Abstract
Background: The trifunctional antibody ertumaxomab bivalently targets the human epidermal growth factor receptor 2 (Her2) on epithelial (tumor) cells and the T cell specific CD3 antigen, and its Fc region is selectively recognized by Fcγ type I/III receptor-positive immune cells. As a trifunctional immunoglobulin, ertumaxomab therefore not only targets Her2 on cancer cells, but also triggers immunological effector mechanisms mediated by T and accessory cells (e.g., macrophages, dendritic cells, natural killer cells). Whether molecular effects, however, might contribute to the cellular antitumor efficiency of ertumaxomab are largely unknown.
Methods: Potential molecular effects of ertumaxomab on Her2-overexpressing BT474 and SK-BR-3 breast cancer cells were evaluated. The dissociation constant Kd of ertumaxomab was calculated from titration curves that were recorded by flow cytometry. Treatment-induced changes in Her2 homodimerization were determined by flow cytometric fluorescence resonance energy transfer measurements on a cell-by-cell basis. Potential activation / deactivation of Her2, ERK1/2, AKT and STAT3 were analyzed by western blotting, Immunochemistry and immunofluorescent cell staining.
Results: The Kd of ertumaxomab for Her2-binding was determined at 265 nM and the ertumaxomab binding epitope was found to not overlap with that of the therapeutic anti-Her2 monoclonal antibodies trastuzumab and pertuzumab. Ertumaxomab caused an increase in Her2 phosphorylation at higher antibody concentrations, but changed neither the rate of Her2-homodimerization /-phosphorylation nor the activation state of key downstream signaling proteins analyzed.
Conclusions: The unique mode of action of ertumaxomab, which relies more on activation of immune-mediated mechanisms against tumor cells compared with currently available therapeutic antibodies for breast cancer treatment, suggests that modular or sequential treatment with the trifunctional bivalent antibody might complement the therapeutic activity of other anti-Her2/anti-ErbB receptor reagents.
Keywords: anti-Her2 targeting, ertumaxomab, trifunctional antibody
Introduction
The human epidermal growth factor receptor 2 (Her2) represents a prominent molecular target for trastuzumab therapy not only in breast cancer, but also in gastric, head and neck and other malignancies.1 Clinical response rates, however, generally do not exceed a rate of ~30% (10% - 60%), an observation that can be reproduced in vitro.2 Although molecular mechanisms causing tumor cell resistance to treatment are manifold, modular Her2 receptor targeting using different antibodies (e. g., pertuzumab) or small molecule kinase inhibitors (e. g., lapatinib) has proven to be a successful strategy to overcome insufficient sensitivity / resistance. Alternate receptor targeting can either be applied as a replacement or complementing treatment.
In contrast to the use of low molecular weight kinase inhibitors, antibody-based molecular targeting using anti-Her2-directed immunoglobulins are thought to not only impair receptor (and downstream signaling) function, but also to trigger the immune system by the activation of T cells and Fcγ-positive accessory cells (e.g., macrophages, dendritic cells, natural killer cells) via Fc-region/Fcγ-receptor binding, which in turn results in a complex immune reaction leading to efficient elimination of tumor-cells by different immunological mechanisms.
Ertumaxomab, a trifunctional bispecific monoclonal antibody (mAb) has the capacity to enhance immunological effector functions such as antibody dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).3 The application of this therapeutic antibody has been proven to be efficient both in vitro and in Phase 1 clinical studies with Her2 positive metastatic breast cancer patients.4 Furthermore, a single agent Phase 2 study with estrogen receptor- or progesterone receptor-positive advanced breast cancer with low Her2 expression proved clinical benefit upon ertumaxomab treatment and indicated a strong immunological response.5 The functional characteristics of ertumaxomab are achieved by two different antigen binding sites (anti-Her2 and anti-CD3) and the typical Fc region. The highly homologous mouse and rat derived IgG2a/IgG2b heavy chains represent two potent and evolutionary related effector subclasses. Ertumaxomab has been designed to induce the formation of tri-cell-complexes consisting of Her2-positive tumor cells, CD3+ T cells, and Fcγ-receptor positive accessory cells (e. g., T cells, mononuclear cells).6 A Th1-type cytokine pattern was observed to efficiently eliminate both Her2 high- and low-expressing tumor cells via cooperation of different classes of immune cells.4,6,7 In peritoneal carcinomatosis patients of gastric and ovarian cancer, a significant increase in tumor-reactive CD4+/CD8+ T lymphocytes by ertumaxomab application/restimulation indicated a specific anti-tumor and potentially long-lasting immunity.8
Combining established target-specific approaches with the administration of ertumaxomab (e.g., in breast cancer) requires, however, extended insight into the molecular effects triggered upon ertumaxomab binding, which have not been studied yet. Thus, we evaluated the direct affects of ertumaxomab on breast cancer cells that may contribute to its anticancer efficiency. We determined the dissociation constant (Kd = 265 nM) of ertumaxomab and found no competition in ertumaxomab/trastuzumab and ertumaxomab/pertuzumab binding. Although ertumaxomab caused an increase in Her2 phosphorylation at higher antibody concentrations, this was not associated with a change in Her2-homodimerization, and no alteration of MAPK, AKT or STAT3 activation was observed.
Results
Binding affinity of ertumaxomab and 2502A
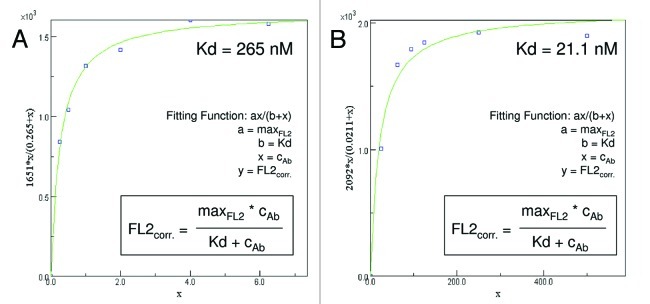
The human Her2-overexpressing breast cancer cell line SK-BR-3 was labeled with ertumaxomab (targeting Her2 the receptor) or 2502A (parental antibody of ertumaxomab targeting the Her2 receptor with each of its binding arms) as primary antibody and a fluorescein isothiocyanate (FITC)-labeled secondary antibody and analyzed by flow cytometry. Antibody titration curves showed that the 2502A antibody reached saturating concentrations at 40 µg/ml. Ertumaxomab-saturating antibody concentrations were reached in the range of 300 to 640 µg/ml. Calculation of the dissociation constant (Kd) show that the binding affinity of ertumaxomab (265 nM, Figure 1A) is lower compared with that of 2502A (21.1 nM, Figure 1B).
Figure 1. Nonlinear regression analysis. Calculation of the binding affinity (Kd) of ertumaxomab (A) and 2502A (B).
Competition of 2502A with 4D5 and 2C4
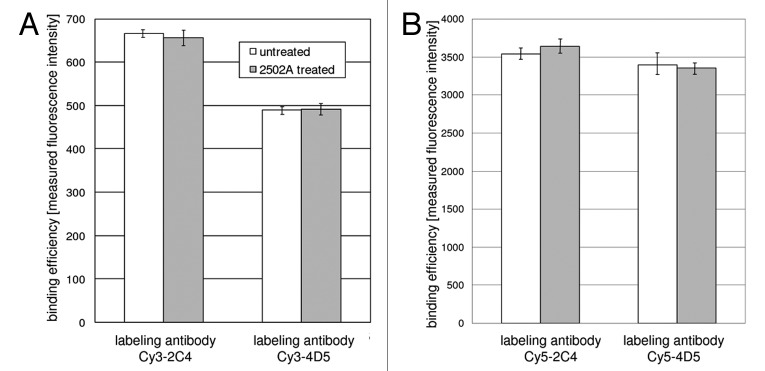
Cells were incubated with 2502A and subsequently labeled with fluorescent dye-conjugated 4D5 (murine counterpart to trastuzumab) or 2C4 (murine counterpart to pertuzumab) antibodies.9Figure 2 shows that pre-incubation with 2502A did not affect the binding capacity of the labeling antibodies. This is represented by equal fluorescence intensities of untreated (white bars) and 2502A treated (gray bars) cells, respectively, that were both labeled with one of the following antibodies as indicated: Cy3–2C4, Cy5–2C4 Cy3–4D5 or Cy5–4D5. Absolute fluorescence intensity values varied for Cy3- and Cy5-labeled antibodies due to the different excitation efficiency achieved by the 488 nm (excites Cy3) and the 635 nm (excites Cy5) laser excitation source of the FACSCalibur Flow Cytometer. Flow cytometric competition experiments (Fig. 2) revealed no competition in binding to the Her2 receptor of 2502A and 4D5 or 2502A and 2C4, respectively. This is a technical prerequisite of the following FRET experiments for which fluorochrome-conjugated labeling antibodies 4D5 or 2C4 are used.
Figure 2. Flow cytometric analysis of fluorescence intensity of Cy3-(A) or Cy5-labeled (B) 4D5 and 2C4 antibodies after cell incubation with 2502A compared with untreated cells. Error bars reprsent standard deviation of the mean (SD).
Flow cytometric FRET analysis of Her2 homodimerization
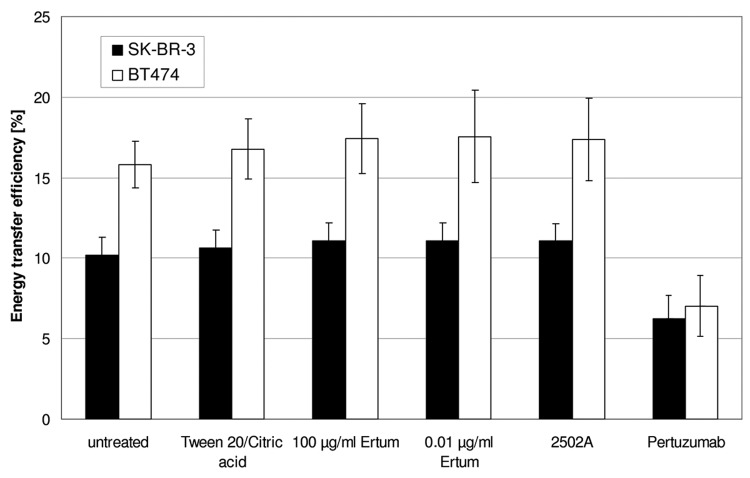
Incubation of SK-BR-3 or BT474 cells with ertumaxomab did not have a significant effect on Her2 homodimerization either at high (100 µg/ml) or at low (0.01 µg/ml) antibody concentration (Fig. 3). Incubation with ertumaxomab vehicle solution and control antibody 2502A, respectively, did not significantly change energy transfer values in both cell lines. However, pertuzumab-treated samples showed reduced energy transfer efficiency-values as previously demonstrated.10
Figure 3. Fluorescence resonance energy transfer (FRET) efficiency of ertumaxomab-incubated SK-BR-3 and BT474 cell samples compared with 2502A, vehicle buffer (Tween/Citric acid) and pertuzumab-incubated samples. Error bars represent standard deviation of the mean (SD).
Her2 activation/phosphorylation analyzed by western blotting
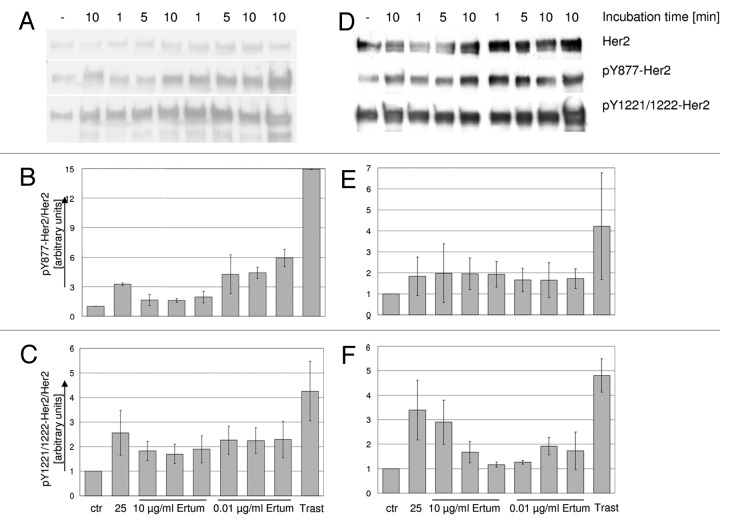
Incubation of SK-BR-3 and BT474 cell samples with trastuzumab (control treatment) caused a significant increase (p < 0.05) in Her2 phosphorylation of Y877 and Y1221/1222 residues compared with untreated control cells (Fig. 4). In SK-BR-3 cells, 2502A and ertumaxomab treatment (0.01 µg/ml; 1 min, 5 min and 10 min) also tend to increase Y877-Her2 phosphorylation, but without reaching significance. In BT474 cells (Fig. 4D-F), incubation with 2502A or ertumaxomab treatment did not increase Y877-Her2 phosphorylation compared with control cells. In contrast, the Y1221/1222-phosphorylation site on Her2 appeared to be activated by 2502A and 10 µg/ml ertumaxomab with incubation for 1 min.
Figure 4. western blot detection of pY877-Her2 and pY1221/1222-Her2 in SK-BR-3 (A) and BT474 cells (D). Cells were treated with 10 µg/ml or 0.01 µg/ml ertumaxomab (Ertum). Control samples were left untreated (ctr) or were treated with 10 µg/ml 2502A (25) or 30 µg/ml trastuzumab (Trast), respectively. Her2 phosphorylation of Y877 and Y1221/1222 in SK-BR-3 (B and C, respectively) and BT474 (E and F, respectively) cell samples was densitometrically quantified. Values are expressed as Her2 phosphorylation relative to Her2 expression and were normalized to control cells which were set equal one. Mean values of independent experiments from two different cell lysates are shown.
Her2 activation/phosphorylation analyzed by immunochemical and immunofluorescent staining
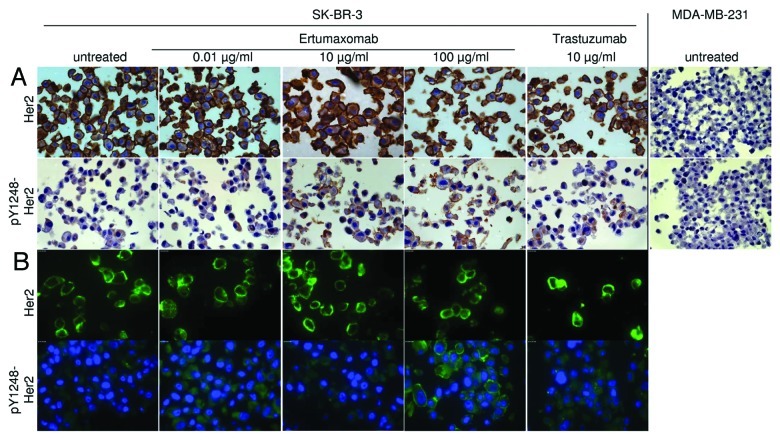
Because available Y1248-Her2 detection antibodies were found to be non-functional in western blot experiments, we performed immunochemical (IC) and immunofluorescent (IF) stainings to identify potential ertumaxomab-caused induction of Y1248-Her2 phosphorylation (Fig. 5). We used SK-BR-3 breast cancer cells because this cell line was the most responsive in terms of ertumaxomab-caused Y877 and Y1221/1222 phosphorylation (see above). In IC experiments, MDA-MB-231 cells (Her2 score 0) were used as negative control and showed no Her2 and anti-phospho-Y1248-Her2 staining indicative for specific binding of the antibodies (Fig. 5A).
Figure 5. IC (A) and IF (B) staining of Her2 and pY1248-Her2 in SK-BR-3 (Her2 Score 3+) upon treatment with different concentrations of ertumaxomab and trastuzumab as reference. MDA-MB-231 (Her2 Score 0, negative control) cells were used in IC as negative control to guarantee antibody specificity (A). Objective magnification 63x.
IC- and IF-stainings (evaluated by AxioVision software Ver. 4.6.3 SP1, Carl Zeiss) of Her2 in SK-BR-3 cells showed that no incubation modality affected the high expression level of Her2 in this cell line (Fig. 5A, B). Phosphorylation of Y1248-Her2 after cell incubation with 0.01 µg/ml ertumaxomab was not changed compared with control cells (IC experiments); however, an increase in Her2 Y1248-phosphoylation was shown by trastuzumab-incubation. The staining intensity seemed to be even higher in 10 µg/ml and 100 µg/ml ertumaxomab-incubated SK-BR-3 cells. In line, IF-staining suggested an increase in Y1248-Her2 phosphorylation upon ertumaxomab incubation with 100 µg/ml and a faint phosphorylation upon trastuzumab incubation (Fig. 5B).
Phosphorylation of intracellular key signaling proteins
The intracellular Her2 receptor-triggered pathway system is highly complex.11 Activation of a Her kinase leads to the phosphorylation of key tyrosine residues within its COOH-terminal residue and thereby provides specific docking sites for cytoplasmic proteins. The main signal transmitting pathways are represented by: (1) Ras/Raf/mitogen-activated protein kinase (MAPK) pathway (cell proliferation pathway), (2) phosphatidylinositol 3-kinase (PI3K)/AKT pathway (anti-apoptosis pathway), and (3) signal transducers and activators of transcription (STAT) pathway. Due to their fundamental role in cellular signaling, the key proteins of these pathways, ERK1/2, AKT and Stat3α/β, were analyzed.
Western blotting detection of phospho-ERK1/2 and phospho-AKT in SK-BR-3 cells showed a constitutive phosphorylation in control cells (Fig. 6A) that remained unchanged by ertumaxomab-, 2502A- or trastuzumab-treatment. In line, in BT474 ERK1/2 and AKT phosphorylation was not reproducibly altered upon cell treatment in three independent experiments (Fig. 6B).
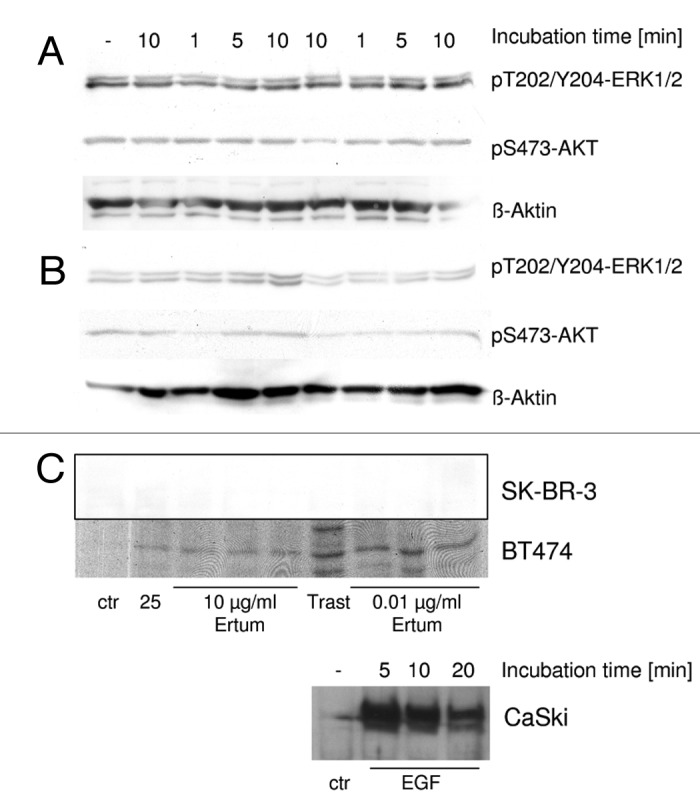
Figure 6. western blot detection of ERK1/2- and AKT phosphorylation in SK-BR-3 (A) and BT474 (B). (C) pY705-STAT3 detection in BT474 cells, SK-BR-3 and CaSki positive control cells (0.031 µg/ml EGF). SK-BR-3 and BT474 cells were treated with 10 µg/ml or 0.01 µg/ml Ertumaxomab (Ertum). Control samples were left untreated (ctr) or were treated with 10 µg/ml 2502A (25) or 30 µg/ml trastuzumab (Trast), respectively.
Although BT474 cells showed a slight activation of STAT3, the achieved signals in western blotting in SK-BR-3 were below the detection limit (Fig. 6C). Therefore, neither SK-BR-3 nor BT474 cells were regarded as showing STAT3 phosphorylation upon ertumaxomab, 2502A or trastuzumab incubation. Control experiments with CaSki cells treated with EGF (Fig. 6C) showed a time dependent phosphorylation of STAT3, which proved that the detection procedure applied here was suitable for analysis of the activation of STAT3 by phosphorylation.
Discussion
Trifunctional antibodies like ertumaxomab are potential powerful tools in immune therapies of cancer. Incorporation of two different antigen-binding sites into one drug that assembles a multi-cell complex including immunologic effector cells enables a broadly efficacious anti-cancer therapy.12 As indicated by promising results derived from preclinical or early clinical studies,13-15 the therapeutic application of trifunctional and bivalent antibodies such as ertumaxomab (anti-Her2, anti-CD3, Fc-region) or catumaxomab (anti–EpCAM, anti-CD3, Fc-region) antibody,12 or bispecific antibody fusion proteins such as MM-111 (anti-Her2, anti-HER-3 single chain variable fragments) and dual-action antibodies such as MEHD7945A (anti-EGFR and anti-Her3 by each antigen binding fragment) might be superior to monospecific immunogobulins. For example, combining the advantages of targeting two different antigens with one drug might be more efficient and could significantly contribute to overcoming resistance.15
Patients benefit from dual Her2 antibody-targeting with trastuzumab plus pertuzumab (in addition to chemotherapy),16-18 which is mainly due to a more effective inhibition of Her2-dependent cellular signaling compared with treatment with either antibody alone.16,19 Although both antibodies verifiably have the capacity to elicit antibody-dependent cell-mediated cytotoxicity (ADCC),20 their (complementary) effect has been mainly attributed to cellular effects,21 e. g., prevention of Her2 cleavage (trastuzumab)21 and inhibition of HER2 homo-10 and heterodimerization (pertuzumab).22,23 Compared with this, ertumaxomab and its parental monospecifc antibody 2502A (anti-Her2) seem to not affect the degree of Her2 homodimerization, a phenomenon that indicates a distinct, and therefore a potential complementary, mechanism of action for the bivalent antibody. In the case of trastuzumab and pertuzumab, it has been shown, however, that the biological activity of an anti-Her2 antibody is connected to specific antibody binding epitopes.10,24 The competition assay we performed in this study revealed that the Her2 epitope recognized by ertumaxomab is distinct from those of trastuzumab (humanized form of 4D5, binding to domain IV)6,25 and pertuzumab (humanized form of 2C4, binding to domain II),26 respectively. The data suggest that the ertumaxomab binding epitope is far from the Her2/Her2 interaction region of the extracellular receptor domain. In line with other data,6 we therefore suggest domain III as the binding epitope of ertumaxomab on the Her2 ectodomain. Hence, a modular or sequential addition of ertumaxomab to trastuzumab- or pertuzumab-based treatment regimes appears reasonable and promising since complementing treatment strategy may enhance antitumor efficiency by combining different modes of action.4,6,7
We found ertumaxomab to induce Her2 phosphorylation at Y877, Y1221/1222 and Y1248. Based on analyses addressing the mechanisms of action of trastuzumab, we previously suggested that an initial induction of Her2 receptor phosphorylation at distinct tyrosine residues does not contradict an anti-proliferative outcome,27 which is in agreement with observations by others.28,29 Hence the phosphorylation events detected in this study upon ertumaxomab and trastuzumab treatment are likely to activate anti-proliferative or pro-apoptotic signaling cascades rather than to lower the intensity of pro-proliferative signaling. This hypothesis is stressed by the induction of apoptosis upon binding of E3 ubiquitin-ligase c-Cbl to pY1112-Her2 and subsequent Her2 protein degradation.30 Moreover, an inhibition of cell proliferation has been attributed to an association of Csk homologous kinase (CHK) to pY1248-Her2.31 Overall, different tyrosine phosphorylation sites positively or negatively modulate the transforming capacity of Her2.32 The Y877 residue, for instance, is located in the activation loop of the Her2 kinase domain. Although the role of Y877-Her2-phosphorylation is the subject of debate,33,34 the Her2 kinase domain has been suggested to be catalytically active only if it is phosphorylated at this regulatory residue. Xu et al.35 reported that autophosphorylation of Y1248 was decreased after mutation of Y877 to phenylalanine and that Y1248 might occur as a result of Y877 phosphorylation. Our data showed, for higher antibody concentrations, concomitant ertumaxomab-caused Y877 and Y1248 phosphorylation in SK-BR-3, and therefore also supports a model of sequential tyrosine residue phosphorylation.
Intracellular signaling, however, was only observed at higher ertumaxomab concentrations (> 10 µg/ml) that largely exceed the clinical dose range. The observed lack of significant changes in the activation of intracellular signaling proteins ERK1/2, AKT and STAT3. are not limiting factors for ertumaxomab’s anti-tumor activity, which mainly relies on activation of immune-mediated mechanisms against tumor cells. This is based on the particular mode of action of a trifunctional antibody that induces simultaneous recruitment and activation of T cells and accessory cells, leading to the destruction of targeted tumor cells by different killer mechanisms.6 Hence, it can be concluded that cytotoxic effects on target cells caused by ertumaxomab treatment occur dose dependently and, if ertumaxomab is applied at lower concentrations, have to be attributed to the Fc part or the anti-CD3 arm of the trifuncional antibody.
Compared with the ertumaxomab parental antibody 2502A (Kd = 21.1 nM), the binding affinity of ertumaxomab (Kd = 265 nM) was significantly lower. This is most probably due to the availability of only one Her2 binding arm in the ertumaxomab antibody compared with two Her2 binding arms in the parental 2502A molecule, a phenomenon that has been previously described.36 It is worth noting that low affinity antibodies, e. g., monospecific anti-Her2 antibodies with Kd in the order of 270 nM, have been shown in vivo to more effectively penetrate tumors and to be internalized and catabolized at a lower rate.37 This can result in more effective antitumor activity37 compared with that of high affinity antibodies, e.g., trastuzumab or the 4D5 antibody (parental trastuzumab) with Kd of 0.1 nM38 or 1.04 nM,39 respectively.
Effective anti-tumor activity by ertumaxomab treatment can, however, only evolve in the presence of a functional immune system. Hematotoxicity caused by chemotherapeutics and radiation therapy is often observed during anticancer treatment. Ertumaxomab was demonstrated to retain its potency for an effective anti-tumor activity in in vitro cytoxicity assays using patient immune effector cells after conventional therapy.40
In this study, we described for the first time molecular effects of ertumaxomab at higher antibody concentrations on its target, Her2. Furthermore, we provided experimental evidence of differences in the effects of ertumaxomab compared with those observed by using trastuzumab. Due to its different and particular mechanism of action, patients with low Her2 expressing tumors40 and with Her2 overexpression but trastuzumab-refractory disease may benefit from ertumaxomab.6 The unique characteristics of trifunctional antibodies would justify a clinical application, particularly in cancer patients with resistance to current standard treatment schemes or minimal residual disease at high risk for tumor recurrence.41,42
Material and Methods
Cells and cell culture
The human breast cancer cell lines BT474, SK-BR-3 and MDA-MB-231 and the human epidermoid cervical carcinoma cell line CaSki were obtained from the American Type Culture Collection (ATCC) and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% (v/v) fetal calf serum (FCS) (both PAA Laboratories). Routine cell culture was performed as described.43
Cell treatment with growth factors and therapeutic drugs
Cells were incubated with different ertumaxomab (anti-Her2 x anti-CD3) concentrations as indicated or incubated with 10 µg/ml parental antibody 2502A (anti-Her2), (provided by Fresenius Biotech, TRION), 30 µg/ml trastuzumab (Herceptin®, Roche Diagnostics) or with 0.031 µg/ml human epidermal growth factor (EGF; ImmunoTools, #11343407) as indicated. Due to an increasing probability of unspecific binding or “sticking” of the antibody to the cell membrane with increasing antibody concentration, a lower concentration (100 µg/ml and 10 µg/ml) than the saturating concentration (approximately 350 µg/ml, data not shown) was used for FRET studies and the analysis of protein phosphorylation. The concentration of 0.01 µg/ml was chosen to represent a concentration that mediates efficient tumor cell killing in vitro6 and to reflect a clinically-relevant concentration.4 Because treatment of Her2 positive SK-BR-3 cells with ≥ 0.05 µg/ml ertumaxomab revealed 100% cytotoxicity,4 we decided to test non-immunological effects on tumor cells upon ertumaxomab treatment within a concentration range of 0.01 µg/ml – 100 µg/ml.
Non-treated samples served as controls. Control samples containing Tween-20 and citric acid were prepared for FRET measurements and served as reference for ertumaxomab- and 2502A-treated samples.
Titration of ertumaxomab and 2502A and calculation of Kd
Cell culture and cell harvesting was performed as described.27 0.5 million Her2 overexpressing SK-BR-3 cells were added per tube, washed twice in 1 ml PBS supplemented with 0.2% (w/v) BSA and centrifuged at 130 g at 4°C for 5 min. The supernatant was discharged and cells were resuspended in the prediluted primary ertumaxomab or 2502A antibody solutions. After an incubation step of 30 min at 4°C, cells were washed twice in PBS/0.2% BSA, centrifuged (130 g, 5 min, 4°C) and labeled with a polyclonal rabbit anti-mouse immunoglobulins/FITC (Dako, F0232, dilution 1:20) for 45 min at 4°C in the dark. After two washing steps in PBS/0.2% BSA, cells were resuspended in 500 µl PBS/0.2% BSA and analyzed on a FACSCalibur Flow Cytometer (BD Biosciences).
The calculation of Kd values was performed online at www.colby.edu/chemistry/PChem/scripts/lsfitpl.html by nonlinear curve fitting. The background corrected fluorescent values of antibody titration curves were inserted into the online matrix and the fitting function y = ax/(b+x) was selected [a = maximum fluorescence in detection channel = maxFL2, b = Kd, x = antibody concentration = c(Ab), y = background corrected fluorescence in detection channel = FL2(corr)]. Antibody concentrations were converted from the unit µg/ml in µmol/ml (1 g antibody = 6.67 µmol; molecular weight ~150 kDa) and added to the online matrix. Finally, curve fitting and calculation of Kd was performed automatically by the software.
Competition of 2502A with 4D5 and 2C4
The binding capacity of anti-Her2 antibodies 4D5 and 2C4 after pretreatment with 2502A was determined and compared with untreated cells (due to the higher binding affinity of 2502A, this antibody and not ertumaxomab was used to determine the relative binding epitope of 2502A compared with 4D5 and 2C4). Three million SK-BR-3 cells were harvested and washed as described above. 1.5 million cells were transferred to an additional tube and incubated with 40 µg/ml 2502A in PBS/2% (w/v) BSA in a total volume of 120 µl. Cells without 2502A were incubated in 120 µl PBS/2% BSA. After 30 min on ice, cells were washed twice in PBS/0.2% BSA as described above and each sample was divided into three tubes (without 2502A: sample no. 1–3; with 2502A: sample no 4–6). Samples 2 and 5 were labeled with Cy3- or Cy5-conjugated 2C4 antibody, and samples 3 and 6 were labeled with Cy3- and Cy5-conjugated 4D5 antibody. The labeling concentrations were 40 µg/ml for each antibody in a volume of 40 µl. Unlabeled cells (samples 1 and 4) were incubated in 40 µl PBS/2% BSA and served for determination of background fluorescence. Cells were incubated for 30 min in the dark, washed twice, resuspended in 500 µl PBS/0.2% BSA and analyzed on a FACSCalibur Flow Cytometer. The results were confirmed by applying two differently labeled antibody sets, conjugated either to Cy3 or Cy5.
Flow cytometric fluorescence resonance energy transfer
Five samples were run for each experimental setup: (1) unlabeled cells, (2) donor-dye labeled cells, (3) acceptor-dye labeled cells and (4) donor- and acceptor-dye labeled cells labeled with Cy3-/Cy5–4D5 or (5) with Cy3-/Cy5–2C4 (gift from János Szöllősi, University of Debrecen, Hungary). Single-stained samples served as control measurements that allow determination of spectral overspill of donor and acceptor-dye, respectively, and dye cross-excitation by two laser illumination. The detailed procedure for analysis of Her2 homodimerization is described in detail elsewhere.10,27 Antibody treatment was performed under cell culture conditions for 45 min. Negative control cells remained untreated or were treated with ertumaxomab vehicle. For samples 2 and 3, final antibody concentrations of 40 µg/ml were used for labeling. The labeling volume was 40 µl. In double labeled samples, donor and acceptor dye-labeled antibodies were mixed to a final concentration of 30 µg/ml for the donor dye and 60 µg/ml for the acceptor dye conjugated antibody and added to the cell pellet in a total volume of 40 µl. Antibody dilutions were prepared in PBS/2% (w/v) BSA.
Flow cytometry instrumentation and data analysis
Flow cytometric measurements were done on a FACSCalibur Flow Cytometer equipped with a blue (488 nm: excitation of FITC and Cy3) air-cooled argon ion laser and a red (635 nm: excitation of Cy5) diode laser. FITC was measured with a 530/30 bandpass filter, Cy3 with a 585/42 nm bandpass filter and Cy5 with a 661/16 nm bandpass filter. Acceptor-dye emission resulting from energy transfer (sensitized emission) was detected with a 670 long pass filter upon 635 nm excitation. Sample measurements and data analysis were performed using Cell Quest (BD Biosciences) on a Macintosh G3 computer. Data was stored in list mode format. Energy transfer efficiency (E) was determined on a cell-by-cell basis as previously described.44 Briefly, both donor quenching and acceptor sensitization were taken into account with corrections for spectral overspills and crosstalks.27 E values were calculated by ReFlex software45 and are presented as mean values from approximately normally distributed, unimodal energy transfer histograms.
Immunoblotting
For preparation of cell lysates for SDS-PAGE, 1 × 106 cells of BT474 or SK-BR-3 were seeded in T175 tissue culture flasks and incubated with therapeutic as indicated. Preparation of cell lysates, determination of protein concentration, SDS-PAGE, western blotting, chemiluminescent detection and data evaluation was done as previously described.27
For protein detection the following primary antibodies were used: rabbit anti-human HER2/ErbB2 polyclonal Ab (#2242), rabbit anti-human phospho-HER2/ErbB2 (Tyr877) polyclonal Ab (#2241), rabbit anti-human phospho-HER2/ErbB2 (Tyr1221/1222) polyclonal Ab (#2249), rabbit anti-human phospho-AKT (Ser473) (clone 193H12) mAb (#4058), rabbit anti-human phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204) (clone 197G2) mAb (#4377), rabbit anti-human phospho-STAT3 (Tyr705) (clone D3A7) mAb (#9145), rabbit anti-human β-actin (clone 13E5) mAb (#4970, all from Cell Signaling Technology), all at a dilution of 1:1,000 in AP-T buffer supplemented with 3% (w/v) BSA at 4°C. After washing blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (anti-rabbit IgG, HRP-linked antibody, dilution 1:1,000, Cell Signaling, #7074S) in AP-T buffer. Amersham ECL-Western Blotting Detection Reagent was used as chemiluminescence substrate for detection (GE Healthcare, #RPN 2209). Exposure time to X-ray film [Amersham Hyperfilm ECL (18 × 24cm), GE Healthcare, #28906837] was adjusted to an optimal signal-to-noise ratio. Signal intensity on X-ray films was quantified densitometrically with Image Quant V5.2 included in the software package IQ Solutions V1.31. The software allows bordering each band by two lines running along the respective lane, to sum up the optical density in between and to calculate the area of the resulting histogram peak. These values were used to calculate the ratio of phosphorylated vs. total Her2 protein and the obtained data were normalized to untreated samples that were set equal to one. Finally, the mean values of different experiments from independent cell lysates were calculated. For evaluation of phosphorylation of signaling proteins, the results of the area calculation were used to normalize treated samples to the control sample.
Microarray construction
Subconfluent growing cells were used for generation of cell pellets for antigen staining with IC or IF. Cells were starved for 24 h, then incubated with Na3VO4 (500 µg/ml, Sigma, # S6508) for 1 h and incubated for 10 min with ertumaxomab or trastuzumab as indicated. Cells were harvested by incubation with accutase (PAA Laboratories, #L11–007) for 10 min according to the manufacturer’s instructions, centrifuged for 3 min at 130 g, washed once in PBS and centrifuged again. Cells were fixed in 4% formalin (SG Planung) and incubated for 24 h at room temperature. Cells were transferred to a 15 ml tube and centrifuged for 8 min at 350 g. The cell pellet was mixed with two drops of Cytoblock Reagent 2 (Shandon Cytoblock Cell Block Preparation System, Thermo Electron Corporation). The cells were then transferred into a fresh tube containing four drops of Cytoblock Reagent 1. The entirely-coated cell pellet was transferred into a Cytoblock Cassette and incubated in 70% (v/v) ethanol for 6 h until further sample processing. Dehydration with an ascending alcohol series and embedding in paraffin was performed automatically with the HyperCenter Excelsior (ThermoFisher Scientific). The dehydration procedure consisted of two successive incubation steps each in 70% (entire incubation time: 1 min, 30 sec) and 96% (entire incubation time: 1 min, 50 sec), followed by three successive steps in 100% ethanol (entire incubation time: 2 min 30 sec). Finally, the samples were transferred to xylene for 1 min 40 sec in two successive steps and embedded in paraffin at 58°C. Microarrays were prepared as described previously.46 Core cylinders with a diameter of 1.5 mm each were punched from the main area of the donor block with a thin-walled stainless steel tube and deposited into a recipient paraffin block. Sections were sliced with a sliding microtome 4 µM thick and mounted on charged slides.
Immunochemistry (IC) and immunofluorescent (IF) staining
IC slides were dried 30 sec at 72°C and deparaffinated by washing in fresh xylene for 3 × 7 min at room temperature, followed by a descending alcohol series (2 min 100% propanol, 2 min 96% ethanol, 2 min 70% ethanol) and a final washing step in distilled water. Demasking of binding epitopes for the primary antibody was performed by microwave treatment at 100°C in citrate buffer and incubation of the slides for 30 min under these conditions. Slides were cooled down by incubation in an ice bath for 20 min. After washing 5 times in distilled water, IC staining was performed in accordance with the manufacturer's instructions. The following antibodies were used: rabbit anti-human c-erbB-2 oncoprotein polyclonal Ab (dilution: 1:400; #A0485), mouse anti-human c-erbB2 /Her2 (phospho-Y1248-specific) mAb-18 (Clone PN2A), (dilution 1:50; #M7269, both from DAKO), ChromeoTM 488 goat anti-rabbit IgG, (dilution 1:2,000; #15041), ChromeoTM 488 goat anti-mouse IgG, (dilution 1:2,000; #15031, both from Active Motif Chromeon). The staining procedure was performed by an automated IC slide staining system NexES® IC (software NexES v9.30, Ventana Medical Systems S.A.). Immunohistochemically stained samples were imaged with an Axio Imager Z.1 (Carl Zeiss MicroImaging) with a Plan-Apochromat lens (63 × , numerical aperture 1.4) and recorded with a CCD (charge-coupled device) camera AxioCam MRm. Images were recorded and digitally processed using AxioVision 4.5 software. Corresponding images were superimposed.
For fluorescence microscopy, slides were pretreated as described above. For the manual staining procedure (Table 1), cover plates (ThermoFisher Scientific) were used. Slides were mounted with DAPI containing mounting medium (Vectashield, #H-1200, Vector Laboratories) and imaged with a Leica DMI6000 B with fixed exposition times for all samples. Each color (for ChromeoTM 488 and DAPI) was recorded and digitally processed separately and corresponding images were superimposed. Equal exposition times to fluorescent excitation and similar conditions for imaging of each sample allowed qualitative evaluation of fluorescent stainings with antibodies, proven in IC to be specific.
Table 1. Manual staining procedure for fluorescence microscopy.
| Reagent | Volume [µl] | Incubation time [min] |
|---|---|---|
| Wash buffer (1:10 diluted) |
1000 |
5 |
| Primary antibody |
100 |
60 |
| Wash buffer (1:10 diluted) |
1000 |
5 |
| ChromeoTM 488 goat anti-rabbit or anti-mouse IgG |
100 |
45 |
| Wash buffer (1:10 diluted) | 1000 | 5 |
Statistical analysis
The Student's t-test for independently acquired samples was applied to calculate mean values ± standard deviation. Each measurement was performed at least in triplicate (n = 3) unless otherwise stated.
Acknowledgments
The authors wish to thank Rosa Kromas and Elisabeth Schmidt-Brücken (both Institute of Pathology, University of Regensburg, Germany) for their indispensable technical assistance. The study was sponsored by Fresenius Biotech GmbH, Munich, Germany.
Glossary
Abbreviations:
- FRET
fluorescence resonance energy transfer
- Fab
antigen binding fragment
- Fc
crystallizable fragment
- Ig
immunoglobulin
- ADCC
antibody-dependent cell-mediated cytotoxicity
- FcγR
Fc gamma receptor
- ADCP
antibody-dependent cellular phagocytosis
- Kd
dissociation constant
- Her2
human epidermal growth factor receptor 2
- IC
immunochemistry
- IF
immunofluorescence
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/21003
References
- 1.Harris M. Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol. 2004;5:292–302. doi: 10.1016/S1470-2045(04)01467-6. [DOI] [PubMed] [Google Scholar]
- 2.Stern HM. Improving treatment of HER2-positive cancers: opportunities and challenges. Sci Transl Med 2012;4:127rv2. doi: 10.1126/scitranslmed.3001539. [DOI] [PubMed]
- 3.Kiewe P, Thiel E. Ertumaxomab: a trifunctional antibody for breast cancer treatment. Expert Opin Investig Drugs. 2008;17:1553–8. doi: 10.1517/13543784.17.10.1553. [DOI] [PubMed] [Google Scholar]
- 4.Kiewe P, Hasmüller S, Kahlert S, Heinrigs M, Rack B, Marmé A, et al. Phase I trial of the trifunctional anti-HER2 x anti-CD3 antibody ertumaxomab in metastatic breast cancer. Clin Cancer Res. 2006;12:3085–91. doi: 10.1158/1078-0432.CCR-05-2436. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F, Dirix L, Conte PF, Semiglazov V, de Placido S, Jaeger M, et al. Phase II study of single agent trifunctional antibody ertumaxomab (Anti-HER-2 & Anti-CD3) in HER-2 low expressing hormone-refractory advanced breast cancer patients (ABC). San Antonio Breast Cancer Symposium 2010; P3-14-21. [Google Scholar]
- 6.Jäger M, Schoberth A, Ruf P, Hess J, Lindhofer H. The trifunctional antibody ertumaxomab destroys tumor cells that express low levels of human epidermal growth factor receptor 2. Cancer Res. 2009;69:4270–6. doi: 10.1158/0008-5472.CAN-08-2861. [DOI] [PubMed] [Google Scholar]
- 7.Zeidler R, Mysliwietz J, Csánady M, Walz A, Ziegler I, Schmitt B, et al. The Fc-region of a new class of intact bispecific antibody mediates activation of accessory cells and NK cells and induces direct phagocytosis of tumour cells. Br J Cancer. 2000;83:261–6. doi: 10.1054/bjoc.2000.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ströhlein MA, Siegel R, Jäger M, Lindhofer H, Jauch KW, Heiss MM. Induction of anti-tumor immunity by trifunctional antibodies in patients with peritoneal carcinomatosis. J Exp Clin Cancer Res. 2009;28:18. doi: 10.1186/1756-9966-28-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagy P, Bene L, Balázs M, Hyun WC, Lockett SJ, Chiang NY, et al. EGF-induced redistribution of erbB2 on breast tumor cells: flow and image cytometric energy transfer measurements. Cytometry. 1998;32:120–31. doi: 10.1002/(SICI)1097-0320(19980601)32:2<120::AID-CYTO7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Diermeier-Daucher S, Hasmann M, Brockhoff G. Flow cytometric FRET analysis of erbB receptor interaction on a cell-by-cell basis. Ann N Y Acad Sci. 2008;1130:280–6. doi: 10.1196/annals.1430.003. [DOI] [PubMed] [Google Scholar]
- 11.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 12.Seimetz D. Novel monoclonal antibodies for cancer treatment: the trifunctional antibody catumaxomab (removab) J Cancer. 2011;2:309–16. doi: 10.7150/jca.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chelius D, Ruf P, Gruber P, Plöscher M, Liedtke R, Gansberger E, et al. Structural and functional characterization of the trifunctional antibody catumaxomab. MAbs. 2010;2:309–19. doi: 10.4161/mabs.2.3.11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonagh CF, Huhalov A, Harms BD, Adams S, Paragas V, Oyama S, et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther. 2012;11:582–93. doi: 10.1158/1535-7163.MCT-11-0820. [DOI] [PubMed] [Google Scholar]
- 15.Kamath AV, Lu D, Gupta P, Jin D, Xiang H, Wong A, et al. Preclinical pharmacokinetics of MEHD7945A, a novel EGFR/HER3 dual-action antibody, and prediction of its human pharmacokinetics and efficacious clinical dose. Cancer Chemother Pharmacol. 2012;69:1063–9. doi: 10.1007/s00280-011-1806-6. [DOI] [PubMed] [Google Scholar]
- 16.Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 17.Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, et al. CLEOPATRA Study Group Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baselga J, Swain SM. CLEOPATRA: a phase III evaluation of pertuzumab and trastuzumab for HER2-positive metastatic breast cancer. Clin Breast Cancer. 2010;10:489–91. doi: 10.3816/CBC.2010.n.065. [DOI] [PubMed] [Google Scholar]
- 19.Ahn ER, Vogel CL. Dual HER2-targeted approaches in HER2-positive breast cancer. Breast Cancer Res Treat. 2012;131:371–83. doi: 10.1007/s10549-011-1781-y. [DOI] [PubMed] [Google Scholar]
- 20.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–6. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 21.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–37. doi: 10.1016/S1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 23.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–6. doi: 10.1158/0008-5472.CAN-03-3856. [DOI] [PubMed] [Google Scholar]
- 24.Klapper LN, Vaisman N, Hurwitz E, Pinkas-Kramarski R, Yarden Y, Sela M. A subclass of tumor-inhibitory monoclonal antibodies to ErbB-2/HER2 blocks crosstalk with growth factor receptors. Oncogene. 1997;14:2099–109. doi: 10.1038/sj.onc.1201029. [DOI] [PubMed] [Google Scholar]
- 25.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr., et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–60. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 26.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–28. doi: 10.1016/S1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 27.Diermeier S, Horváth G, Knuechel-Clarke R, Hofstaedter F, Szöllosi J, Brockhoff G. Epidermal growth factor receptor coexpression modulates susceptibility to Herceptin in HER2/neu overexpressing breast cancer cells via specific erbB-receptor interaction and activation. Exp Cell Res. 2005;304:604–19. doi: 10.1016/j.yexcr.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Scott GK, Dodson JM, Montgomery PA, Johnson RM, Sarup JC, Wong WL, et al. p185HER2 signal transduction in breast cancer cells. J Biol Chem. 1991;266:14300–5. [PubMed] [Google Scholar]
- 29.Ekerljung L, Steffen AC, Carlsson J, Lennartsson J. Effects of HER2-binding affibody molecules on intracellular signaling pathways. Tumour Biol. 2006;27:201–10. doi: 10.1159/000093023. [DOI] [PubMed] [Google Scholar]
- 30.Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–40. doi: 10.1016/S1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- 31.Zrihan-Licht S, Deng B, Yarden Y, McShan G, Keydar I, Avraham H. Csk homologous kinase, a novel signaling molecule, directly associates with the activated ErbB-2 receptor in breast cancer cells and inhibits their proliferation. J Biol Chem. 1998;273:4065–72. doi: 10.1074/jbc.273.7.4065. [DOI] [PubMed] [Google Scholar]
- 32.Dankort DL, Wang Z, Blackmore V, Moran MF, Muller WJ. Distinct tyrosine autophosphorylation sites negatively and positively modulate neu-mediated transformation. Mol Cell Biol. 1997;17:5410–25. doi: 10.1128/mcb.17.9.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bose R, Molina H, Patterson AS, Bitok JK, Periaswamy B, Bader JS, et al. Phosphoproteomic analysis of Her2/neu signaling and inhibition. Proc Natl Acad Sci U S A. 2006;103:9773–8. doi: 10.1073/pnas.0603948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang HT, O’Rourke DM, Zhao H, Murali R, Mikami Y, Davis JG, et al. Absence of autophosphorylation site Y882 in the p185neu oncogene product correlates with a reduction of transforming potential. Oncogene. 1998;16:2835–42. doi: 10.1038/sj.onc.1201820. [DOI] [PubMed] [Google Scholar]
- 35.Xu W, Yuan X, Beebe K, Xiang Z, Neckers L. Loss of Hsp90 association up-regulates Src-dependent ErbB2 activity. Mol Cell Biol. 2007;27:220–8. doi: 10.1128/MCB.00899-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colcher D, Pavlinkova G, Beresford G, Booth BJ, Choudhury A, Batra SK. Pharmacokinetics and biodistribution of genetically-engineered antibodies. Q J Nucl Med. 1998;42:225–41. [PubMed] [Google Scholar]
- 37.Rudnick SI, Lou J, Shaller CC, Tang Y, Klein-Szanto AJ, Weiner LM, et al. Influence of affinity and antigen internalization on the uptake and penetration of Anti-HER2 antibodies in solid tumors. Cancer Res. 2011;71:2250–9. doi: 10.1158/0008-5472.CAN-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldenberg MM. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther. 1999;21:309–18. doi: 10.1016/S0149-2918(00)88288-0. [DOI] [PubMed] [Google Scholar]
- 39.Park BW, Zhang HT, Wu C, Berezov A, Zhang X, Dua R, et al. Rationally designed anti-HER2/neu peptide mimetic disables P185HER2/neu tyrosine kinases in vitro and in vivo. Nat Biotechnol. 2000;18:194–8. doi: 10.1038/72651. [DOI] [PubMed] [Google Scholar]
- 40.Schroeder P, Lindemann C, Dettmar K, Brieger J, Gosepath J, Pogorzelski B, et al. Trifunctional antibodies induce efficient antitumour activity with immune cells from head and neck squamous cell carcinoma patients after radio-chemotherapy treatment. Clin Transl Oncol. 2011;13:889–98. doi: 10.1007/s12094-011-0751-5. [DOI] [PubMed] [Google Scholar]
- 41.Ruf P, Lindhofer H. Induction of a long-lasting antitumor immunity by a trifunctional bispecific antibody. Blood. 2001;98:2526–34. doi: 10.1182/blood.V98.8.2526. [DOI] [PubMed] [Google Scholar]
- 42.Morecki S, Lindhofer H, Yacovlev E, Gelfand Y, Ruf P, Slavin S. Induction of long-lasting antitumor immunity by concomitant cell therapy with allogeneic lymphocytes and trifunctional bispecific antibody. Exp Hematol. 2008;36:997–1003. doi: 10.1016/j.exphem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Brockhoff G, Heckel B, Schmidt-Bruecken E, Plander M, Hofstaedter F, Vollmann A, et al. Differential impact of Cetuximab, Pertuzumab and Trastuzumab on BT474 and SK-BR-3 breast cancer cell proliferation. Cell Prolif. 2007;40:488–507. doi: 10.1111/j.1365-2184.2007.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trón L, Szöllósi J, Damjanovich S, Helliwell SH, Arndt-Jovin DJ, Jovin TM. Flow cytometric measurement of fluorescence resonance energy transfer on cell surfaces. Quantitative evaluation of the transfer efficiency on a cell-by-cell basis. Biophys J. 1984;45:939–46. doi: 10.1016/S0006-3495(84)84240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szentesi G, Horváth G, Bori I, Vámosi G, Szöllosi J, Gáspár R, et al. Computer program for determining fluorescence resonance energy transfer efficiency from flow cytometric data on a cell-by-cell basis. Comput Methods Programs Biomed. 2004;75:201–11. doi: 10.1016/j.cmpb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Sassen A, Rochon J, Wild P, Hartmann A, Hofstaedter F, Schwarz S, et al. Cytogenetic analysis of HER1/EGFR, HER2, HER3 and HER4 in 278 breast cancer patients. Breast Cancer Res. 2008;10:R2. doi: 10.1186/bcr1843. [DOI] [PMC free article] [PubMed] [Google Scholar]