Abstract
The family of Eph tyrosine kinase receptors is an important part of signaling pathways involved in development, tissue homeostasis and tumorigenesis. Binding and activation of the receptors by their ligands, the ephrins, result in bidirectional signaling into both receptor and ligand expressing cells. Adult stem cell niches and tumors frequently express receptors and ligands, although their function is only beginning to be understood. Thus, Eph receptors and ephrins have become important molecules for understanding basic biological processes as well as tumorigenesis, and are promising targets for potential therapeutic intervention in human disease.
Introduction
The Eph receptors constitute the largest family of tyrosine kinase receptors with 14 members found in mammals. EphA1 was the first receptor of this family to be cloned in 1987 from an erythropoietin-producing hepatocellular carinoma cell line,1 but it would take several years before the Eph receptor-interacting proteins, the ephrins, were identified as Eph ligands.2,3 Ever since, there has been an exponential increase in the number of Eph/ephrin related scientific reports published each year,4 reflecting the growing interest and knowledge in Eph receptor biology. This review will provide an overview of Eph and ephrin function in normal tissue homeostasis, particularly focusing on their regulation of adult epithelial tissue stem cells, as well as discussing the different ways Ephs and ephrins modulate tissues and cells in tumor development and progression.
Eph Signaling Biology
The Eph/ephrin system is traditionally seen as a chemotactic/chemorepulsive guidance system, steering moving cells or axons to a specific position or maintaining cellular organization by preventing cell intermingling. Eph signaling can result in either repulsion or attraction and adhesion depending on the cellular context or developmental stage,5,6 outcomes most of which can be explained by Eph mediated rearrangement of the cellular cytoskeleton. There are however also findings pointing in the direction of Eph regulated cellular functions that are not explained by the regulation of the cytoskeleton, such as synapse formation, cell survival and proliferation.7
Ephrins are divided into the glycosylphosphatidylinositol (GPI)-anchored type A ephrins and the transmembrane type B ephrins, whereas receptors are grouped based on structural features in their ligand binding domain and their ephrin-binding preferences so that EphA receptors bind ephrin-A ligands and EphB receptors bind ephrin-Bs. It is now becoming increasingly clear that this distinction may be over simplified, as several Ephs are able to be activated by both ephrin-A and ephrin-Bs albeit at higher concentrations then their preferred ligand.8,9 Examples include EphA4 binding to ephrin-B2 and ephrin-B3,10,11 and EphB2 binding to ephrin-A5.8
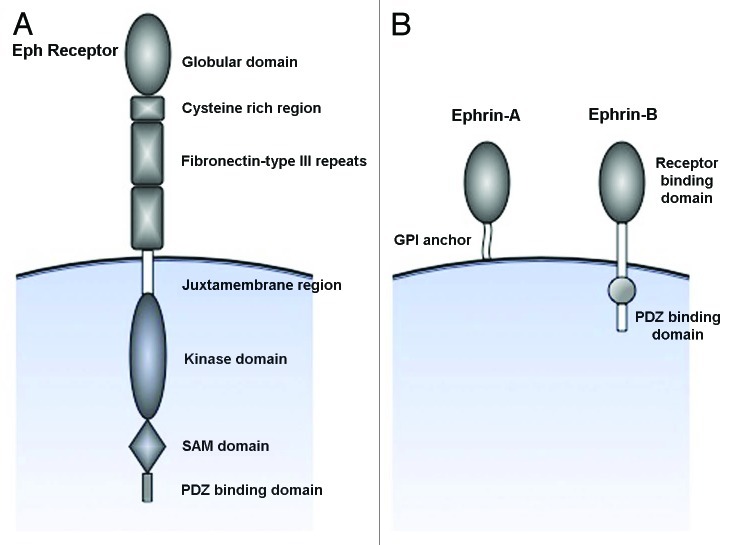
Ephrins bind Eph receptors with high affinity at sites of direct cell-to-cell contact, forming heterodimers which creates complementary interaction surfaces that result in the joining of dimer pairs into tetrameric complexes. The formation of high order complexes is necessary for proper activation and signaling to occur. Engagement of ligand to the receptor also induces a conformational change in the cytoplasmic portion of the Eph receptor12 by phosphorylation of tyrosine residues in the juxtamembrane domain, relieving the structural inhibition imposed by the juxtamembrane domain on the kinase domain, and allowing for activation of the kinase domain. Similarly, ephrin-B ligands also become phosphorylated at a conserved tyrosine residue in the cytoplasmic portion, resulting in a conformational change and activation of signaling.13-15 Ephrin-A ligands lack the cytoplasmic part present in the B class, and presumably convey intracellular signals through a co-receptor16 (Fig. 1A and B). The notion that signals cannot only be conveyed into the receptor-expressing cell (forward signaling) but also by the ligand-expressing cell (reverse signaling) adds an extra layer of complexity to the possible biological outcomes upon ligand-receptor binding.
Figure 1. Schematic representation of the structure of Eph receptors and ephrins. (A) Eph receptors are divided into two subclasses based on their sequence homology of the extracellular ephrin-binding globular domain. Eph receptors also have an extracellular cystein rich region followed by two fibronectin-type III repeats. The juxtamembrane domain contains tyrosines that can undergo autophosphrylation, and is followed by a tyrosine kinase domain. The SAM and PDZ binding domains serves as docking sites for interacting proteins and mediators of downstream signaling. (B) Ephrins are subdivided based on how they are linked to the membrane; ephrin-As are linked through a glycosylphosphatidyl anchor, whereas ephrin-Bs contain a transmembrane domain as well as a cytoplasmic tail involved in signaling processes.
Ephs and Ephrins in Adult Epithelial Stem Cell Niches
It is becoming more and more clear that Ephs and ephrins are not only crucial regulators of developmental processes, but also pivotal in controlling the physiology of adult organs and tumor initiation and progression. There have been an increasing number of studies focusing on the role of Ephs and ephrins in regulating adult stem cell function in various organs. Eph receptors and ephrins are commonly expressed in adult stem cell niches although their effect on the stem cells usually varies depending on the cellular context. EphB receptor forward signaling have been shown to regulate proliferation and migration of intestinal stem and progenitor cells17 whereas ephrin-A2 ligand reverse signaling negatively determines the rate of progenitor amplification in the adult subventricular zone in the later wall of the lateral brain.18 Hence, forward and reverse signaling have been shown to have direct and opposing roles on stem cell function. Similarly, Eph receptors and ephrins are present in most cancer cells. However, both increased and decreased expression has been linked to tumor progression, underlining the complexity of Eph/ephrin biology.
Intestine
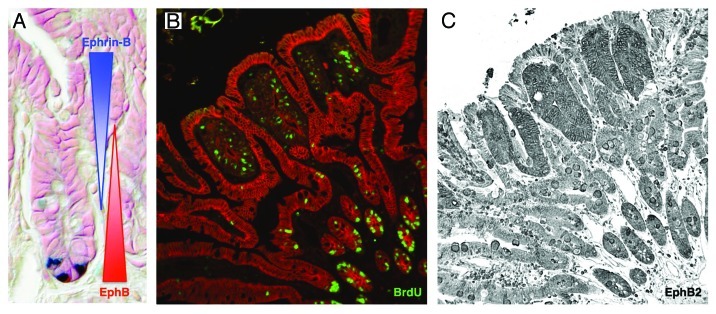
Perhaps the most well characterized adult epithelial stem cell system in terms of Eph function is the intestine. Stem cells reside at the bottom of crypts in the monolayered intestinal epithelium, where they divide and give rise to progenitor cells, which continue to divide as they migrate up the crypt axis. As cells leave the crypt, they also leave the cell cycle and start to differentiate. Canonical Wnt signaling is a pivotal mitogenic regulator for intestinal stem cells and it also transcriptionally regulates the expression of EphB receptors.19,20 EphB receptors and their ephrin-B ligands are expressed in counter gradients in the intestinal epithelium, where EphB2 and EphB3 are present at high levels in the intestinal stem cells (Fig. 2A). Progenitor cells express decreasing levels of EphB2 and EphB3 as they migrate away from the crypt bottom. Conversely, ephrin-B1 and ephrin-B2 is negatively regulated by Wnt signaling and is predominantly expressed by the differentiated cells outside the crypt with decreasing expression in cells closer to the crypt bottom. Genetic removal of EphB2 and EphB3 results in decreased stem/progenitor cell proliferation17 and independently to a misregulation of cell migration.19 Ephrin-B1 null mice also displays misplaced cells, however without any effect on cell proliferation.21
Figure 2. EphB expression in intestinal crypts and adenomas. (A) EphB receptors are expressed in a decreasing gradient as cells move up the crypt, whereas differentiating cells upregulate ephrin-B. Ligand and receptor expression form a counter gradient guiding the migrating progenitor cells and at the same time promoting their proliferation. Paneth cells (blue) are positioned at the bottom of the crypt, where they are in close contact with intestinal stem cells. (B) Highly proliferative adenoma cells incorporate BrdU and are compartmentalized by untransformed villi cells. (C) Intestinal tumor cells express high levels of EphB2, promoting their proliferating as well as suppressing tumor progression.
The intestine is one example where EphB receptors have been clearly demonstrated to have dual roles, acting both as tumor promoters and suppressors in the same organ, although at different stages of tumor progression. Gain-of-function mutations in the canonical Wnt pathway leads to the formation of intestinal adenomas characterized by high EphB expression leading to tumor cell proliferation. Cells forming the adenoma are surrounded and repelled by untransformed ephrin-B expressing cells keeping the adenoma in situ. As tumors progress toward carcinomas, EphB expression is downregulated22 and the ligand-imposed repulsion is lost allowing cells to invade the surrounding tissues (Fig. 2B and C). When the APCmin mouse model of intestinal tumorigenesis is crossed to mice lacking either EphB3, ephrin-B1 or mice expressing a dominant negative EphB2 receptor they develop predominantly more aggressive tumors, clearly suggesting that EphB receptors have tumor suppressor functions during tumor progression.21,22 Loss of EphB correlates not only to carcinoma transition in mice but also to shorter survival in patients.23,24
Mammary gland
Estrogen-dependent regulation of EphB4 and its preferred ligand ephrin-B2 have been suggested to regulate epithelial cell proliferation and mammary gland branching25,26 during the estrous cycle in mouse mammary epithelium. EphB4, as well as EphB2, have also been detected in epithelial cells from human breast tissue.27,28 Members of the A class family, EphA2 and its ligand ephrin-A1 are reported to be expressed by the mouse mammary epithelial cells,29 where genetic removal of EphA2 leads to reduced epithelial proliferation and branching. Interestingly, both EphA2 and EphB4 are commonly expressed in human breast cancer cell lines,30,31 and transfection of normal human breast epithelial cells with EphA2 is sufficient to induce transformation. When EphA2 is genetically removed, or EphB4 overexpressed, in a Neu overexpressing mouse model (MMTV-Neu), EphA2 deficiency impairs and EphB4 overexpression promotes tumor initiation and metastatic progression25,32 corroborating a role of Eph receptors as tumor promoters. In line with these findings are reports stating that in humans, high EphA2 and EphB2 expression correlate to poor disease prognosis27,33,34 although there are also indications that the tumors overexpressing Eph receptors might be the less malignant ones (similar to the epithelial tumors of the intestine, prostate and skin).28
Skin
In skin, Eph and ephrins are so far known to be expressed by two distinct epithelial stem cell populations. Adult hair follicle bulge stem cells express high levels of EphA4, EphB4 and ephrin-B135 whereas EphA2 and ephrin-A1 are detected in a complimentary pattern in the epidermis, allowing for signaling to occur only in the proliferative stem cell-harboring basal layer.36 Proliferation in both epithelial adult stem cell compartments is negatively regulated by Ephs and ephrins37 and hence genetic removal of ephrin-B2 results in increased epidermal proliferation.38 Similarly, human epidermis ubiquitously expresses several Eph receptors and ligands from both classes, and only EphA7 has been shown to be exclusively localized to the cells in the basal layer.39
In contrast to the mammary epithelium, genetic ablation of EphA2 in the skin epithelium leads to increased tumor cell proliferation and progression to invasive squamous cell carcinomas in a DMBA/TPA carcinogenesis assay36 suggesting that EphA2 acts as a tumor suppressor in the skin.
Prostate
In human prostate cancer, EphA2 and EphB4 are found to be upregulated when compared with untransformed tissue40,41 whereas mutations leading to the inactivation of EphB2 have been found in about 8% of prostate tumors.42 Reintroduction of EphB2 suppresses clonogenic growth of metastatic tumor cells, suggesting that EphB2 might be important in the progression and metastasis of prostate cancer. Furthermore, silencing of EphA7 through promoter methylation has been reported and shown to correlate to tumor progression43 suggesting that different members of Eph receptors act discretely to influence cell growth and tumor progression.
Eph Signaling in Cancer Cells
Cancer cells seem to have different mechanisms to minimize the tumor suppressor functions of Eph forward signaling. Taking into account the relatively high levels of EphB4 and EphA2 in breast cancer cells, the level of receptor tyrosine phosphorylation is lower in cancer cells than in untransformed breast epithelial cell lines30,31 although endogenous ligands are present at low levels. Stimulation of the receptors using soluble ligands could however enhance receptor tyrosine phosphorylation and inhibit receptor-mediated transformation and tumorigenesis. These data suggest that silencing of kinase dependent functions of Eph receptors in breast cancer cells is mediated through downregulation of the ephrin ligand. Loss of ephrin-B2 has been reported in mammary tumor models as well as in colorectal cancer cells,20,44 suggesting that cancer cells downregulate ephrins in order to avoid the tumor suppressor functions of Eph receptors. Alternatively, as seen in developing colon cancers, Eph expressing tumor cells are compartmentalized by the ligand expressing cells, allowing for little contact and hence receptor forward signaling between transformed and non-transformed cells.
However, kinase-dependent as well as -independent functions have been demonstrated in both colorectal and prostate cancer cells45,46 suggesting that Eph receptors are able to convey signals even in the apparent lack of ligand activation. Furthermore, Eph receptors have been shown to be able to bind the p100γ PI 3-kinase subunit in a kinase-independent manner47 and PI 3-kinase signaling has been shown to influence the in vivo cell sorting of intestinal progenitor cells in an EphB kinase dead mouse model.48 It is possible that in contrast to oncogenic mechanisms proposed for other receptor tyrosine kinases, Eph receptors may exert parts of their tumor promotor functions independently of receptor phosphorylation or that Eph receptor clustering due to high level of expression lead to somewhat increased kinase activity even in the absence of ligand.
Perspectives
Our understanding of the complex roles of Eph and ephrins in stem cell and tumor biology is still evolving, and more research is needed to resolve contradictory and confusing issues. The range of activities, interaction partners and molecular mechanisms underlying Eph and ephrin function has steadily increased, resulting in a continuous revision of established models. Characterization of Eph signaling complexes and pathways are necessary to fully understand the outcome of Eph/ephrin signaling on a cell, and to fully exploit the possible avenues in designing Eph and ephrin targeting drugs in order to specifically be able to inhibit growth and progression of tumors.
Acknowledgments
I thank Jonas Frisén for valuable comments on the manuscript. M.G.’s work was supported by the Damon Runyon Cancer Research Foundation.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/18932
References
- 1.Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717–20. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- 2.Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, et al. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–9. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- 3.Beckmann MP, Cerretti DP, Baum P, Vanden Bos T, James L, Farrah T, et al. Molecular characterization of a family of ligands for eph-related tyrosine kinase receptors. EMBO J. 1994;13:3757–62. doi: 10.1002/j.1460-2075.1994.tb06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lackmann M, Boyd AW. Eph, a protein family coming of age: more confusion, insight, or complexity? Sci Signal. 2008;1:re2. doi: 10.1126/stke.115re2. [DOI] [PubMed] [Google Scholar]
- 5.Holmberg J, Clarke DL, Frisen J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature. 2000;408:203–6. doi: 10.1038/35041577. [DOI] [PubMed] [Google Scholar]
- 6.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Himanen JP, Saha N, Nikolov DB. Cell-cell signaling via Eph receptors and ephrins. Curr Opin Cell Biol. 2007;19:534–42. doi: 10.1016/j.ceb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, et al. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–9. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- 9.Lackmann M, Mann RJ, Kravets L, Smith FM, Bucci TA, Maxwell KF, et al. Ligand for EPH-related kinase (LERK) 7 is the preferred high affinity ligand for the HEK receptor. J Biol Chem. 1997;272:16521–30. doi: 10.1074/jbc.272.26.16521. [DOI] [PubMed] [Google Scholar]
- 10.Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/S0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 11.Gale NW, Flenniken A, Compton DC, Jenkins N, Copeland NG, Gilbert DJ, et al. Elk-L3, a novel transmembrane ligand for the Eph family of receptor tyrosine kinases, expressed in embryonic floor plate, roof plate and hindbrain segments. Oncogene. 1996;13:1343–52. [PubMed] [Google Scholar]
- 12.Wybenga-Groot LE, Baskin B, Ong SH, Tong J, Pawson T, Sicheri F. Structural basis for autoinhibition of the Ephb2 receptor tyrosine kinase by the unphosphorylated juxtamembrane region. Cell. 2001;106:745–57. doi: 10.1016/S0092-8674(01)00496-2. [DOI] [PubMed] [Google Scholar]
- 13.Brückner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–3. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- 14.Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–5. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- 15.Kalo MS, Yu HH, Pasquale EB. In vivo tyrosine phosphorylation sites of activated ephrin-B1 and ephB2 from neural tissue. J Biol Chem. 2001;276:38940–8. doi: 10.1074/jbc.M105815200. [DOI] [PubMed] [Google Scholar]
- 16.Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF, O'Leary DD. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–58. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmberg J, Genander M, Halford MM, Anneren C, Sondell M, Chumley MJ, et al. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125:1151–63. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Holmberg J, Armulik A, Senti KA, Edoff K, Spalding K, Momma S, et al. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005;19:462–71. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–63. doi: 10.1016/S0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 20.Clevers H, Batlle E. EphB/EphrinB receptors and Wnt signaling in colorectal cancer. Cancer Res. 2006;66:2–5. doi: 10.1158/0008-5472.CAN-05-3849. [DOI] [PubMed] [Google Scholar]
- 21.Cortina C, Palomo-Ponce S, Iglesias M, Fernandez-Masip JL, Vivancos A, Whissell G, et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376–83. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- 22.Batlle E, Bacani J, Begthel H, Jonkheer S, Gregorieff A, van de Born M, et al. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–30. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- 23.Davalos V, Dopeso H, Castano J, Wilson AJ, Vilardell F, Romero-Gimenez J, et al. EPHB4 and survival of colorectal cancer patients. Cancer Res. 2006;66:8943–8. doi: 10.1158/0008-5472.CAN-05-4640. [DOI] [PubMed] [Google Scholar]
- 24.Guo DL, Zhang J, Yuen ST, Tsui WY, Chan AS, Ho C, et al. Reduced expression of EphB2 that parallels invasion and metastasis in colorectal tumours. Carcinogenesis. 2006;27:454–64. doi: 10.1093/carcin/bgi259. [DOI] [PubMed] [Google Scholar]
- 25.Munarini N, Jager R, Abderhalden S, Zuercher G, Rohrbach V, Loercher S, et al. Altered mammary epithelial development, pattern formation and involution in transgenic mice expressing the EphB4 receptor tyrosine kinase. J Cell Sci. 2002;115:25–37. doi: 10.1242/jcs.115.1.25. [DOI] [PubMed] [Google Scholar]
- 26.Nikolova Z, Djonov V, Zuercher G, Andres AC, Ziemiecki A. Cell-type specific and estrogen dependent expression of the receptor tyrosine kinase EphB4 and its ligand ephrin-B2 during mammary gland morphogenesis. J Cell Sci. 1998;111:2741–51. doi: 10.1242/jcs.111.18.2741. [DOI] [PubMed] [Google Scholar]
- 27.Wu Q, Suo Z, Risberg B, Karlsson MG, Villman K, Nesland JM. Expression of Ephb2 and Ephb4 in breast carcinoma. Pathol Oncol Res. 2004;10:26–33. doi: 10.1007/BF02893405. [DOI] [PubMed] [Google Scholar]
- 28.Berclaz G, Flutsch B, Altermatt HJ, Rohrbach V, Djonov V, Ziemiecki A, et al. Loss of EphB4 receptor tyrosine kinase protein expression during carcinogenesis of the human breast. Oncol Rep. 2002;9:985–9. [PubMed] [Google Scholar]
- 29.Vaught D, Chen J, Brantley-Sieders DM. Regulation of mammary gland branching morphogenesis by EphA2 receptor tyrosine kinase. Mol Biol Cell. 2009;20:2572–81. doi: 10.1091/mbc.E08-04-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noren NK, Foos G, Hauser CA, Pasquale EB. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol. 2006;8:815–25. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- 31.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–6. [PubMed] [Google Scholar]
- 32.Brantley-Sieders DM, Zhuang G, Hicks D, Fang WB, Hwang Y, Cates JM, et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fournier MV, Martin KJ, Kenny PA, Xhaja K, Bosch I, Yaswen P, et al. Gene expression signature in organized and growth-arrested mammary acini predicts good outcome in breast cancer. Cancer Res. 2006;66:7095–102. doi: 10.1158/0008-5472.CAN-06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin KJ, Patrick DR, Bissell MJ, Fournier MV. Prognostic breast cancer signature identified from 3D culture model accurately predicts clinical outcome across independent datasets. PLoS ONE. 2008;3:e2994. doi: 10.1371/journal.pone.0002994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo H, Miao H, Gerber L, Singh J, Denning MF, Gilliam AC, et al. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006;66:7050–8. doi: 10.1158/0008-5472.CAN-06-0004. [DOI] [PubMed] [Google Scholar]
- 37.Genander M, Holmberg J, Frisen J. Ephrins negatively regulate cell proliferation in the epidermis and hair follicle. Stem Cells. 2010;28:1196–205. doi: 10.1002/stem.442. [DOI] [PubMed] [Google Scholar]
- 38.Egawa G, Osawa M, Uemura A, Miyachi Y, Nishikawa SI. Transient expression of ephrin b2 in perinatal skin is required for maintenance of keratinocyte homeostasis. J Invest Dermatol. 2009;129:2386–95. doi: 10.1038/jid.2009.105. [DOI] [PubMed] [Google Scholar]
- 39.Hafner C, Becker B, Landthaler M, Vogt T. Expression profile of Eph receptors and ephrin ligands in human skin and downregulation of EphA1 in nonmelanoma skin cancer. Mod Pathol. 2006;19:1369–77. doi: 10.1038/modpathol.3800660. [DOI] [PubMed] [Google Scholar]
- 40.Xia G, Kumar SR, Masood R, Zhu S, Reddy R, Krasnoperov V, et al. EphB4 expression and biological significance in prostate cancer. Cancer Res. 2005;65:4623–32. doi: 10.1158/0008-5472.CAN-04-2667. [DOI] [PubMed] [Google Scholar]
- 41.Walker-Daniels J, Coffman K, Azimi M, Rhim JS, Bostwick DG, Snyder P, et al. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275–80. doi: 10.1002/(SICI)1097-0045(19991201)41:4<275::AID-PROS8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 42.Huusko P, Ponciano-Jackson D, Wolf M, Kiefer JA, Azorsa DO, Tuzmen S, et al. Nonsense-mediated decay microarray analysis identifies mutations of EPHB2 in human prostate cancer. Nat Genet. 2004;36:979–83. doi: 10.1038/ng1408. [DOI] [PubMed] [Google Scholar]
- 43.Guan M, Xu C, Zhang F, Ye C. Aberrant methylation of EphA7 in human prostate cancer and its relation to clinicopathologic features. Int J Cancer. 2009;124:88–94. doi: 10.1002/ijc.23890. [DOI] [PubMed] [Google Scholar]
- 44.Andres AC, Ziemiecki A. Eph and ephrin signaling in mammary gland morphogenesis and cancer. J Mammary Gland Biol Neoplasia. 2003;8:475–85. doi: 10.1023/B:JOMG.0000017433.83226.22. [DOI] [PubMed] [Google Scholar]
- 45.Miao H, Strebhardt K, Pasquale EB, Shen TL, Guan JL, Wang B. Inhibition of integrin-mediated cell adhesion but not directional cell migration requires catalytic activity of EphB3 receptor tyrosine kinase. Role of Rho family small GTPases. J Biol Chem. 2005;280:923–32. doi: 10.1074/jbc.M411383200. [DOI] [PubMed] [Google Scholar]
- 46.Taddei ML, Parri M, Angelucci A, Onnis B, Bianchini F, Giannoni E, et al. Kinase-dependent and -independent roles of EphA2 in the regulation of prostate cancer invasion and metastasis. Am J Pathol. 2009;174:1492–503. doi: 10.2353/ajpath.2009.080473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu C, Park S. The EphA8 receptor regulates integrin activity through p110gamma phosphatidylinositol-3 kinase in a tyrosine kinase activity-independent manner. Mol Cell Biol. 2001;21:4579–97. doi: 10.1128/MCB.21.14.4579-4597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Genander M, Halford MM, Xu NJ, Eriksson M, Yu Z, Qiu Z, et al. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell. 2009;139:679–92. doi: 10.1016/j.cell.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]