Abstract
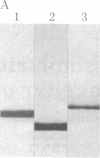
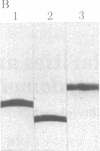
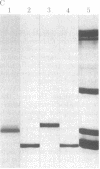
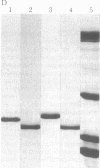
A protein that resembles vertebrate calmodulins and troponin C has been isolated from Chlamydomonas flagella by using a calmodulin purification protocol that included calcium-dependent affinity-based adsorption chromatography on phenothiazine-Sepharose conjugates. The flagellar protein resembled calmodulin in elution from reverse-phase columns, had a peptide map similar to that of calmodulin, and competed with vertebrate calmodulin in a radioimmunoassay using antisera against vertebrate calmodulin. However, this flagellar protein did not activate phosphodiesterase, lacked N epsilon-trimethyllysine, and had an isoelectric point approximately 0.3 pH unit higher than that of vertebrate calmodulin. When analyzed by polyacrylamide gel electrophoresis under various conditions, the Chlamydomonas protein migrated between vertebrate calmodulins and rabbit skeletal muscle troponin C and did not manifest a large calcium-dependent mobility shift. This calmodulin-like protein was identified as one of the approximately 200 35S-labeled components in Chlamydomonas flagella resolved by two-dimensional gel electrophoresis. These studies indicate that calmodulin and a structurally and functionally homologous protein are present in the same cell. These studies also demonstrate that caution is necessary: (i) in identifying a protein as a calmodulin, (ii) in using phenothiazines or antisera directed against vertebrate calmodulins as specific probes for calmodulin, and (iii) in the interpretation of experiments on biological systems in which calmodulin is substituted for the homologous calmodulin-like protein.
Full text
PDF
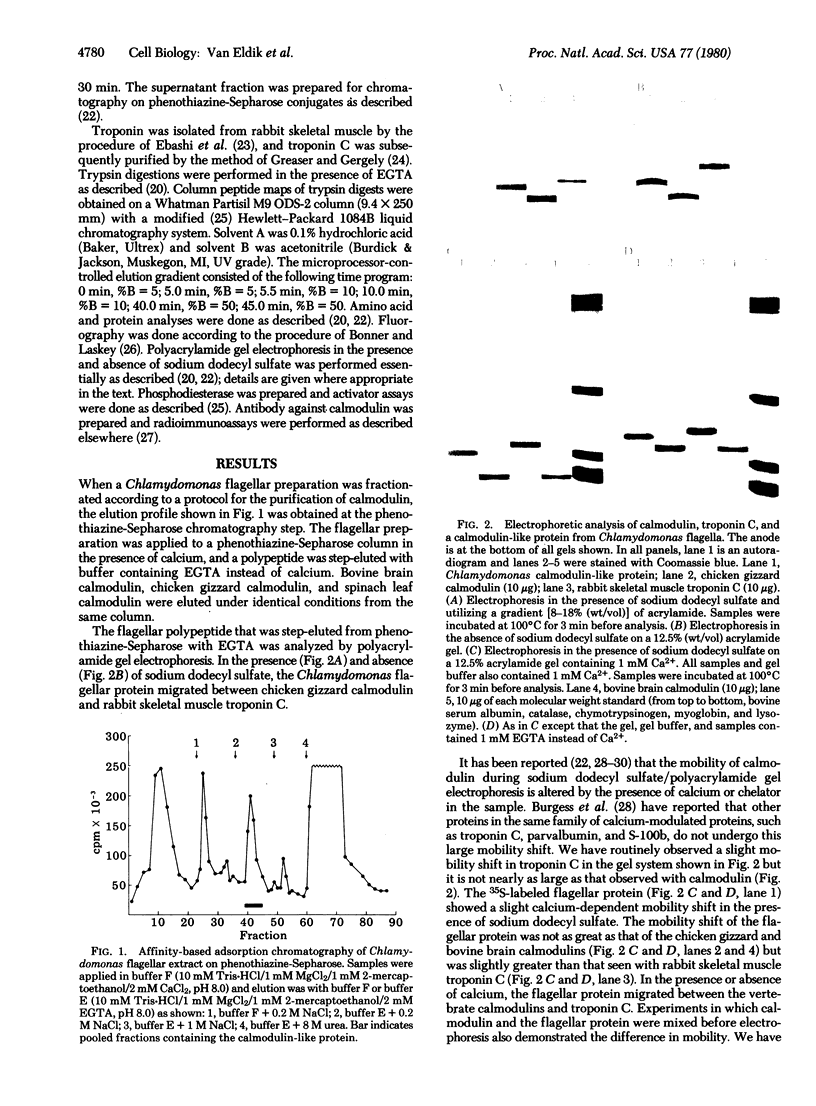
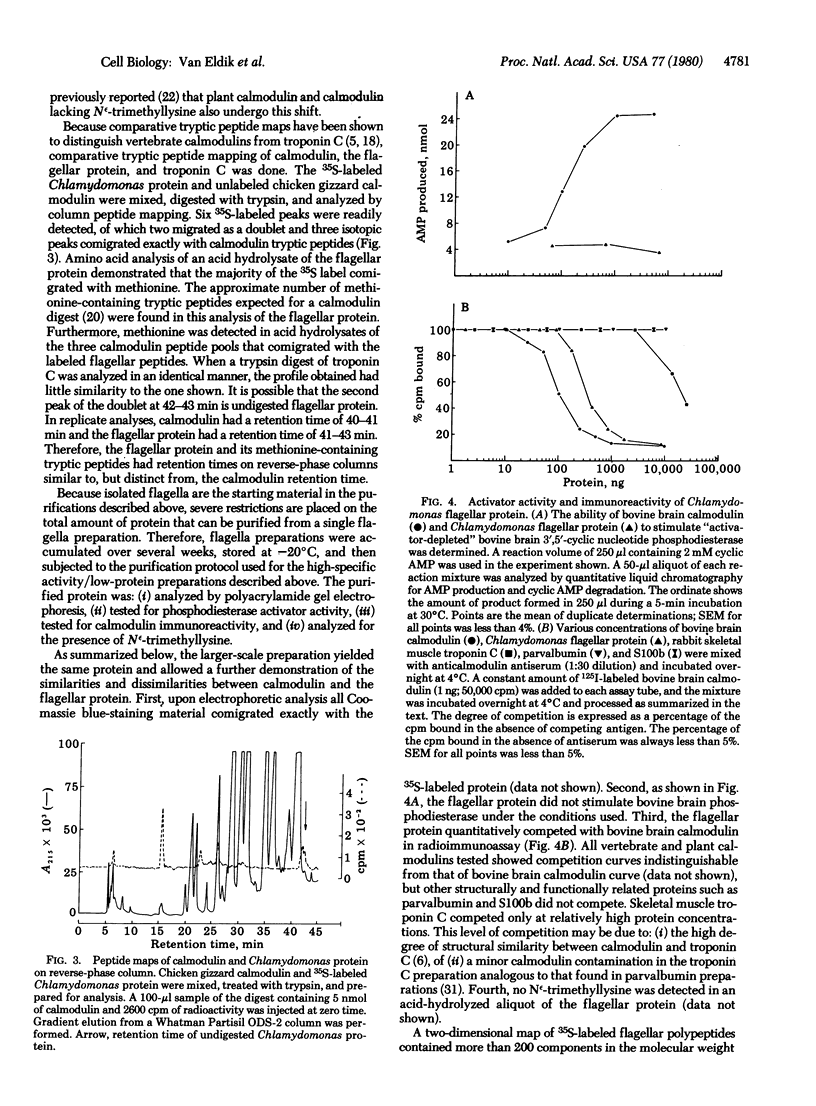


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J., Josslin R., Bobrow L. Calcium ion regulation of flagellar beat symmetry in reactivated sea urchin spermatozoa. Biochem Biophys Res Commun. 1974 Jun 4;58(3):795–800. doi: 10.1016/s0006-291x(74)80487-0. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Dedman J. R., Munjaal R. P., Means A. R. Calmodulin. Development and application of a sensitive radioimmunoassay. J Biol Chem. 1979 Oct 25;254(20):10262–10267. [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Wakabayashi T., Ebashi F. Troponin and its components. J Biochem. 1971 Feb;69(2):441–445. doi: 10.1093/oxfordjournals.jbchem.a129486. [DOI] [PubMed] [Google Scholar]
- Eldik L. J., Grossman A. R., Iverson D. B., Watterson D. M. Isolation and characterization of calmodulin from spinach leaves and in vitro translation mixtures. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1912–1916. doi: 10.1073/pnas.77.4.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grab D. J., Berzins K., Cohen R. S., Siekevitz P. Presence of calmodulin in postsynaptic densities isolated from canine cerebral cortex. J Biol Chem. 1979 Sep 10;254(17):8690–8696. [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Reconstitution of troponin activity from three protein components. J Biol Chem. 1971 Jul 10;246(13):4226–4233. [PubMed] [Google Scholar]
- Holwill M. E., McGregor J. L. Control of flagellar wave movement in Crithidia oncopelti. Nature. 1975 May 8;255(5504):157–158. doi: 10.1038/255157a0. [DOI] [PubMed] [Google Scholar]
- Huang B., Piperno G., Luck D. J. Paralyzed flagella mutants of Chlamydomonas reinhardtii. Defective for axonemal doublet microtubule arms. J Biol Chem. 1979 Apr 25;254(8):3091–3099. [PubMed] [Google Scholar]
- Huang B., Rifkin M. R., Luck D. J. Temperature-sensitive mutations affecting flagellar assembly and function in Chlamydomonas reinhardtii. J Cell Biol. 1977 Jan;72(1):67–85. doi: 10.1083/jcb.72.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyams J. S., Borisy G. G. Isolated flagellar apparatus of Chlamydomonas: characterization of forward swimming and alteration of waveform and reversal of motion by calcium ions in vitro. J Cell Sci. 1978 Oct;33:235–253. doi: 10.1242/jcs.33.1.235. [DOI] [PubMed] [Google Scholar]
- Jamieson G. A., Jr, Vanaman T. C., Blum J. J. Presence of calmodulin in Tetrahymena. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6471–6475. doi: 10.1073/pnas.76.12.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDonne N. C., Jr, Coffee C. J. Inability of parvalbumin to function as a calcium-dependent activator of cyclic nucleotide phosphodiesterase activity. J Biol Chem. 1979 Jun 10;254(11):4317–4320. [PubMed] [Google Scholar]
- Mannherz H. G., Goody R. S. Proteins of contractile systems. Annu Rev Biochem. 1976;45:427–465. doi: 10.1146/annurev.bi.45.070176.002235. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Switzer R. C., Van Keuren M. L. Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4335–4339. doi: 10.1073/pnas.76.9.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Kaneko H. Reactivated triton-extracted models o paramecium: modification of ciliary movement by calcium ions. Science. 1972 May 5;176(4034):523–524. doi: 10.1126/science.176.4034.523. [DOI] [PubMed] [Google Scholar]
- Piperno G., Huang B., Luck D. J. Two-dimensional analysis of flagellar proteins from wild-type and paralyzed mutants of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1600–1604. doi: 10.1073/pnas.74.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Luck D. J. An actin-like protein is a component of axonemes from Chlamydomonas flagella. J Biol Chem. 1979 Apr 10;254(7):2187–2190. [PubMed] [Google Scholar]
- Piperno G., Luck D. J. Axonemal adenosine triphosphatases from flagella of Chlamydomonas reinhardtii. Purification of two dyneins. J Biol Chem. 1979 Apr 25;254(8):3084–3090. [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Eckert R. Calcium couples flagellar reversal to photostimulation in Chlamydomonas reinhardtii. Nature. 1976 Aug 19;262(5570):713–715. doi: 10.1038/262713a0. [DOI] [PubMed] [Google Scholar]
- Stevens F. C., Walsh M., Ho H. C., Teo T. S., Wang J. H. Comparison of calcium-binding proteins. Bovine heart and brain protein activators of cyclic nucleotide phosphodiesterase and rabbit skeletal muscle troponin C. J Biol Chem. 1976 Aug 10;251(15):4495–4500. [PubMed] [Google Scholar]
- Van Eldik L. J., Watterson D. M. Characterization of a calcium-modulated protein from transformed chicken fibroblasts. J Biol Chem. 1979 Oct 25;254(20):10250–10255. [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]
- Watterson D. M., Iverson D. B., Van Eldik L. J. Rapid separation and quantitation of 3',5'-cyclic nucleotides and 5'-nucleotides in phosphodiesterase reaction mixtures using high-performance liquid chromatography. J Biochem Biophys Methods. 1980 Mar;2(3):139–146. doi: 10.1016/0165-022x(80)90021-4. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Mendel P. A., Vanaman T. C. Comparison of calcium-modulated proteins from vertebrate brains. Biochemistry. 1980 Jun 10;19(12):2672–2676. doi: 10.1021/bi00553a020. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Sharief F., Vanaman T. C. The complete amino acid sequence of the Ca2+-dependent modulator protein (calmodulin) of bovine brain. J Biol Chem. 1980 Feb 10;255(3):962–975. [PubMed] [Google Scholar]