Abstract
Eph receptors and their membrane-bound ligands are intimately involved in the control of morphogenic processes during embryonic development and adult tissue homeostasis. By their ability to orchestrate cell migration, pattern formation and tissue integrity they are also prone to be involved in carcinogenic growth. In this review we concentrate on their involvement in the normal and carcinogenic development of the breast. In this context we summarize their multi-faceted functions as tumor suppressors, tumor promoters, angiogenic inducers and regulators of stem cell homeostasis.
Keywords: angiogenesis, differentiation, EphA, EphB, mammary gland, stem cells, tumorigenesis
Introduction
The large families of signaling molecules, Eph and ephrin, play a key role in the specification of cell fate, mobility, lineage determination and compartmentalization of cell populations. The Eph receptors and their ephrin ligands can be divided into two classes, A and B, based on sequence similarity, common structural features and binding affinities. The EphA receptors interact in general with ephrin-A ligands which are attached to the plasma membrane by a glycosylphosphatidylinositol (GPI) tail. EphB receptors bind preferentially to ephrin-B ligands which are classical transmembrane proteins. Complexes between Eph receptors and the ephrin ligands, assembled through cell-cell contact, transduce forward signals from the Eph receptors and reverse signals from the ephrin ligands, a signaling mechanism referred to as bidirectional signaling. The classical forward signal is initiated when ligand binding initiates oligomerization of the receptor and activates its kinase catalytic domain leading to phosphorylation of the tyrosine residues in the intracellular part of the Eph receptor. At the same time, the ephrin-Bs can also initiate signaling as a consequence of phosphorylation of the five conserved tyrosine residues in the cytoplasmic domain. Despite lacking a cytoplasmic domain, the ephrin-A ligands can also transduce reverse signals through interaction with integrins and src family members or, as shown in neurons, by employing co-receptors to specify the reverse signal.1,2 Although certain preferences exist, receptor ligand binding is highly promiscuous and signaling can be further modulated by the formation of heterodimers between different EphA or EphB receptors or even between EphA and EphB receptors.3 Moreover, Ephs and ephrins can also function independently by cross-talk with a variety of other signal transduction pathways. With respect to mammary gland biology and carcinogenesis, pathways including signaling elicited by wnt’s,4 integrins,5 E-cadherin,6,7 FGFs,5 EGFs and especially their receptors HER-1 and HER-2,8-10 as well as ILGF,11 are noteworthy, since all are major regulators of mammary epithelial growth and differentiation. In addition, ephrin-B ligands are capable of switching from a tyrosine-phosphorylation-dependent reverse signaling to PDZ domain-dependent signaling. Ephrin-B ligands thereby interact with G-protein signaling via PDZ-RGS3 or they can operate in a serine-phosphorylation-dependent manner binding the adaptor protein GRIP.12-14 As complex as the signaling cascades affecting or affected by Ephs and ephrins are, equally as wide are the variations in cellular responses, ranging from cell death and survival to cellular movements, adhesion and repulsion.1 Thus, it is not astonishing that this family of molecules is involved in many aspects of both normal and particularly carcinogenic developmental processes. In the following review, we will concentrate on their often controversial involvement in the development of breast cancer.
The Tumor Suppressing Role of Eph/Ephrin Signaling
Evidence that Eph or ephrin genes act as tumor suppressor genes in breast carcinogenesis has been found for the EphA2, EphB4 and EphB6 receptors. It has been shown that EphA2 negatively regulates tumor growth after interaction with its preferred ligand ephrin-A1.15 Similarly, treatment of breast cancer cell lines overexpressing EphA2 with soluble ephrin-A1 ligand suppresses their growth in vivo and in vitro.15 EphA2 is a direct transcriptional target of the ras-raf-MAPK pathway and functions after interaction with ephrin-A1 as a negative feed-back regulator of growth factor-activated ras signaling.16 Additionally, it has recently been shown that ligand-stimulated EphA2 also attenuates the Akt-mTor survival pathway in prostate cancer cells. This tumor suppressing function, however, does not appear to operate in breast cancer cell lines.17 Therapeutically, it has been shown that activating EphA2-specific antibodies which mimic the action of ephrin-A1, reduce growth of EphA2 overexpressing tumor cells in culture.18 Of the A-class receptors, EphA5 has also been ascribed a tumor suppressing function, since expression profiling analyses revealed its downregulation in cancerous vs. normal human breast epithelium.19 There is, however, no experimental evidence demonstrating this inhibitory role.
EphB4 forward signaling also exerts tumor suppressing functions by reducing cell viability, proliferation, motility and invasion. After interaction with its cognate ligand ephrin-B2, EphB4 forward signaling activates the anti-oncogenic Abl-Crk pathway and downregulates the expression of the matrix metalloprotease MMP-2.20 Interestingly, EphB4 expression has been found to be downregulated in the majority of tumor cells of human breast carcinomas, while a minority of cells at the periphery of the tumor mass exhibited strong overexpression of this receptor.21 This observation may point to the dual role of Ephs in tumor progression (see below).
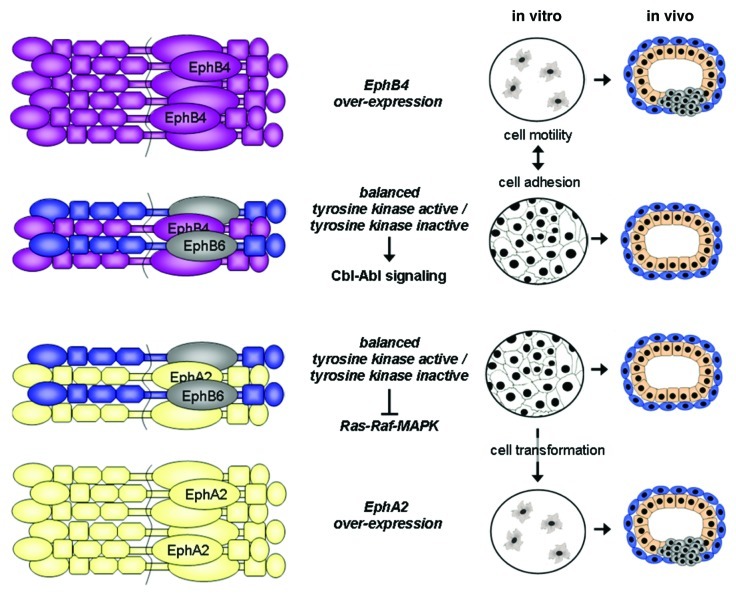
The most clear cut tumor suppressing function is exerted by EphB6. EphB6 is one of the two family members lacking kinase activity due to six amino acid alterations in the kinase domain. The receptor has a high affinity for ephrin-B1 and ephrin-B2 and is phosphorylated by and participates in signal transduction by forming heterodimers with EphB1 and EphB4.22 In addition, the formation of EphB6-EphA2 heterodimers has been described recently, representing the first evidence that A-type receptors can interact with B-type receptors.23 EphB6 is highly expressed in normal mammary epithelial cells and non-invasive breast neoplasias, however, it is downregulated or absent in invasive and metastatic breast cancer.24,25 This downregulation is accomplished at the transcriptional level by hypermethylation of the EphB6 gene promoter.26 Thus, EphB6 seems to be intimately involved in the suppression of invasive and metastatic cancer growth. Interestingly, interaction with ephrin-B2 leads to heterodimer formation between EphB4 and EphB6, the latter being transphosphorylated and initiating the signaling of the Cbl-Abl pathway, thereby favoring adhesion vs. motility.27 These observations may illustrate the mechanism of the observed tumor-suppressing function of EphB4 described by Noren et al.20 and probably also of EphA215 suggesting that the outcome of the transformation process depends on the balance between EphB6 and kinase-active Eph receptor expression (Fig. 1).
Figure 1. Schematic representation of possible Eph receptor interactions involved in the tumor suppressing mechanisms of Eph/ephrin signaling. The kinase-dead EphB6 receptor forms ligand-dependent hetero-oligomers with EphB4 or EphA2 which then activate the Cbl-Abl or inhibit the Ras-Raf-MAPK pathways, respectively, leading to growth arrest and cell adhesion. An imbalance between kinase active and inactive receptors as in the absence of EphB6 or with overexpression of EphB4 or EphA2 can lead to ligand-independent receptor activation, and stimulation of cell growth and migration.
Eph/Ephrin Signaling Promotes Carcinogenesis
Overexpression of Eph receptors has been described in a variety of cancer types.1 In breast cancer, RNA profiling studies revealed that the overexpression of EphA2, EphA4, EphA7 and EphB2 is correlated with overall and disease-free survival, while conflicting data are available about the clinical significance of EphB4 overexpression.21,28,29 Interestingly, EphB10, the other kinase-dead member of this family with similar but not identical amino acid changes as EphB6, is overexpressed in metastatic breast epithelial cells and thus, seems to exert a tumor promoting effect.24 Of all the members of the Eph/ephrin family, the role of EphA2 and EphB4 has been most extensively studied.
EphA2 is overexpressed in about 60–80% of breast cancers29-31 and may be associated with an estrogen receptor-positive status.32 Moreover, EphA2 is involved in the development of resistance toward treatment with trastuzumab, a monoclonal antibody directed against the HER2 receptor. Interestingly, prolonged exposure to trastuzumab seems to activate the src kinase which phosphorylates EphA2 thereby activating the PI3K/Akt survival and the mitogenic PI3K/MAPK pathways.10 Experimentally, overexpression of EphA2 was sufficient to transform non-malignant MCF-10 human breast epithelial cells,31 while the expression of a dominant negative EphA2 mutant reversed the metastatic phenotype of 4T1 metastatic mouse mammary tumor cells.33 Moreover, crossing transgenic MMTV-Neu (murine homolog of HER2) mice, which develop invasive mammary tumors, with EphA2 deficient animals prevented tumor initiation and invasion.34 The mechanism(s) involved in this obvious tumor-promoting effect of EphA2 are not yet completely understood. It is conceivable that the loss of E-cadherin mediated cell contacts in tumor cells prevents the interaction between EphA2 and ephrin-A1 on neighboring cells and thereby abolishes its tumor suppressing function. High levels of un-engaged EphA2 receptors have the ability to interact with HER1 and HER2 receptors, thereby activating mitogenic pathways.8,34 In addition, EphA2 stimulation by EGF leads to its binding to the guanine nucleotide exchange factor ephexin-4 which in turn activates the formation of lamellipodia protrusions and cellular motility.9 Thus, blocking EphA2 activity could provide an effective treatment of EphA2 induced tumor growth. Attempts in this direction include the administration of siRNAs downregulating EphA2 expression as well as ephrin-A1-coupled cytotoxins to kill EphA2 overexpressing cells.35-37 Moreover, the kinase inhibitor Dasatinib inhibits, among others, also the kinase activity of EphA2 and may thereby contribute to its synergizing effect with doxorubicin in the treatment of breast cancer.38 In addition, magnetic ephrin-A1 presenting nanoparticles have been developed to remove circulating EphA2 expressing tumor cells.39
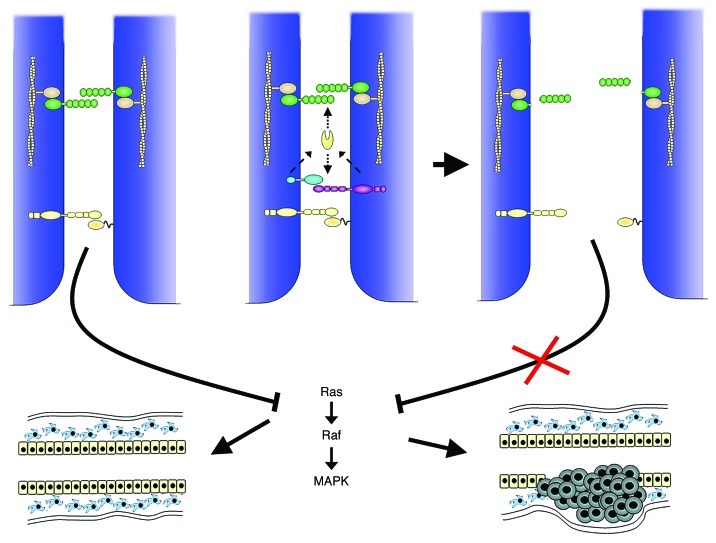
EphB4 is overexpressed in up to 58% of human breast tumors and it has been found that this overexpression is driven either by gene amplification or by crosstalk with HER1.29,40 There is substantial evidence that EphB4 acts as a tumor promoting oncogene. EphB4 is (over)-expressed in the majority of human breast cancer cell lines and its knock-down by siRNA led to reduced proliferation and increased apoptosis in vitro and in vivo.40 More recently, it has been demonstrated that one of the tumor promoting effects of EphB4, the induction of anchorage independence and motility, does not depend on the activation by its cognate ligand ephrin-B2, however, requires the phosphorylation of EphB4 intracellular tyrosines.41 This demonstrates that the activity of Eph receptors is not only brought about by their ligand but can also be induced by other signaling cascades thereby altering the cellular response. In support of this notion, Rutkowski et al.42 recently have shown that overexpression of EphB4 in non-malignant MCF-10 human breast epithelial cells is sufficient to induce anchorage independent growth, migration and invasion. This effect could be reversed by the addition of the ephrin-B2 ligand, partly through downregulation of the EphB4 protein levels. In vivo, overexpression of EphB4 or ephrin-B2 is not sufficient to transform mammary epithelial cells. Transgenic mice exhibiting MMTV-driven EphB4 expression in the mammary epithelium did not develop mammary tumor formation. However, when crossed with MMTV-NeuT transgenic mice, developing non-metastasizing tumors after a latency time of 6 mo, the NeuT induced tumor latency was significantly reduced and the tumors were rapidly metastasizing to the lung.43 Overexpression of ephrin-B2 in the same experimental setting was not able to alter either the tumor latency or growth characteristics. In contrast, epithelial overexpression of a dominant negative ephrin-B2 mutant, capable of receptor interaction but unable to elicit reverse signaling, conferred aggressive and metastatic growth on NeuT induced tumors in a similar fashion as EphB4.44 These results demonstrate that deregulated EphB4/ephrin-B2 signaling promotes tumor progression. Moreover, they support the notion that their tumor promoting function is only effective in the absence of ephrin-B2 reverse signaling. It seems that in the context of the mammary gland, epithelial ephrin-B2 expression exerts a surveillance function controlling the responses to EphB4 activation. The underlying mechanism is not completely clear. Interference of deregulated EphB4/ephrin-B2 signaling with the E-cadherin mediated cell contacts seems highly probable, since overexpression of EphB4 or the dominant negative ephrin-B2 mutant led to the downregulation and cytoplasmic localization of E-cadherin as well as impaired polarization in the single transgenic animals.44 Indeed, it has been shown, that EphB’s can complex and activate the membrane-bound protease ADAM10, which is not only capable of cleaving Eph/ephrin complexes45 but also leads to the shedding of E-cadherin from the cell membrane.46 Moreover, Eph/ephrin signaling is involved in the assembly of tight junctions during the polarization process of epithelial cells.47 Thus, by interfering with the formation of cell-cell contacts, deregulated EphB4 signaling may favor cell motility and invasiveness. A potential downstream effect on the EphA2/ephrin-A1 interaction is also conceivable and might reveal a new facet of EphB/ephrin-B and EphA/ephrin-A inter-dependency (Fig. 2).
Figure 2. Schematic model summarizing a possible scenario of the tumor promoting mechanism involving simultaneous EphA/ephrin-A and EphB/ephrin-B signaling. E-cadherin-mediated cell adhesion (green) allows the interaction between EphAs and ephrin-As (yellow), thereby blocking the MAPK induced cell proliferation. EphB (purple)/ephrin-B (blue) interactions can lead to the activation of the ADAM10 protease (yellow ovoid) which cleaves not only the Eph complex but also the extracellular part of E-cadherin. As a consequence cell adhesion is disrupted and impedes the interaction of EphAs with their ligands and thus relieves the block of MAPK activation promoting tumor formation/growth.
Eph/Ephrin Signaling and Tumor Angiogenesis
Blood vessel formation is indispensable for cancer growth, invasion and metastasis formation. As hyperplastic nodules or tumors grow, they require their own vasculature in order to ensure sufficient supply of nutrients and oxygen. In addition, the presence of intra-tumoral vessels is the major requirement for metastatic spreading of evading tumor cells. Members of the Eph-ephrin family, in particular EphB4 and ephrin-B2, have been implicated not only in the development of the embryonic vasculature but also in neo-vascularization in the adult.48 Endothelial expression of EphB4 and ephrin-B2 ensure the critical communication between arterial and venous endothelia whereas non-endothelial expression is instrumental in endothelial cell attraction and guidance.48 In addition, ephrin-B2 is instrumental in the spatial activation of VEGF-receptor internalization, thereby regulating the endothelial tip cell filopodial extension.49,50 Thus, it is evident that Eph/ephrin signaling must be involved in breast carcinogenesis also by their capacity to induce and guide tumor angiogenesis. Again, EphA2/ephrinA1 and EphB4/ephrin-B2 signaling seem to play a central role. In this scenario, however, the tumor cell derived EphA2 expression is not the driving force, but rather the EphA2 receptor localized on the endothelial cells stimulated by the tumor-derived ephrin-A1 ligand. It has been shown that tumor cells xenografted into the mammary gland of EphA2 knockout mice exhibit a reduced microvascular density and consequently also reduced growth.51 In addition, downregulation of ephrin-A1 in metastatic tumor cells reduced the intratumoral microvascular density and metastasis formation after implantation into a normal host environment. In contrast, overexpression of ephrin-A1 in non-metastatic tumor cells increased the microvascular density and endothelial attraction after implantation into a normal but not into an EphA2 deficient host environment.52 These experiments indicate that tumor derived ephrin-A1 promotes angiogenesis by stimulating EphA2 on endothelial cells.
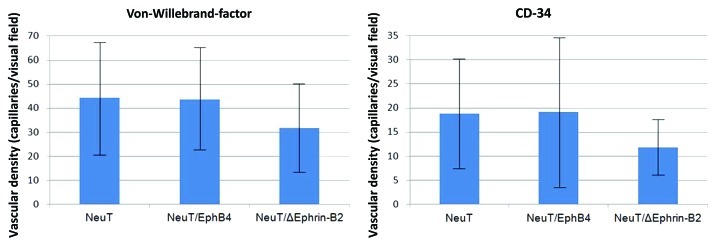
Ephrin-B2 expression has been found in the microvasculature surrounding and within a variety of solid tumors including breast cancers.53 Thus, it is conceivable that tumor cell derived EphB4 expression may stimulate angiogenesis by interacting with endothelial ephrin-B2 ligands. Indeed, overexpression of a cytoplasmatically truncated EphB4 receptor on human breast cancer cells resulted in accelerated growth of tumors with significantly enlarged blood vessels in a mouse xenograft model. In vitro, the engineered tumor cells promoted endothelial migration, proliferation and survival.54 These results indicate that angiogenesis is independent of EphB4 forward signaling but requires the reverse signaling of ephrin-B2 in endothelial cells. Therapeutically, small interfering peptides antagonizing ephrin-B2 action, have been shown to inhibit capillary tube formation of primary human endothelial cells and thus may be also effective in the inhibition of tumor angiogenesis in vivo.55,56 The reciprocal stimulation, tumor cell-derived ephrin-B2 stimulation of endothelial EphB4 seems also to be effective, since EphB4 activation on circulating endothelial precursors increases their recruitment to sites of neo-vascularization.57 In agreement with these observations, we have found that the deregulated EphB4 and ephrin-B2 expression in the mammary epithelium of transgenic mice not only affects the epithelium but also the architecture of the mammary vasculature.58 Thus, the acquisition of a metastatic phenotype of NeuT induced tumors by the additional expression of EphB4 or a dominant negative ephrin-B2 mutant43,44 might also have been brought about by a stimulation of tumor angiogenesis. Strikingly, determination of the microvascular density by immuno-histochemical detection of von Willebrand-factor for mature vessels or CD34 for developing capillaries did not support this hypothesis. The microvascular density was similar between the NeuT and the NeuT/EphB4 derived tumors and the highly metastasizing NeuT/dominant negative ephrin-B2 induced tumors exhibited an even significantly lower vascularization (unpublished observation) (Fig. 3). This result suggests that NeuT expression alone was sufficient to promote adequate tumor vascularization and that the extent of the vasculature is not the main determinant of the Eph/ephrin induced metastatic potential.
Figure 3. Microvascular density in mammary tumors derived from NeuT, NeuT/EphB4 and NeuT/mutant ephrin-B2 (NeuT/ΔEphrin-B2) transgenic mice. Mature vessels were detected with an antibody against the von Willebrand-factor, whereas developing vessels were detected with an antibody against CD34.
Eph/Ephrin Signaling in Normal Mammary Gland Development
Although there is accumulating evidence that Eph/ephrin signaling is an important determinant of breast tumor development and progression, relatively little is known about this signaling in the control of normal mammary gland development. The epithelial unit of the mammary gland is organized in an extensively branched network composed of secretory and ductal epithelial cells, myoepithelial cells, their progenitors and mammary stem cells. The two main compartments of epithelial and myoepithelial cells are spatially separated and are also differentially regulated. The hormonal status of estrogen, progesterone, growth hormone and prolactin coordinates the developmental events through puberty, repeated estrous cycles and the development at pregnancy, lactation and involution by employing local, paracrine mediators and cell-cell and cell matrix interactions.59 Although Eph/ephrin signaling plays a key role in morphogenic processes, information on their participation in mammary gland morphogenesis is only available for the EphA2 and EphB4 receptors as well as for the ephrin-B2 ligand.
The EphA2 receptor is expressed in the mammary epithelium and is induced during pubertal expansion of the mammary ductal tree, differentially regulated during the estrous cycle and diminishes during pregnancy and lactation.60,61 The mammary epithelium of EphA2 knockout mice was characterized by a reduced epithelial expansion and branching at puberty, which was resumed during adulthood. This observation indicates that EphA2 is involved in branching morphogenesis and acts down-stream of HGF, the main inducer of the pubertal branching patterning in the mammary gland.62 In vitro, EphA2 expression is downregulated in normal mammary epithelial cells undergoing growth arrest, polarization and differentiation,63 suggesting that EphA2 signaling is involved in the growth phase of the mammary epithelial cells but incompatible with their terminal differentiation.
Similar to EphA2, EphB4 is also expressed in mammary epithelial and myoepithelial cells and is induced at puberty and differentially regulated during the estrous cycle in mice and humans.21,60 Studies in ovariectomized mice revealed that EphB4 expression is transcriptionally regulated by estrogen.64 In contrast, the epithelial expression of its ligand ephrin-B2 is estrogen independent and present throughout all phases of the mammary epithelial life cycle.64
In order to investigate the role of EphB4 and ephrin-B2 signaling in mammary gland development, we established transgenic mice exhibiting overexpression of EphB or ephrin-B2, as well as of a dominant negative ephrin-B2 mutant able to interact with the receptor but unable to elicit reverse signaling. The MMTV-LTR promoter was used to direct transgene expression predominantly to the mammary epithelial cells. Overexpression of EphB4 resulted in the delayed development of the mammary epithelium at puberty and during pregnancy. During pregnancy fewer lobules were formed, these however, exhibited more numerous but smaller alveolar units. The alveoli themselves were characterized by a fragile, irregular morphology at lactation. The most striking phenotypic consequence of transgene expression in the mammary gland was the unscheduled epithelial apoptotic cell death during pregnancy and untimely DNA synthesis in the epithelium at early post-lactational involution indicating a disturbed response to proliferative/apoptotic signals.43
Expression of the MMTV-ephrin-B2 transgene was induced at the onset of puberty and led to a significant growth retardation of the epithelial ducts. Proliferation, however, resumed in mature animals and the epithelium was almost normally developed at 12 weeks of age. During pregnancy and lactation overexpression of native ephrin-B2 resulted in precocious differentiation of the epithelium and in a high secretory activity already in the last third of pregnancy. Although secretory alveoli were less abundant than in control animals, they exhibited a normal organization. As observed in the native ephrin-B2 transgenic line, overexpression of mutant ephrin-B2 at the onset of puberty inhibited epithelial outgrowth, which was resumed in mature animals. During pregnancy and lactation, however, distinct effects of the two transgenes became evident. In contrast to the native ephrin-B2, overexpression of the ephrin-B2 mutant delayed differentiation and led to a disturbed alveolar structure, with loose cell-cell contacts and epithelial cells exfoliating into the lumen. These phenotypic changes were accompanied by reduced expression and cytoplasmic localization of E-cadherin.44 These results demonstrate that correct ephrin-B2 reverse signaling is indispensable for the maintenance of tissue integrity in the mammary gland and that unbalanced forward signaling prevents proper epithelial differentiation. Furthermore, we have established transgenic mice exhibiting a conditional ephrinB2 knockout in the mammary epithelium. In homozygote double transgenic MMTVCre/ephrin-B2Lox mice, the specific knockout of ephrinB2 was induced in the mammary epithelium during the first pregnancy-lactating period. Abolishing ephrin-B2 function led to severe interference with the architecture and functioning of the mammary gland at lactation. The morphology of the transgenic lactating glands resembled that of involuting controls, with decreased epithelial cell number and collapsed lobulo-alveolar structures. Accordingly, massive epithelial cell death and expression of involution-specific genes were observed.65 These results emphasize the critical role of epithelial ephrin-B2 expression in the establishment of a functional glandular structure in the mammary gland.
The Involvement of Eph/Ephrin Signaling in Mammary Stem Cell Homeostasis
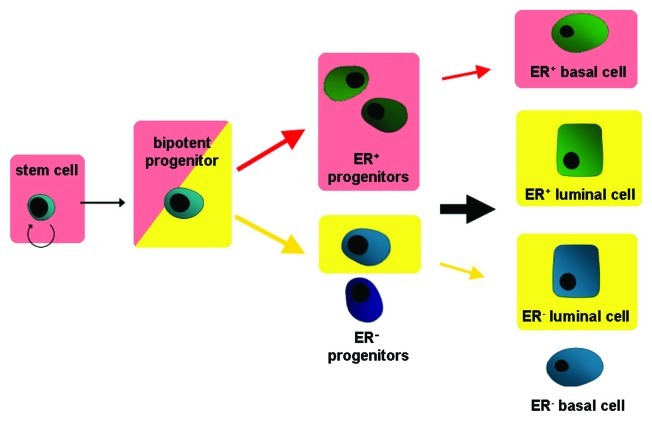
There is growing evidence that Ephs and ephrins are involved in the control of the stem cell niche and the regulation of the progenitor cell evasion and migration. The most clear cut experimental evidence is available for the nervous system, intestine and epidermis. In these experimental settings, Ephs and ephrins of both A and B class have been implicated in positive or negative regulation of stem and progenitor cell proliferation, in the regulation of the phenotype of the niche cells harboring the stem cells and in the control of the directed migration of precursor cells.66 Especially EphBs were shown to be directly correlated with niche cell identity and lineage specification by regulating differentiation and transition of cells. Thereby they enable plasticity in the adult neural stem cell niche.67 EphBs are also implicated in the control of stem cell expansion. On the one hand they can initiate cell proliferation via cyclin-D1 as shown in the intestine,68 however, on the other hand they inhibit progenitor cell expansion in a p53-dependent manner in the brain.69 According to the cancer stem cell theory, stem or progenitor cells are the origin of malignant growth. Moreover, the theory implies that cancerous tissue exhibits a hierarchy similar to normal tissue and is composed of cancer stem cells, capable of self-renewal, invasion and metastasis formation, fast dividing progenitor cells and the “differentiated” cancer cells which make up the tumor mass.70 Thus, it is conceivable that deregulated Eph/ephrin signaling contributes to breast carcinogenesis also by interference with the stem cell homeostasis. It has been shown that the EphA1, 2, 3 and EphB2, 3, 4 receptors as well as the ephrin-B1 and -B2 proteins are enriched in the mouse mammary basal/stem cell-enriched fraction as compared with the luminal precursors and the mature luminal epithelial cells, suggesting their involvement in stem cell homeostasis.71 Indeed, we have shown that the metastasizing NeuT/EphB4 tumors were enriched in CD24 expressing progenitor cells, whereas the metastasizing NeuT/mutant ephrin-B2 tumors contained in addition significant amounts of CD49f expressing stem-like cells which were scarce or absent in the non-invasive NeuT induced tumors. The same cell populations were also enriched in the respective lung metastases. Interestingly, mammary glands of single transgenic MMTV-EphB4 and MMTV-mutant ephrin-B2 females also exhibited a significant increase in CD24-positive cells (MMTV-EphB4) or CD49-positive cells (MMTV-mutant ephrin-B2) indicating that deregulated EphB4/ephrin-B2 signaling interferes with the homeostasis of the stem/progenitor cell pool before tumor formation is initiated.72 To investigate the role of ephrin-B2 in the control of the mammary stem cell niche in more detail, we analyzed the mammary stem and progenitor cell populations in transgenic mice overexpressing the native or mutant ephrin-B2 gene. Quantification by FACS analysis of mammary epithelial cells of mutant ephrin-B2 transgenic mammary glands revealed a significant increase of cells in the basal/alveolar cell-, the bi-potent progenitor- and notably, the stem cell-enriched fractions. Moreover, the supposed precursors of estrogen receptor positive cells were elevated in the stem cell-enriched fraction. In contrast, the epithelium from transgenic mice overexpressing the native ephrin-B2 gene showed no alteration in the stem cell-enriched fraction, but rather an augmentation of the luminal cell- and the bi-potent progenitor-enriched fractions. Repopulation assays revealed that the epithelial cells of mutant ephrin-B2 transgenic epithelial cells are able to repopulate epithelial-free mammary fat pads of normal recipient mice more extensively and faster than those of controls and of native ephrin-B2 transgenic mice, confirming the augmentation of stem cells. Morphologically, these outgrowths exhibited an abnormal basal/luminal compartmentalization and impaired epithelial polarization.73 These results demonstrate that deregulated ephrin-B2 expression interferes with the regulation of the stem cell niche and leads to a shift in the binary decision pathway during progenitor cell differentiation (Fig. 4). Moreover, the expression of the dominant negative ephrin-B2 mutant, but not the native ephrin-B2 gene, increases the mammary stem cell pool indicating that the reverse ephrin-B2 signaling is responsible for the homeostasis of the mammary epithelial stem cell niche. Thus, the absence of reverse signaling or the reinforced forward signaling may have contributed to the acquisition of the potential for metastasis formation long before carcinogenic growth became apparent.
Figure 4. Schematic representation of the effects of deregulated ephrin-B2 expression on the mammary epithelial differentiation pathway. Expression of truncated ephrin-B2 (red arrows) leads to an accumulation of the stem and bipotent progenitor population and shifts differentiation toward the estrogen receptor-positive lineage, thereby further favoring the basal lineage (shaded in red). Overexpression of the native ephrin-B2 leads (yellow arrows) to an accumulation of the bipotent progenitor population and shifts differentiation toward the luminal lineage (shaded in yellow), thereby not affecting steroid receptor expression.
Conclusions
The evidence gained so far clearly indicates that the same Eph receptors can fulfill both tumor promoting and tumor suppressing functions; however, it seems that the promoting effects prevail. Strikingly, there is almost no indication that ephrins are able to elicit tumorigenic growth. In contrast, it is mostly because of the intact reverse signaling that Ephs suppress carcinogenic growth. Thus, it seems that Ephs are mostly the bad and ephrins the good. This may also be reflected by the observation that at least in the mammary gland the expression of Eph is more tightly controlled than the expression of ephrins.61,64 It is also conceivable, that Ephs switch their promoting/suppressing function during the process of carcinogenesis. To date, the best example for the switch between promoting and suppressing function of Eph signaling is observed in colorectal cancer. In the intestine, the stem cells are localized at the base of the crypts and their regenerative descendants proliferate and differentiate while migrating toward the tip of the intestinal villi. The wnt growth factor is the driving force and induces the expression of EphBs. Wnts, on the other hand, repress the expression of ephrin-B ligands which is consequently low at the base but high in the differentiated cells at the tips. The interaction between receptor and ligand attenuates the cellular migration and induces differentiation.74 Most colorectal cancers are initiated by activating mutations in the wnt signaling pathway, leading to hyper-proliferation of stem and progenitor cells and to the induction of EphB expression. This in turn favors their proliferation, favors their outpocketing and the formation of adenomas. Thus, in this phase of the carcinogenic process EphBs act as tumor promoters. The adenomatous growth, however, is limited and becomes arrested when Eph positive cells encounter the ephrin-B positive cells at the tip of the villi. Further tumor progression cannot occur unless EphB expression is downregulated and the growth attenuating effect of ephrins over-ridden. Thus, in the later phases of neoplastic development, Eph acts as a tumor suppressor gene.75
An additional complicating aspect in the prediction of the actual outcome of Eph signaling are the facts, that receptor ligand interaction is highly promiscuous and modulates the cellular response and that both receptors and ligands can act independently via cross-talk with other signaling pathways. Moreover, recent evidence gained in prostate cancer cells revealed that signaling of different Eph/ephrin complexes may cooperate with each other to support carcinogenesis. Contact inhibition of locomotion describes the mechanism by which growing cells stop migration or change direction after collision with another cell. The loss of contact inhibition is a major hallmark of tumor cells facilitating their spreading into the surrounding tissue. Interestingly, contact inhibition is maintained after homotypic collision of two tumor cells.76 Astin et al.77 elegantly showed that prostate cancer cells express EphA2 and A4 as well as ephrin-As and that the interaction of the EphAs with ephrin-As on neighboring cells activates the contact inhibition response. Prostate cancer cells also express EphB3 and EphB4 while only low amounts of ephrin-B2. Ligand expression, however, is high on the surrounding stromal and endothelial cells and the EphB-ephrin-B interaction exerts an attractive response and abolishes contact inhibition, thereby allowing the unimpeded invasion of tumor cells into the surrounding tissue.77 Thus the decision of tumor cell adhesion or invasion depends on the constellation of Ephs on the tumor cells and the profile of ligands on the neighboring cells. Similar mechanisms may also be decisive for their role as tumor promoters or suppressors.
In summary, the role of Ephs and ephrins in breast cancer is extremely versatile and multi-faceted. It effects positively or negatively proliferation and survival, dictates compartmentalization and spreading, effects the stem cell population and thereby the pool of tumor initiating cells responsible for invasion, supports the homing process78 and influences the surrounding tissue, most notably the vasculature. Undoubtedly Ephs and ephrins are key regulators of carcinogenic outcome which would make them important targets for therapeutic intervention. Although several strategies are undertaken in this direction, the validation of the different compounds is still in the pre-clinical stage. Considering the many-sided effects of Eph/ephrin signaling it may turn out to be extremely difficult to design effective and safe therapeutic interventions. On the one hand, targeting one specific receptor would most probably not be enough for a significant benefit, since many different receptors cooperate in the establishment of a given tumor phenotype. On the other hand, the therapeutic design, inhibition or induction of activity, will depend on the stage of tumor development when the therapy is applied. Furthermore, Eph-targeted therapies would have to be applied strictly locally, since tilting the balance between Ephs and ephrins systemically may indeed relieve the burden of the advised tumor but may at the same time initiate carcinogenic growth at another site. In this context, for example, the use of Gleevec might have to be reconsidered, since this highly effective inhibitor of c-abl activity used successfully in the treatment of chronic myelogenous leukemia, may also block the tumor supressing function of EphB420 and thereby facilitate the development of other malignancies especially in the breast.79 Thus it seems that extensive and challenging research is still needed to comprehensively understand the role of Eph/ephrin signaling in the control of normal and neoplastic cell behavior in order to be able to design safe and efficient Eph/ephrin based therapeutic interventions.
Acknowledgments
We wish to thank Robert Strange for helpful discussions and critical reading of the manuscript. The financial support by the Swiss National Science Foundation (31003A_127168), the Swiss Cancer League (KLS-2825-08-2011) and the Schweizerische Stiftung für Klinisch-Experimentelle Tumorforschung is gratefully acknowledged.
Glossary
Abbreviations:
- ADAM
A desintegrin and metalloprotease
- CD
cluster of differentiation or designation (cell surface molecules)
- EGF
epidermal growth factor
- FACS
fluorescence-activated cell sorting
- FGF
fibroblast growth factor
- GRIP
glutamate receptor interacting protein
- HER
human epidermal growth factor receptor
- HGF
hepatocyte growth factor
- ILGF
insulin-like growth factor
- LTR
long terminal repeat
- MAPK
mitogen activated protein kinase
- MMTV
mouse mammary tumor virus
- PDZ
post synaptic density protein, disc large tumor suppressor, zonula occludens protein
- RGS3
regulator of G-protein signaling 3
- siRNA
small interfering RNA
- VEGF
v endothelial growth factor
- Wnt
wingless/int-1
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/20154
References
- 1.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF, O’Leary DD. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–58. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janes PW, Griesshaber B, Atapattu L, Nievergall E, Hii LL, Mensinga A, et al. Eph receptor function is modulated by heterooligomerization of A and B type Eph receptors. J Cell Biol. 2011;195:1033–45. doi: 10.1083/jcb.201104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solanas G, Batlle E. Control of cell adhesion and compartmentalization in the intestinal epithelium. Exp Cell Res. 2011;317:2695–701. doi: 10.1016/j.yexcr.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes Dev. 2008;22:416–29. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orsulic S, Kemler R. Expression of Eph receptors and ephrins is differentially regulated by E-cadherin. J Cell Sci. 2000;113:1793–802. doi: 10.1242/jcs.113.10.1793. [DOI] [PubMed] [Google Scholar]
- 7.Cortina C, Palomo-Ponce S, Iglesias M, Ferńndez-Masip JL, Vivancos A, Whissell G, et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376–83. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- 8.Larsen AB, Pedersen MW, Stockhausen MT, Grandal MV, van Deurs B, Poulsen HS. Activation of the EGFR gene target EphA2 inhibits epidermal growth factor-induced cancer cell motility. Mol Cancer Res. 2007;5:283–93. doi: 10.1158/1541-7786.MCR-06-0321. [DOI] [PubMed] [Google Scholar]
- 9.Hiramoto-Yamaki N, Takeuchi S, Ueda S, Harada K, Fujimoto S, Negishi M, et al. Ephexin4 and EphA2 mediate cell migration through a RhoG-dependent mechanism. J Cell Biol. 2010;190:461–77. doi: 10.1083/jcb.201005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang G, Brantley-Sieders DM, Vaught D, Yu J, Xie L, Wells S, et al. Elevation of receptor tyrosine kinase EphA2 mediates resistance to trastuzumab therapy. Cancer Res. 2010;70:299–308. doi: 10.1158/0008-5472.CAN-09-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashyap AS, Hollier BG, Manton KJ, Satyamoorthy K, Leavesley DI, Upton Z. Insulin-like growth factor-I:vitronectin complex-induced changes in gene expression effect breast cell survival and migration. Endocrinology. 2011;152:1388–401. doi: 10.1210/en.2010-0897. [DOI] [PubMed] [Google Scholar]
- 12.Qiu R, Wang J, Tsark W, Lu Q. Essential role of PDZ-RGS3 in the maintenance of neural progenitor cells. Stem Cells. 2010;28:1602–10. doi: 10.1002/stem.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Essmann CL, Martinez E, Geiger JC, Zimmer M, Traut MH, Stein V, et al. Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat Neurosci. 2008;11:1035–43. doi: 10.1038/nn.2171. [DOI] [PubMed] [Google Scholar]
- 14.Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- 15.Noblitt LW, Bangari DS, Shukla S, Knapp DW, Mohammed S, Kinch MS, et al. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. Cancer Gene Ther. 2004;11:757–66. doi: 10.1038/sj.cgt.7700761. [DOI] [PubMed] [Google Scholar]
- 16.Macrae M, Neve RM, Rodriguez-Viciana P, Haqq C, Yeh J, Chen C, et al. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell. 2005;8:111–8. doi: 10.1016/j.ccr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Yang NY, Fernandez C, Richter M, Xiao Z, Valencia F, Tice DA, et al. Crosstalk of the EphA2 receptor with a serine/threonine phosphatase suppresses the Akt-mTORC1 pathway in cancer cells. Cell Signal. 2011;23:201–12. doi: 10.1016/j.cellsig.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carles-Kinch K, Kilpatrick KE, Stewart JC, Kinch MS. Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res. 2002;62:2840–7. [PubMed] [Google Scholar]
- 19.Fu DY, Wang ZM, Wang BL, Chen L, Yang WT, Shen ZZ, et al. Frequent epigenetic inactivation of the receptor tyrosine kinase EphA5 by promoter methylation in human breast cancer. Hum Pathol. 2010;41:48–58. doi: 10.1016/j.humpath.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Noren NK, Foos G, Hauser CA, Pasquale EB. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol. 2006;8:815–25. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- 21.Berclaz G, Flütsch B, Altermatt HJ, Rohrbach V, Djonov V, Ziemiecki A, et al. Loss of EphB4 receptor tyrosine kinase protein expression during carcinogenesis of the human breast. Oncol Rep. 2002;9:985–9. [PubMed] [Google Scholar]
- 22.Truitt L, Freywald A. Dancing with the dead: Eph receptors and their kinase-null partners. Biochem Cell Biol. 2011;89:115–29. doi: 10.1139/O10-145. [DOI] [PubMed] [Google Scholar]
- 23.Fox BP, Kandpal RP. A paradigm shift in EPH receptor interaction: biological relevance of EPHB6 interaction with EPHA2 and EPHB2 in breast carcinoma cell lines. Cancer Genomics Proteomics. 2011;8:185–93. [PubMed] [Google Scholar]
- 24.Fox BP, Kandpal RP. Invasiveness of breast carcinoma cells and transcript profile: Eph receptors and ephrin ligands as molecular markers of potential diagnostic and prognostic application. Biochem Biophys Res Commun. 2004;318:882–92. doi: 10.1016/j.bbrc.2004.04.102. [DOI] [PubMed] [Google Scholar]
- 25.Fox BP, Kandpal RP. EphB6 receptor significantly alters invasiveness and other phenotypic characteristics of human breast carcinoma cells. Oncogene. 2009;28:1706–13. doi: 10.1038/onc.2009.18. [DOI] [PubMed] [Google Scholar]
- 26.Fox BP, Kandpal RP. Transcriptional silencing of EphB6 receptor tyrosine kinase in invasive breast carcinoma cells and detection of methylated promoter by methylation specific PCR. Biochem Biophys Res Commun. 2006;340:268–76. doi: 10.1016/j.bbrc.2005.11.174. [DOI] [PubMed] [Google Scholar]
- 27.Truitt L, Freywald T, DeCoteau J, Sharfe N, Freywald A. The EphB6 receptor cooperates with c-Cbl to regulate the behavior of breast cancer cells. Cancer Res. 2010;70:1141–53. doi: 10.1158/0008-5472.CAN-09-1710. [DOI] [PubMed] [Google Scholar]
- 28.Wu Q, Suo Z, Risberg B, Karlsson MG, Villman K, Nesland JM. Expression of Ephb2 and Ephb4 in breast carcinoma. Pathol Oncol Res. 2004;10:26–33. doi: 10.1007/BF02893405. [DOI] [PubMed] [Google Scholar]
- 29.Brantley-Sieders DM, Jiang A, Sarma K, Badu-Nkansah A, Walter DL, Shyr Y, et al. Eph/ephrin profiling in human breast cancer reveals significant associations between expression level and clinical outcome. PLoS One. 2011;6:e24426. doi: 10.1371/journal.pone.0024426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043–52. doi: 10.1038/sj.onc.1204004. [DOI] [PubMed] [Google Scholar]
- 31.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–6. [PubMed] [Google Scholar]
- 32.Pan M. Over-expression of EphA2 gene in invasive breast cancer and its association with hormone receptor status. J Clin Oncol. 2005;23:9583. [Google Scholar]
- 33.Fang WB, Brantley-Sieders DM, Parker MA, Reith AD, Chen J. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene. 2005;24:7859–68. doi: 10.1038/sj.onc.1208937. [DOI] [PubMed] [Google Scholar]
- 34.Brantley-Sieders DM, Zhuang G, Hicks D, Fang WB, Hwang Y, Cates JM, et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landen CN, Merritt WM, Mangala LS, Sanguino AM, Bucana C, Lu C, et al. Intraperitoneal delivery of liposomal siRNA for therapy of advanced ovarian cancer. Cancer Biol Ther. 2006;5:1708–13. doi: 10.4161/cbt.5.12.3468. [DOI] [PubMed] [Google Scholar]
- 36.Wykosky J, Gibo DM, Debinski W. A novel, potent, and specific ephrinA1-based cytotoxin against EphA2 receptor expressing tumor cells. Mol Cancer Ther. 2007;6:3208–18. doi: 10.1158/1535-7163.MCT-07-0200. [DOI] [PubMed] [Google Scholar]
- 37.Jackson D, Gooya J, Mao S, Kinneer K, Xu L, Camara M, et al. A human antibody-drug conjugate targeting EphA2 inhibits tumor growth in vivo. Cancer Res. 2008;68:9367–74. doi: 10.1158/0008-5472.CAN-08-1933. [DOI] [PubMed] [Google Scholar]
- 38.Pichot CS, Hartig SM, Xia L, Arvanitis C, Monisvais D, Lee FY, et al. Dasatinib synergizes with doxorubicin to block growth, migration, and invasion of breast cancer cells. Br J Cancer. 2009;101:38–47. doi: 10.1038/sj.bjc.6605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scarberry KE, Mezencev R, McDonald JF. Targeted removal of migratory tumor cells by functionalized magnetic nanoparticles impedes metastasis and tumor progression. Nanomedicine (Lond) 2011;6:69–78. doi: 10.2217/nnm.10.103. [DOI] [PubMed] [Google Scholar]
- 40.Kumar SR, Singh J, Xia G, Krasnoperov V, Hassanieh L, Ley EJ, et al. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am J Pathol. 2006;169:279–93. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noren NK, Yang NY, Silldorff M, Mutyala R, Pasquale EB. Ephrin-independent regulation of cell substrate adhesion by the EphB4 receptor. Biochem J. 2009;422:433–42. doi: 10.1042/BJ20090014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutkowski R, Mertens-Walker I, Lisle JE, Herington AC, Stephenson SA. Evidence for a dual function of EphB4 as tumor promoter and suppressor regulated by the absence or presence of the ephrin-B2 ligand. Int J Cancer. 2012;131:E614–24. doi: 10.1002/ijc.27392. [DOI] [PubMed] [Google Scholar]
- 43.Munarini N, Jäger R, Abderhalden S, Zuercher G, Rohrbach V, Loercher S, et al. Altered mammary epithelial development, pattern formation and involution in transgenic mice expressing the EphB4 receptor tyrosine kinase. J Cell Sci. 2002;115:25–37. doi: 10.1242/jcs.115.1.25. [DOI] [PubMed] [Google Scholar]
- 44.Haldimann M, Custer D, Munarini N, Stirnimann C, Zürcher G, Rohrbach V, et al. Deregulated ephrin-B2 expression in the mammary gland interferes with the development of both the glandular epithelium and vasculature and promotes metastasis formation. Int J Oncol. 2009;35:525–36. doi: 10.3892/ijo_00000364. [DOI] [PubMed] [Google Scholar]
- 45.Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, et al. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005;123:291–304. doi: 10.1016/j.cell.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 46.Solanas G, Cortina C, Sevillano M, Batlle E. Cleavage of E-cadherin by ADAM10 mediates epithelial cell sorting downstream of EphB signalling. Nat Cell Biol. 2011;13:1100–7. doi: 10.1038/ncb2298. [DOI] [PubMed] [Google Scholar]
- 47.Miao H, Wang B. Eph/ephrin signaling in epithelial development and homeostasis. Int J Biochem Cell Biol. 2009;41:762–70. doi: 10.1016/j.biocel.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Hughes S. Role of the ephrin and Eph receptor tyrosine kinase families in angiogenesis and development of the cardiovascular system. J Pathol. 2006;208:453–61. doi: 10.1002/path.1937. [DOI] [PubMed] [Google Scholar]
- 49.Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, et al. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–91. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–6. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 51.Brantley-Sieders DM, Fang WB, Hicks DJ, Zhuang G, Shyr Y, Chen J. Impaired tumor microenvironment in EphA2-deficient mice inhibits tumor angiogenesis and metastatic progression. FASEB J. 2005;19:1884–6. doi: 10.1096/fj.05-4038fje. [DOI] [PubMed] [Google Scholar]
- 52.Brantley-Sieders DM, Fang WB, Hwang Y, Hicks D, Chen J. Ephrin-A1 facilitates mammary tumor metastasis through an angiogenesis-dependent mechanism mediated by EphA receptor and vascular endothelial growth factor in mice. Cancer Res. 2006;66:10315–24. doi: 10.1158/0008-5472.CAN-06-1560. [DOI] [PubMed] [Google Scholar]
- 53.Héroult M, Schaffner F, Augustin HG. Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp Cell Res. 2006;312:642–50. doi: 10.1016/j.yexcr.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 54.Noren NK, Lu M, Freeman AL, Koolpe M, Pasquale EB. Interplay between EphB4 on tumor cells and vascular ephrin-B2 regulates tumor growth. Proc Natl Acad Sci U S A. 2004;101:5583–8. doi: 10.1073/pnas.0401381101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salvucci O, de la Luz Sierra M, Martina JA, McCormick PJ, Tosato G. EphB2 and EphB4 receptors forward signaling promotes SDF-1-induced endothelial cell chemotaxis and branching remodeling. Blood. 2006;108:2914–22. doi: 10.1182/blood-2006-05-023341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noberini R, Koolpe M, Peddibhotla S, Dahl R, Su Y, Cosford ND, et al. Small molecules can selectively inhibit ephrin binding to the EphA4 and EphA2 receptors. J Biol Chem. 2008;283:29461–72. doi: 10.1074/jbc.M804103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foubert P, Silvestre JS, Souttou B, Barateau V, Martin C, Ebrahimian TG, et al. PSGL-1-mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J Clin Invest. 2007;117:1527–37. doi: 10.1172/JCI28338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andres AC, Djonov V. The mammary gland vasculature revisited. J Mammary Gland Biol Neoplasia. 2010;15:319–28. doi: 10.1007/s10911-010-9186-9. [DOI] [PubMed] [Google Scholar]
- 59.Sternlicht MD, Kouros-Mehr H, Lu P, Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006;74:365–81. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andres AC, Reid HH, Zürcher G, Blaschke RJ, Albrecht D, Ziemiecki A. Expression of two novel eph-related receptor protein tyrosine kinases in mammary gland development and carcinogenesis. Oncogene. 1994;9:1461–7. [PubMed] [Google Scholar]
- 61.Kouros-Mehr H, Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn. 2006;235:3404–12. doi: 10.1002/dvdy.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaught D, Chen J, Brantley-Sieders DM. Regulation of mammary gland branching morphogenesis by EphA2 receptor tyrosine kinase. Mol Biol Cell. 2009;20:2572–81. doi: 10.1091/mbc.E08-04-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fournier MV, Martin KJ, Kenny PA, Xhaja K, Bosch I, Yaswen P, et al. Gene expression signature in organized and growth-arrested mammary acini predicts good outcome in breast cancer. Cancer Res. 2006;66:7095–102. doi: 10.1158/0008-5472.CAN-06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nikolova Z, Djonov V, Zuercher G, Andres AC, Ziemiecki A. Cell-type specific and estrogen dependent expression of the receptor tyrosine kinase EphB4 and its ligand ephrin-B2 during mammary gland morphogenesis. J Cell Sci. 1998;111:2741–51. doi: 10.1242/jcs.111.18.2741. [DOI] [PubMed] [Google Scholar]
- 65.Weiler S, Rohrbach V, Pulvirenti T, Adams R, Ziemiecki A, Andres AC. Mammary epithelial-specific knockout of the ephrin-B2 gene leads to precocious epithelial cell death at lactation. Dev Growth Differ. 2009;51:809–19. doi: 10.1111/j.1440-169X.2009.01140.x. [DOI] [PubMed] [Google Scholar]
- 66.Genander M, Frisén J. Ephrins and Eph receptors in stem cells and cancer. Curr Opin Cell Biol. 2010;22:611–6. doi: 10.1016/j.ceb.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 67.Nomura T, Göritz C, Catchpole T, Henkemeyer M, Frisén J. EphB signaling controls lineage plasticity of adult neural stem cell niche cells. Cell Stem Cell. 2010;7:730–43. doi: 10.1016/j.stem.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Genander M, Halford MM, Xu NJ, Eriksson M, Yu Z, Qiu Z, et al. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell. 2009;139:679–92. doi: 10.1016/j.cell.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Theus MH, Ricard J, Bethea JR, Liebl DJ. EphB3 limits the expansion of neural progenitor cells in the subventricular zone by regulating p53 during homeostasis and following traumatic brain injury. Stem Cells. 2010;28:1231–42. doi: 10.1002/stem.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumor initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Disc. 2009;8:806–23. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- 71.Ji H, Goode RJA, Vaillant F, Mathivanan S, Kapp EA, Mathias RA, et al. Proteomic profiling of secretome and adherent plasma membranes from distinct mammary epithelial cell subpopulations. Proteomics. 2011;11:4029–39. doi: 10.1002/pmic.201100102. [DOI] [PubMed] [Google Scholar]
- 72.Kaenel P, Schwab C, Mülchi K, Wotzkow C, Andres AC. Preponderance of cells with stem cell characteristics in metastasising mouse mammary tumours induced by deregulated EphB4 and ephrin-B2 expression. Int J Oncol. 2011;38:151–60. [PubMed] [Google Scholar]
- 73.Kaenel P, Antonijevic M, Richter S, Küchler S, Sutter N, Wotzkow C, et al. Deregulated ephrin-B2 signaling in mammary epithelial cells alters the stem cell compartment and interferes with the epithelial differentiation pathway. Int J Oncol. 2012;40:357–69. doi: 10.3892/ijo.2011.1238. [DOI] [PubMed] [Google Scholar]
- 74.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–63. doi: 10.1016/S0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 75.Merlos-Súrez A, Batlle E. Eph-ephrin signalling in adult tissues and cancer. Curr Opin Cell Biol. 2008;20:194–200. doi: 10.1016/j.ceb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 76.Wang B. Cancer cells exploit the Eph-ephrin system to promote invasion and metastasis: tales of unwitting partners. Sci Signal. 2011;4:pe28. doi: 10.1126/scisignal.2002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Astin JW, Batson J, Kadir S, Charlet J, Persad RA, Gillatt D, et al. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat Cell Biol. 2010;12:1194–204. doi: 10.1038/ncb2122. [DOI] [PubMed] [Google Scholar]
- 78.Héroult M, Schaffner F, Pfaff D, Prahst C, Kirmse R, Kutschera S, et al. EphB4 promotes site-specific metastatic tumor cell dissemination by interacting with endothelial cell-expressed ephrinB2. Mol Cancer Res. 2010;8:1297–309. doi: 10.1158/1541-7786.MCR-09-0453. [DOI] [PubMed] [Google Scholar]
- 79.Wang JYJ. Eph tumour suppression: the dark side of Gleevec. Nat Cell Biol. 2006;8:785–6. doi: 10.1038/ncb0806-785. [DOI] [PubMed] [Google Scholar]