical and chemical characterization of control (C) and transgenic aspen
| Plant No. | Lignin Composition
|
Xylem
CAOMT Activity
|
Wood Colorationa | |||||
|---|---|---|---|---|---|---|---|---|
| G | 5-OH G | S | S/G | CA | 5HFA | 5HFA/CA | ||
| μmol g−1 Klason lignin | pkat mg−1 protein | |||||||
| C | 700 ± 33 | – | 1485 ± 106 | 2.12 | 114.6 ± 7.2 | 265.0 ± 7.8 | 2.3 | −a |
| S10 | 757 ± 28 | – | 1323 ± 44 | 1.75 | 144.0 ± 2.5 | 261.2 ± 11.0 | 1.8 | +b |
| S7 | 651 ± 18 | – | 1089 ± 29 | 1.67 | 136.1 ± 1.6 | 311.9 ± 26.1 | 2.3 | + |
| S12 | 706 ± 12 | – | 1166 ± 43 | 1.65 | 123.3 ± 9.2 | 220.7 ± 5.5 | 1.8 | ++c |
| S14 | 938 ± 42 | – | 1355 ± 49 | 1.44 | 101.8 ± 3.5 | 210.9 ± 4.6 | 2.1 | +++d |
| S3 | 875 ± 16 | – | 1360 ± 15 | 1.55 | 78.2 ± 3.1 | 180.7 ± 14.3 | 2.3 | +++ |
| S13 | 905 ± 29 | 118 ± 4 | 506 ± 17 | 0.56 | 32.5 ± 0.7 | 61.2 ± 0.7 | 1.9 | ++++e |
5-OH G, 5-Hydroxyguaiacyl unit; CA, caffeic acid; 5HFA, 5-hydroxyferulic acid.
−, No coloration.
+, Slightly colored.
++, Moderately colored.
+++, Highly colored.
++++, Entirely colored.
In addition to the alteration of the S and G contents, the abnormal 5-hydroxyconiferyl alcohol (Fig. 1, 15) units were also detected in transgenic line S13 (Table I). Although these units could be associated with the altered lignins in CAOMT-down-regulated transgenic plants (Atanassova et al., 1995; Van Doorsselaere et al., 1995) and in CAOMT-deficient maize bm3 mutants (Lapierre et al., 1988), they are not chromophoric and would not respond to the Wiesner reaction. Furthermore, except for line S13, these 5-hydroxyconiferyl alcohol units were not detected in other transgenic aspen reported here, which nevertheless have red-brown woody stems. Therefore, the red coloration could not be attributed to 5-hydroxyguaiacyl lignin units. Instead, as shown by the Wiesner color reaction described above, the presence of abnormal amounts of cinnamyl aldehyde moieties in lignin could be the origin of the coloration associated with the transgenic aspen reported here. Hence, to further demonstrate that the coloration was due to the incorporation of abnormal amounts of cinnamyl aldehyde residues in lignin, dioxane lignins were isolated from the control and four transgenic plants (S10, S12, S13, and S14), representing four levels of coloration (Table I), and were subjected to UV spectrophotometric analysis, the Wiesner reaction, and thioacidolysis.
UV-Spectrophotometric Analysis, the Wiesner Reaction, and Thioacidolysis of Dioxane Lignins Isolated from Transgenic and Control Aspen
Figure 5a reveals that the UV spectra of dioxane lignins from transgenic plants exhibit a shoulder at approximately 340 nm, which is a typical absorption maximum of coniferyl and sinapyl aldehyde moieties in lignins (Goldschmid, 1971). The shoulder absorption at approximately 340 nm was particularly obvious for lignin isolated from S13. After NaBH4 reduction, the shoulder absorption of lignins from transgenic plants was completely diminished (Fig. 5b). Although the control lignin displayed no apparent shoulder at approximately 340 nm (Fig. 5a), NaBH4 reduction also decreased its absorption near 320 to 340 nm (Fig. 5b). This was likely due to the reduction of basal amounts of coniferyl aldehyde moieties present in the lignins of woody plants (Adler and Ellmer, 1948).
Figure 5.
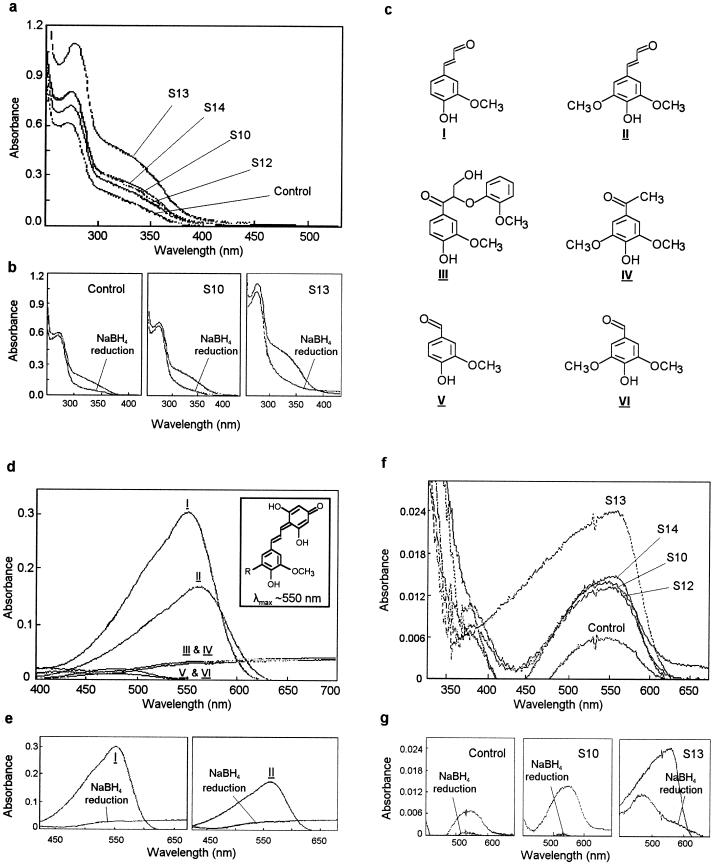
Spectrophotometric characterization of dioxane lignins from control and several lines of transgenic aspen. a, Neutral UV spectra of isolated dioxane lignins. b, UV spectra of the isolated dioxane lignins before and after NaBH4 reduction. c, Various aldehydes used in the Wiesner reaction. d, Absorption spectra of phenolic aldehydes (compounds I–VI, as in c) after the Wiesner reaction. The inset shows the structure of the condensation products from coniferyl aldehyde (R = H) and sinapyl aldehyde (R = OCH3). e, Absorption spectra of Wiesner-reaction products from coniferyl (I) and sinapyl (II) aldehydes before and after NaBH4 reduction. f, Absorption spectra of isolated dioxane lignins after the Wiesner reaction. g, Absorption spectra of Wiesner-reaction products from dioxane lignins before and after NaBH4 reduction.
The UV-spectrophotometric analysis results provide evidence that abnormal amounts of cinnamyl aldehyde units were incorporated into the lignins of transgenic aspen. To further demonstrate that the abnormal amount of cinnamyl aldehyde derivatives was the origin of the red-brown color in wood that could be intensified instantaneously by the Wiesner reaction (Fig. 2, g–l), several lignin-related phenolic aldehyde derivatives (compounds shown in Fig. 5c) were selected and subjected to the Wiesner reaction. These compounds include coniferyl (compound I, same as Fig. 1, 11) and sinapyl (compound II, same as Fig. 1, 13) aldehydes and various aryl-α-CO-containing moieties, representing all possible carbonyl units that are present in lignin (Goldschmid, 1971). For instance, compound III represents the most frequent arylglycerol-β-aryl dimeric compounds having a ketonic aryl-α-CO group. Compound IV denotes S units containing a ketonic aryl-α-CO group and compounds V and VI exemplify G and S units, respectively, containing an aldehydic aryl-α-CO functionality.
However, only coniferyl and sinapyl aldehydes reacted positively with phloroglucinol in the Wiesner reaction, as revealed by the Wiesner-reaction spectra exhibiting an absorption maximum at approximately 550 nm (Fig. 5d, curves I and II) due to the extended conjugation system (Nakamura and Kitaura, 1957) present in the reaction product (Fig. 5d). This extended conjugation system gave rise to a deep reddish-purple seen on the cross-sections of the transgenic aspen (Fig. 2, g–l). In contrast, the Wiesner-reaction solutions for compounds III, IV, V, and VI were colorless and exhibited no absorption maximum at 550 nm (Fig. 5d, curves III, IV, V, and VI) even at a concentration that was 10 times higher than that of coniferyl or sinapyl aldehyde. Therefore, based on the negative response of these ketonic and aldehydic aryl-α carbonyl lignin units to the Wiesner reaction, it is evident that these carbonyl functionalities are not the origin of the red-brown color in lignified stems of transgenic aspen, which can be intensified to a dark red by the Wiesner reaction. Thus, the dark red shown on the Wiesner reagent-treated cross-sections of the transgenic aspen stem is very likely to originate from the carbonyl functionality in coniferyl aldehyde derivatives. Furthermore, NaBH4 reduction of coniferyl and sinapyl aldehydes, to destroy their carbonyl functionality prior to the Wiesner reaction, completely eliminated the color of the resulting Wiesner-reaction solutions, as revealed by the abolished absorption maximum at 550 nm (Fig. 5e). For the same reason, NaBH4 reduction of cinnamyl aldehydes eliminated the red-brown wood coloration, and the NaBH4-treated cross-sections of the transgenic aspen responded negatively to the Wiesner reaction (Fig. 2o).
When dioxane lignins were subjected to the Wiesner reaction, they also exhibited the same absorption maximum at approximately 550 nm (Fig. 5f) as the pure coniferyl or sinapyl aldehydes (Fig. 5d), and NaBH4 reduction diminished their absorption maximum at 550 nm (Fig. 5g), confirming the presence of these aldehydes in these lignins. Furthermore, the relative intensity at 550 nm (Fig. 5f) indicates that lignins from transgenic aspen, especially from S13, contained a significantly higher amount of coniferyl aldehyde derivatives than the basal amount of these aldehydes in control lignin. This was further substantiated by the detection of a higher amount of coniferyl aldehyde released from thioacidolysis of the dioxane lignins from transgenic plants than was released from the control plant (Table II). However, no sinapyl or 5-hydroxyconiferyl aldehydes could be detected in these lignins. Therefore, based on the molar extinction coefficient of the Wiesner-reaction product from coniferyl aldehyde, the quantities of coniferyl aldehyde in dioxane lignins were calculated and matched well with those obtained from thioacidolysis (Table II), which revealed an approximately 3-fold increase in coniferyl aldehyde residues in dioxane lignin of S13.
Table II.
Yield of coniferaldehyde units released from dioxane lignin by the Wiesner color reaction and by thioacidolysis
| Plant No. | Wiesner Reaction | Thioacidolysis |
|---|---|---|
| μmol g−1 Klason lignin | ||
| Ca | 9.9 | 6.2 |
| S10 | 16.0 | 16.2 |
| S7 | 17.8 | 14.2 |
| S12 | 15.3 | 14.7 |
| S14 | 16.7 | 11.7 |
| S3 | 14.1 | 18.9 |
| S13 | 27.4 | ndb |
C, Control.
nd, Not determined.
In summary, the results from the characterization of model compounds and lignin preparations presented above provide several lines of direct evidence that (a) novel lignins with the incorporation of a higher amount of coniferyl aldehyde residues were deposited in woody xylems of these transgenic plants and (b) the presence of this higher amount of coniferyl aldehyde in lignin contributes to the red-brown coloration in stems of transgenic aspen.
DISCUSSION
From this study we report that homologous transgene-mediated sense suppression of CAOMT in developing xylem of transgenic aspen resulted in the formation of a red-brown woody stem. Common to all previous and present studies of down-regulation of CAOMT gene expression in transgenic plants (Dwivedi et al., 1994; Ni et al., 1994; Atanassova et al., 1995; Van Doorsselaere et al., 1995; Boerjan et al., 1997) is the observation of inhibited CAOMT activity and decreased S/G ratio in the xylem of these plants. However, the striking difference between this and previous studies is the observation here of mottled reddish-brown coloration in woody stems of the transgenic plants. The occurrence of such unique coloration was a consistent event in all transgenic aspen produced, and the degree of coloration was correlated inversely with the residual CAOMT activity in the xylem and the S/G ratio (Table I). In contrast, in transgenic hybrid poplar expressing an antisense CAOMT cDNA fragment, a barely visible pale-rose coloration on the surface of the woody stem was reported for only two transgenic plants exhibiting a 95% reduction in CAOMT activity (Van Doorsselaere et al., 1995). The cause of this obscure coloration was not discussed, and no discoloration was observed for any other transgenic hybrid poplar lines with lesser degrees of reduction (up to 50%) in CAOMT activity. Similarly, when a homologous antisense CAOMT cDNA fragment was transferred into the same aspen clone as used in this study, we did not observe any wood discoloration in any of the antisense lines regenerated (Boerjan et al., 1997). The difference between these two previous studies and the current study could then be ascribed to the different regulatory mechanisms for antisense inhibition and sense cosuppression, suggesting that the sense CAOMT construct produces transgenic aspen with colored phenotypes that antisense CAOMT constructs do not.
Although this phenomenon in transgenic trees is reported here for the first time, to our knowledge, Jorgensen et al. (1996) demonstrated a similar result in transgenic petunia, in which the sense chalcone synthase transgene could produce flower color patterns that the antisense transgene could not. In addition, the use of a homologous (this paper) versus heterologous (Van Doorsselaere et al., 1995) gene manipulation strategy in the two Populus spp. systems might also be responsible for the phenotypic difference in these two types of transgenic plants, although the species variation and/or the complex nature of a hybrid genome in the latter case may not be excluded. Species variation could be an important factor. For instance, when the same gene-manipulation strategy of using homologous sense CAOMT cDNA was applied in tobacco (Nicotiana tabacum L.), no wood discoloration was reported for any of the transgenic lines (Atanassova et al., 1995). The implication of this species variation is that tobacco might not always represent an applicable model plant to tree species.
Primer-extension analysis of the CAOMT endogene and transgene mRNAs in the xylem of transgenic plants indicated that, although the endogenous CAOMT transcripts in different transgenic plants were reduced to various levels, the transgene messages in these plants were completely undetectable (Fig. 3c). This indicates that the expression levels of the endogenous CAOMT gene in the xylem of various transgenic lines were apparently higher than that of the CAOMT transgene. Consequently, the expression of the CAOMT transgene could be silenced by the CAOMT endogene, resulting in the cosuppression of both homologous transgene and endogene expression, as seen in S13. Although one may argue that transcripts differ in their 5′ and 3′ untranslated regions and may have different stabilities and turnover rates, thereby contributing to their different levels of steady-state transcripts, we do not think that the apparently low level of CAOMT transgene expression in the xylem is due to an inherent instability of the transgene transcript, since the transgene was strongly expressed and conferred significant CAOMT activity in leaves and sclerenchyma of all transgenic lines (Figs. 2 and 4).
The inability to detect CAOMT transgene transcripts in the xylem of the transgenic plants could be due to an increased susceptibility of the transgene to cosuppression in a tissue in which the endogenous gene is normally expressed at high levels. An alternative explanation is that the transgene was not transcribed in the xylem because of the inefficiency of 35S promoter in such tissue (Nilsson et al., 1996). Thus, in this model cosuppression would be a homology-based, posttranscriptional gene-silencing event that is similar to that in the studies of Van Blokland et al. (1994) and Stam et al. (1997) in which promoterless chalcone synthase transgene constructs were able to silence the endogenous gene expression. Since transcription assays were not done, silencing effects at the transcription level cannot be ruled out. The variation in the reduction (or cosuppression) level of endogenous CAOMT transcripts could be attributed to variable transgene insertional events or positional effects occurring in different transgenic plants (Cannon et al., 1990; Napoli et al., 1990). This position effect, together with the not-yet-understood homology-based, gene-silencing mechanisms, could also be responsible for the diversity of the mottled coloration patterns observed in the xylem of these transgenic aspen.
In addition to the sense cosuppression of the CAOMT gene expression in the xylem, abundant introduced CAOMT transcripts (Fig. 3, a and b) accompanied with up-regulated CAOMT activities (Fig. 4) were consistently observed in both young leaves and in developing sclerenchyma of all 13 transgenic plants analyzed, as compared with the essentially nondetectable endogenous CAOMT expression in these two tissue types. These results strongly suggest that the introduction of a homologous sense transgene could result in an ectopic expression of the transgene in tissues with insignificant endogene expression and a cosuppression of endogene and transgene in tissues where the endogene is highly expressed. Thus, the cosuppression effect could be dependent on the level of target endogene expression (Stam et al., 1997) and the degree of sequence homology between the endogene and transgene.
It has recently been shown that cosuppression is graft transmissible (Palauqui et al., 1997) and that a signal molecule, very likely a nucleic acid, could be involved in systemic gene silencing of virus resistance in plants (Voinnet and Baulcombe, 1997). However, this (transmissible cosuppression and/or signal molecule) model might not be applicable to the case that we described here, because the overexpression of the CAOMT transgene in sclerenchyma and leaves is clearly independent of the cosuppression effect that we observed in the xylem of the transgenic plants. In spite of the engineered CAOMT activities in leaves and developing sclerenchyma, the S/G ratio and content remained unaltered in these tissues of transgenic plants (data not shown). This could suggest that distinct lignin biosynthesis pathways are compartmentalized and are probably independent of CAOMT function in these two tissue types. In contrast, the lignin biosynthesis in aspen xylem could be affected by disturbing the CAOMT-mediated methylation flux to yield lignin with a unique structure that give rises to red-brown coloration in the xylem of the transgenic aspen.
The red coloration observed in CAD-deficient mutants and CAD-down-regulated transgenic plants has always been referred to as the consequence of a high amount of coniferyl aldehyde derivatives in the lignin of these plants. Only Higuchi et al. (1994) further showed that the incorporation of a large amount of coniferyl aldehyde in dehydrogenative polymer had resulted in the production of wine-red synthetic lignin, suggesting the association of augmented coniferyl aldehyde residues in lignin with red coloration in wood. In this study we provided several lines of evidence, including those from the analyses of isolated lignins by the Wiesner reaction, coupled with spectrophotometric characterization and thioacidolysis, to demonstrate that a high amount of coniferyl aldehyde residues in lignin is the chromophoric origin of the reddish-brown color in woody stems of CAOMT down-regulated transgenic plants (Fig. 5; Table II).
A basal amount of coniferyl aldehyde has been found in cambial sap of several tree species and confirmed to be one of the genuine monomeric precursors for lignins in plants (Harkin, 1967; Freudenberg and Neish, 1968). Evidently, the sense-cosuppression mechanisms induced coniferyl aldehyde levels above the basal value (Fig. 5f; Table II), which could represent a threshold level for wood coloration. The induction of a higher amount of coniferyl aldehyde into the lignin polymer could be the result of a shift in the lignin precursor synthesis pathway, favoring the G lignin route in these sense CAOMT transgenic aspens. For instance, in S13, although a drastic reduction in S lignin occurred, the G lignin was increased. However, one would have expected a similar reduction in S and G lignins in the xylem of S13, since a nearly identical level of inhibition of CAOMT activities with caffeic acid and 5-hydroxyferulic acid was observed (Table I). This would strongly suggest that O-methyltransferases other than CAOMT, such as CCoAOMT (Ye et al., 1994), could be involved in channeling precursors to the synthesis of G lignin in plant S13. Thus, in this enhanced G lignin synthesis flux, it could be expected that a higher amount of G lignin precursors would accumulate, including coniferyl aldehyde, which could be efficiently polymerized into the lignin polymer (Connors et al., 1970; Higuchi et al., 1994), as well as be converted to coniferyl alcohol.
Whereas coniferyl aldehyde can be efficiently polymerized with coniferyl alcohol for the synthesis of in vitro lignin (Higuchi et al., 1994), sinapyl and 5-hydroxyconiferyl aldehydes were proven to be poor precursors for in vitro lignin (T. Higuchi, personal communication). Therefore, instead of being polymerized into lignin, sinapyl and 5-hydroxyconiferyl aldehydes could be converted to sinapyl and 5-hydroxyconiferyl alcohols, respectively, to be polymerized with other precursors to form lignin in plant S13. This would explain the detection of sinapyl and 5-hydroxyconiferyl alcohols in lignin of plant S13 but not in their corresponding aldehydes. It also appears that the quantity of coniferyl aldehyde residues in lignin governs the intensity of the red coloration in wood. For instance, our CAOMT sense transgenic aspen with 1- to 3-fold increases in coniferyl aldehyde (Table II) displayed reddish-brown coloration in woody stems, whereas CAD-down-regulated transgenic tobacco plants with 6- to 9-fold increases in coniferyl aldehyde exhibited dark, red-wine-colored wood (Higuchi et al., 1994).
It is not surprising that a significant amount of coniferyl aldehyde was found in the xylem lignin of CAD-down-regulated tobacco, since coniferyl aldehyde is a primary substrate for CAD, and down-regulating CAD would directly augment the coniferyl aldehyde pool to be incorporated into lignin. In our case, since the CAD activity was not altered in the xylem of transgenic aspen, the accumulation of additional coniferyl aldehyde in the xylem must be indirectly modulated by the down-regulation of CAOMT. Therefore, we conclude that, irrespective of the gene(s) manipulated, any disturbance of the lignin biosynthesis pathways in xylem tissue that induces the incorporation of coniferyl aldehyde into the lignin polymer exceeding a threshold level would result in a red coloration in the wood.
ACKNOWLEDGMENTS
The authors thank Prof. Takayoshi Higuchi (Kyoto University, Japan) for stimulating discussions and Dr. C.P. Joshi for helpful comments concerning the manuscript.
Abbreviations:
- CAD
cinnamyl alcohol dehydrogenase
- CAOMT
caffeic acid O-methyltransferase
- CCoAOMT
caffeoyl CoA 3-O-methyltransferase
- 4CL
p-coumarate CoA ligase
- G
guaiacyl
- S
syringyl
Footnotes
This work was supported by grants from the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (nos. 92-37301-7598 and 95-37103-2061) and the CPBR-Department of Energy from the Biomass Program (no. OR22072-55).
LITERATURE CITED
- Adler E, Bjorkquist KJ, Haggroth S. Über die Ursache der Farbreaktionen des Holzes. Acta Chem Scand. 1948;2:93–94. [Google Scholar]
- Adler E, Ellmer LR. Coniferylaldehydgruppen im Holz und isolierten Ligninpraparaten. Acta Chem Scand. 1948;2:839–840. [Google Scholar]
- Atanassova R, Favet N, Martz F, Chabbert B, Tollier M-T, Monties B, Fritig B, Legrand M. Altered lignin composition in transgenic tobacco expressing O-methyltransferase sequences in sense and antisense orientation. Plant J. 1995;8:465–477. [Google Scholar]
- Baucher M, Chabbert B, Pilate G, Van Doorsselaere J, Tollier M-T, Petit-Conil M, Cornu D, Monties B, Van Montagu M, Inzé D and others. Red xylem and higher lignin extractability by down-regulating a cinnamyl alcohol dehydrogenase in poplar. Plant Physiol. 1996;112:1479–1490. doi: 10.1104/pp.112.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Baucher M, Chabbert B, Petit-Conil M, Leple JC, Pilate G, Cornu D, Monties B, Van Montagu M, Van Doorsselaere J, and others (1997) Genetic modification of lignin biosynthesis in quaking aspen (Populus tremuloides) and poplar (Populus tremula × Populus alba). In NB Klopfenstein, YW Chun, MS Kim, MR Ahuja, eds, Micropropagation, Genetic Engineering, and Molecular Biology of Populus. U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO, pp 193–205
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bucholtz DL, Cantrell RP, Axtell JD, Lechtenberg VL. Lignin biochemistry of normal and brown midrib mutant sorghum. J Agric Food Chem. 1980;28:1239–1241. [Google Scholar]
- Bugos RC, Chiang VL, Campbell WH. cDNA cloning, sequence analysis and seasonal expression of lignin-bispecific caffeic acid/5-hydroxyferulic acid O-methyltransferase of aspen. Plant Mol Biol. 1991;17:1203–1215. doi: 10.1007/BF00028736. [DOI] [PubMed] [Google Scholar]
- Bugos RC, Chiang VL, Campbell WH. Characterization of bispecific caffeic acid/5-hydroxyferulic acid O-methyltransferase from aspen. Phytochemistry. 1992;31:1495–1498. doi: 10.1016/0031-9422(92)83093-e. [DOI] [PubMed] [Google Scholar]
- Bugos RC, Chiang VL, Zhang X-H, Campbell ER, Podila GK, Campbell WH. RNA isolation from plant tissues recalcitrant to extraction in guanidine. Biotechniques. 1995;19:734–737. [PubMed] [Google Scholar]
- Cannon M, Platz J, O'Leary M, Sookdeo C, Cannon F. Organ-specific modulation of gene expression in transgenic plants using antisense RNA. Plant Mol Biol. 1990;15:39–47. doi: 10.1007/BF00017722. [DOI] [PubMed] [Google Scholar]
- Chang H-M, Sarkanen KV. Species variation in lignin: effect of species on the rate of kraft delignification. Tech Assoc Pulp Pap Ind. 1973;56:132–134. [Google Scholar]
- Cherney JH, Cherney DJR, Akin DE, Axtell JD. Potential of brown-midrib, low-lignin mutants for improving forage quality. Adv Agron. 1991;46:137–198. [Google Scholar]
- Chiang VL, Funaoka M. The dissolution and condensation reactions in guaiacyl and syringyl units in residual lignin during kraft delignification of sweetgum. Holzforschung. 1990;44:147–155. [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors WJ, Chen CL, Pew JC. Enzymic dehydrogenation of the lignin model coniferaldehyde. J Org Chem. 1970;35:1920–1924. [Google Scholar]
- Datla RSS, Bekkaoui F, Hammerlindl JK, Pilate G, Dunstan DI, Crosby WL. Improved high-level constitutive foreign gene expression in plants using an AMV RNA4 untranslated leader sequence. Plant Sci. 1993;94:139–149. [Google Scholar]
- Depicker A, van Montagu M. Post-transcriptional gene silencing in plants. Curr Opin Cell Biol. 1997;9:373–382. doi: 10.1016/s0955-0674(97)80010-5. [DOI] [PubMed] [Google Scholar]
- Dwivedi UN, Campbell WH, Yu J, Datla RSS, Bugos RC, Chiang VL, Podila GK. Modification of lignin biosynthesis in transgenic Nicotiana through expression of an antisense O-methyltransferase gene from Populus. Plant Mol Biol. 1994;26:61–71. doi: 10.1007/BF00039520. [DOI] [PubMed] [Google Scholar]
- Freudenberg K, Neish AC. Constitution and Biosynthesis of Lignin. New York: Springer-Verlag; 1968. [Google Scholar]
- Goldschmid O. Ultraviolet spectra. In: Sarkanen KV, Ludwig CH, editors. Lignins: Occurrence, Formation, Structures, and Reactions. New York: Wiley-Interscience; 1971. pp. 241–266. [Google Scholar]
- Halpin C, Knight ME, Foxon GA, Campbell MM, Boudet AM, Boon JJ, Chabbert B, Tollier M-T, Schuch W. Manipulation of lignin quality by down-regulation of cinnamyl alcohol dehydrogenase. Plant J. 1994;6:339–350. [Google Scholar]
- Harkin JM (1967) Lignin: a natural polymeric product of phenol oxidation. In WI Taylor, AR Battersby, eds, Oxidative Coupling of Phenols. Marcel Dekker, New York, pp 243–321
- Higuchi T. Biosynthesis of lignin. In: Higuchi T, editor. Biosynthesis and Biodegradation of Wood Components. Orlando, FL: Academic Press; 1985. pp. 141–160. [Google Scholar]
- Higuchi T, Ito T, Umezawa T, Hibino T, Shibata D. Red-brown color of lignified tissues of transgenic plants with antisense CAD gene: wine-red lignin from coniferyl aldehyde. J Biotechnol. 1994;37:151–158. [Google Scholar]
- Holsters M, De Waele D, Depicker A, Messens E, Van Montagu M, Schell J. Transfection and transformation of Agrobacterium tumefaciens. Mol Gen Genet. 1978;163:181–187. doi: 10.1007/BF00267408. [DOI] [PubMed] [Google Scholar]
- Hu W-J, Kawaoka A, Tsai C-J, Lung J, Osakabe K, Ebinuma H, Chiang VL (1998) Compartmentalized expression of two structurally and functionally distinct 4-coumarate: coenzyme A ligase (4CL) genes in aspen (Populus tremuloides). Proc Natl Acad Sci USA (in press) [DOI] [PMC free article] [PubMed]
- Jorgensen RA, Cluster PD, English J, Que Q, Napoli CA. Chalcone synthase cosuppression phenotypes in petunia flowers: comparison of sense versus antisense constructs and single-copy versus complex T-DNA sequences. Plant Mol Biol. 1996;31:957–973. doi: 10.1007/BF00040715. [DOI] [PubMed] [Google Scholar]
- Kajita S, Katayama Y, Omori S. Alterations in the biosynthesis of lignin in transgenic plants with chimeric genes for 4-courmarate:coenzyme A ligase. Plant Cell Physiol. 1996;37:957–965. doi: 10.1093/oxfordjournals.pcp.a029045. [DOI] [PubMed] [Google Scholar]
- Kirk TK, Chang H-M. Decomposition of lignin. II. Characterization of heavily degraded lignins from decayed spruce. Holzforschung. 1975;29:56–64. [Google Scholar]
- Koncz C, Schell J. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric gene carried by a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- Kondo R, Tsutsumi Y, Imamura H. Kinetics of β-aryl ether cleavage of phenolic syringyl type lignin model compounds in soda and kraft systems. Holzforschung. 1987;41:83–88. [Google Scholar]
- Lapierre C, Tollier M, Monties B. Occurrence of additional monomeric units in the lignins from internodes of a brown-midrib mutant of maize bm3. CR Acad Sci Paris. 1988;307:723–728. [Google Scholar]
- Li L, Popko JL, Zhang X-H, Osakabe K, Tsai C-J, Joshi CP, Chiang VL. A novel multifunctional O-methyltransferase implicated in a dual methylation pathway associated with lignin biosynthesis in loblolly pine. Proc Natl Acad Sci USA. 1997;94:5461–5466. doi: 10.1073/pnas.94.10.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz T, Grisebach H. Enzymic synthesis of lignin precursors comparison of cinnamoyl-CoA reductase and cinnamyl alcohol:NADP+ dehydrogenase from spruce (Picea abies L.) and soybean (Glycine max L.) Eur J Biochem. 1981;119:115–124. doi: 10.1111/j.1432-1033.1981.tb05584.x. [DOI] [PubMed] [Google Scholar]
- MacKay JJ, O'Malley DM, Presnell T, Booker FL, Campbell MM, Whetten RW, Sederoff RR. Inheritance, gene expression, and lignin characterization in a mutant pine deficient in cinnamyl alcohol dehydrogenase. Proc Natl Acad Sci USA. 1997;94:8255–8260. doi: 10.1073/pnas.94.15.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Kitaura S. Lignin color reactions. Ind Eng Chem. 1957;49:1388. [Google Scholar]
- Napoli C, Lemieux C, Jorgensen R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell. 1990;2:279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Paiva NL, Dixon R. Reduced lignin in transgenic plants containing a caffeic acid O-methyltransferase antisense gene. Transgenic Res. 1994;3:120–126. [Google Scholar]
- Nilsson O, Little CHA, Sandberg G, Olsson O. Expression of two heterologous promoters, Agrobacterium rhizogenes rolC and cauliflower mosaic virus 35S, in the stem of transgenic hybrid aspen plants during the annual cycle of growth and dormancy. Plant Mol Biol. 1996;31:887–895. doi: 10.1007/BF00019475. [DOI] [PubMed] [Google Scholar]
- Palauqui J-C, Elmayan T, Pollien J-M, Vaucheret H. Systemic acquired silencing: transgene-specific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J. 1997;15:4738–4745. doi: 10.1093/emboj/16.15.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillonel C, Mulder MM, Boon JJ, Forster B, Binder A. Involvement of cinnamoyl-alcohol dehydrogenase in the control of lignin formation in Sorghum bicolor L. Moench. Planta. 1991;185:538–544. doi: 10.1007/BF00202964. [DOI] [PubMed] [Google Scholar]
- Provan GJ, Scobbie L, Chesson A. Characterisation of lignin from CAD and OMT deficient Bm mutants of maize. J Sci Food Agric. 1997;73:133–142. [Google Scholar]
- Ranjeva R, Boudet AM, Faggion R. Phenolic metabolism in petunia tissues. IV. Properties of p-coumarate: coenzyme A ligase isoenzymes. Biochimie. 1976;58:1255–1262. doi: 10.1016/s0300-9084(76)80125-3. [DOI] [PubMed] [Google Scholar]
- Rolando C, Monties B, Lapierre C. Thioacidolysis. In: Lin SY, Dence CW, editors. Methods in Lignin Chemistry. Berlin: Springer-Verlag; 1992. pp. 334–349. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sarkanen KV. Precursors and their polymerization. In: Sarkanen KV, Ludwig CH, editors. Lignins: Occurrence, Formation, Structures, and Reactions. New York: Wiley-Interscience; 1971. pp. 95–163. [Google Scholar]
- Stam M, De Bruin R, Kenter S, Van der Hoorn RAL, Van Blokland R, Mol JNM, Kooter JM. Post-transcriptional silencing of chalcone synthase in Petunia by inverted transgene repeats. Plant J. 1997;12:63–82. [Google Scholar]
- Stumpf W, Freudenberg K. Losliches Lignin aus Fichten und Buchenholz. Angew Chem. 1950;62:537. [Google Scholar]
- Tsai C-J, Mielke MR, Podila GK, Chiang VL. 3′ Cycle-labeled oligonucleotides with predictable length for primer extension and transgene analysis. Nucleic Acids Res. 1996;24:5056–5061. doi: 10.1093/nar/24.24.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C-J, Podila GK, Chiang VL. Agrobacterium-mediated transformation of quaking aspen (Populus tremuloides) and regeneration of transgenic plants. Plant Cell Rep. 1994;14:94–97. doi: 10.1007/BF00233768. [DOI] [PubMed] [Google Scholar]
- Van Blokland R, Van der Geest N, De Lange P, Stam M, Mol JNM, Kooter JM. Transgene-mediated suppression of chalcone synthase expression in Petunia hybrida results from an increase in RNA turnover. Plant J. 1994;6:861–877. [Google Scholar]
- Van Doorsselaere J, Baucher M, Chognot E, Chabbert B, Tollier M-T, Petit-Conil M, Leple J-C, Pilate G, Cornu D, Monties B and others. A novel lignin in poplar trees with a reduced caffeic acid/5-hydroxyferulic acid O-methyltransferase activity. Plant J. 1995;8:855–864. [Google Scholar]
- Vignols F, Rigau J, Torres MA, Capellades M, Puigdomenech P. The brown midrib3 (bm3) mutation in maize occurs in the gene encoding caffeic acid O-methyltransferase. Plant Cell. 1995;7:407–416. doi: 10.1105/tpc.7.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O, Baulcombe DC. Systemic signalling in gene silencing. Nature. 1997;389:553. doi: 10.1038/39215. [DOI] [PubMed] [Google Scholar]
- Ye ZH, Kneusel RE, Matern U, Varner JE. An alternative methylation pathway in lignin biosynthesis in Zinnia. Plant Cell. 1994;6:1427–1439. doi: 10.1105/tpc.6.10.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]