Abstract
Bones cannot properly form or be maintained without cell-cell interactions through ephrin ligands and Eph receptors. Cell culture analysis and evaluation of genetic mouse models and human diseases reveal various ephrins and Eph functions in the skeletal system. Migration, attachment and spreading of mesenchymal stem cells are regulated by ephrinB ligands and EphB receptors. ephrinB1 loss-of-function is associated with craniofrontonasal syndrome (CFNS) in humans and mice. In bone remodeling, ephrinB2 is postulated to act as a “coupling stimulator.” In that case, bidirectional signaling between osteoclastic ephrinB2 and osteoblastic EphB4 suppresses osteoclastic bone resorption and enhances osteoblastic bone formation, facilitating the transition between these two states. Parathyroid hormone (PTH) induces ephrinB2 in osteoblasts and enhances osteoblastic bone formation. In contrast to ephrinB2, ephrinA2 acts as a “coupling inhibitor,” since ephrinA2 reverse signaling into osteoclasts enhances osteoclastogenesis and EphA2 forward signaling into osteoblasts suppresses osteoblastic bone formation and mineralization. Furthermore, ephrins and Ephs likely modulate pathological conditions such as osteoarthritis, rheumatoid arthritis, multiple myeloma and osteosarcoma. This review focuses on ephrin/Eph-mediated cell-cell interactions in bone biology.
Keywords: chondrocyte, osteoclast, osteoblast, coupling factor, coupling inhibitor, cell-cell interaction, osteoarthritis, rheumatoid arthritis, multiple myeloma, osteosarcoma
Introduction
Bone cells such as osteoblasts and osteoclasts must proliferate, migrate, attach, spread and differentiate from precursor cells originating from mesenchymal or hematopoietic stem cells. Cell-cell interaction through ephrins and Ephs regulates these processes. Analysis of animal models and human disease indicates that ephrins and Eph function in somitogenesis, craniofacial development and limb development. Once they form, bones are maintained by bone remodeling, which is also influenced by ephrins and Ephs. In addition, in bone diseases such as osteoarthritis, rheumatoid arthritis or bone-associated tumors such as multiple myeloma and osteosarcoma, signaling mediated by ephrins and Ephs likely affects disease progression.
Eph receptors belong to a subfamily of receptor tyrosine kinases activated by ligands called ephrins (Eph receptor interacting proteins).1 Both Ephs and ephrins are divided into two A and B groups. Generally, EphA receptors (EphA1–A8, A10) interact with ephrinA (ephrinA1–A5) and EphB receptors (EphB1–B6) interact with ephrinB ligands (ephrinB1–B3), with some exceptions.2,3 As an exception, EphA4 binds to ephrinB2 and ephrinB3 as well as to ephrinAs. From the extracellular N-terminus, Eph receptors are composed of an ephrin-binding domain, a cysteine-rich region, two fibronectin type III domains, a juxtamembrane region, a kinase domain, a sterile α motif (SAM) and a C-terminal PDZ-domain binding motif. ephrinA ligands attach to the cell membrane via a glycosylphospatidyl inositol (GPI) anchor, while ephrinB ligands are transmembrane proteins containing a conserved cytoplasmic tail and a C-terminal PDZ-domain binding motif. Eph receptors interact with ephrins at the cell surface, triggering bidirectional signaling: forward through Eph receptors and reverse through ephrins.4 Forward Eph signaling depends on both Eph kinase activity and kinase-independent signals, while reverse ephrin signaling depends on Src family kinases and other effector molecules.5 In communication between neurons and glia, arteries and veins, and many other cell types, activation of ephrin/Eph-mediated bidirectional signaling alters cell adhesion, migration and proliferation. Here we review literature relevant to expression and function of ephrins and Ephs in the field of bone biology (Fig. 1).
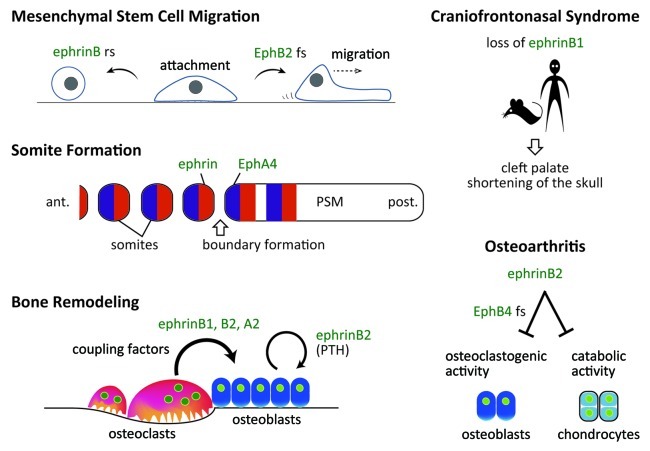
Figure 1. Ephrins and Ephs in bone biology (an overview). rs, reverse signaling; fs, forward signaling; ant., anterior; post., posterior; PSM, presomitic mesoderm.
Stem Cell Attachment and Migration
Ephrin/Eph signaling reportedly mediates cancer cell migration and attachment and regulates similar migratory behavior of mesenchymal stromal/stem cells (MSCs) and dental pulp stem cells (DPSCs). MSCs contribute to skeletal tissue formation by differentiating into chondrocytes, osteoblasts, and tendon cells. When cultured human MSCs are treated with soluble EphB-Fc, MSCs become rounder and smaller, indicating that reverse signaling through ephrinB inhibits MSC attachment (Fig. 1).6 By contrast, treatment of MSCs with ephrinB-Fc promotes MSC migration. This activity is likely mediated by forward signaling through EphB2, as MSCs lacking EphB2 are unaffected by this treatment.6
DPSCs reside in the perivascular niche of dental pulp, and their attachment and migration are dependent on ephrin/Eph signaling.7 Like human MSCs human DPSCs treated with EphB2-Fc become rounder and smaller, although MSCs and DPSCs respond differently to certain ephrin-Fc or Eph-Fc molecules in morphological and migration assays. Since ephrinB1 gene expression is downregulated following tooth injury, ephrin/Eph signaling in DPSCs may function in tooth repair.7
Somite Formation and EphA4
EphA4 signaling regulates formation of somites, which are blocks of mesodermal cells located on both sides of the notochord. Somites, which are derived from unsegmented paraxial mesoderm called presomitic mesoderm (PSM), give rise to vertebrae and ribs, muscle and other connective tissues. In fact, the ventral part of somite becomes the sclerotome and the caudal compartment of one sclerotome fuses to the rostral component of the consecutive sclerotome to form one vertebra through a process called resegmentation.8 Among factors that drive somite formation in early embryos, the basic helix-loop-helix transcription factor Mesp2 is crucial for defining the segmental border. Notch signaling induces Mesp2 transcription at a future somitic compartment,9 and Mesp2, in turn, downregulates Notch signaling in the anterior half of each presumptive segment.10
In the anterior PSM cells posterior to the presumptive somite boundary, EphA4 is induced as a direct transcriptional target of Mesp2,11 while cells in the posterior half of presumptive segment express ephrins.12 Interaction between EphA4 and ephrins is required for somitogenic boundary formation (Fig. 1). Consistently, ectopic Mesp2 expression induces EphA4, and leads to skeletal malformation.11 Similarly, forced expression of cMeso-1, the chicken homolog of mouse Mesp2, in chick embryos results in ectopic boundary formation in the PSM.13 Of genes downstream of cMeso-1, only EphA4 is required for somitic boundary formation.13 Nonetheless, severe skeletal phenotypes have not been reported in EphA4 knockout mice,14 presumably due to EphA receptor redundancy.
Craniofacial Development and EphrinB1
In humans, ephrinB1 is encoded by the X-linked EFNB1 gene, and EFNB1 mutations are associated with craniofrontonasal syndrome (CFNS).15,16 Unlike other X-linked disorders, females are more severely affected than males (see below). CFNS is characterized by cleft palate, hypertelorism, frontonasal dysplasia, agenesis of the corpus callosum, hypoplasia of the maxilla and other anomalies of neurological and skeletal development (Fig. 1).17 Mice lacking ephrinB1 generated by crossing Efnb1 floxed mice with a line ubiquitously expressing Cre recombinase (Pgk-Cre) exhibit multiple skeletal malformations.18
In mice, proliferation of palatal mesenchyme cells controls palatal shelf outgrowth initiated from the invaginated surface of the maxillary processes beginning around embryonic day 11.5 (E11.5). The palatal shelves are vertically positioned lateral to the tongue by E13.5 and then are elevated to a horizontal position, grow and fuse. ephrinB1 is required for this elevation and the PDZ target site of ephrinB1 is crucial for that activity. Forward signaling through EphB2 (previously called Nuk) and EphB3 (previously called Sek4) cooperate in palate formation.19,20
Why are female CFNS patients more severely affected than males? In female ephrinB1 heterozygous mutants, X inactivation generates ephrin-B1-expressing and -non-expressing cells, and these two populations of cells segregate via “homophilic sorting,” causing abnormal tissue boundaries in calvaria, palate and other tissues.21,22 This homophilic sorting process, termed “cellular interference,”23 cannot occur in males. Inhibition of gap junction communication at ectopic ephrin/Eph boundaries may underlie CFNS.24
Osteoblast-specific conditional eprhrinB1 knockout (KO) mice created using collagen α2 (I) promoter-Cre transgene show exencephaly due to reduced size of calvarial bones such as frontal, parietal and interparietal bones in both mutant hemizygous males and homozygous females,25 in contrast to ubiquitous ephrinB1 KO mice, which show skull defects exclusively in heterozygous females.18 Long bones of mice harboring conditional KO ephrinB1 in osteoblasts are also reduced in size and bone mineral density. Consistently, in vitro gain-of-function experiments of ephrinB1 suggest that ephrinB1 reverse signaling dephosphorylates TAZ (a transcriptional coactivator with a PDZ binding motif) within a protein complex, releasing TAZ from the complex to translocate into nucleus and to induce expression of osteoblast-specific transcription factor osterix, osteoblastic differentiation and mineralization.25
Limb Development and Polydactyly
Besides craniofacial phenotypes, heterozygous female ephrinB1 KO mice show polydactyly restricted to digits I or II in forelimbs or hindlimbs.26 Although sonic hedgehog (Shh) and homeobox A13 (Hoxa13) are implicated in polydactyly, their expression is not altered in the absence of ephrinB1.26 Curiously, expression of EphA7 (ephrinA5 is a cognate ligand) is markedly reduced in affected digits of Hoxa13 mutant mice and in mutant mesenchymal cells together with loss of chondrogenic capacity.27 Ectopic EphA4 expression in limb buds of ephrinB1 heterozygous female mice at E12.5 is associated with the presence of an excess number of mesenchymal condensations.26 EphA4 expression is also altered in the polydactylous talpid3 mutant in the chick.28 These data suggest that multiple ephrin/Eph family members including ephrinB1, EphA4 and EphA7 contribute to patterning of digits.
Bone Cells
Bone cells such as chondrocytes, osteoblasts, osteocytes and osteoclasts express a subset of ephrin ligands and Eph receptors. Chondrocytes and osteoblasts are of mesenchymal origin. Osteoblasts differentiate through activation of Runt-related transcription factor 2 (Runx2, also called Cbfa1) and osterix, and secrete extracellular matrix proteins.29 A population of osteoblasts becomes embedded in bone matrix and terminally differentiates into osteocytes, which communicate with each other through gap junctions at the tips of dendrites extending into osteocytic canaliculi.30 Osteoclasts are specialized macrophages of hematopoietic origin that resorb bone.31 Osteoblasts and osteocytes express the receptor activator of NFκB ligand (RANKL), which, together with macrophage-colony stimulating factor (M-CSF), induces osteoclastic differentiation through activation of the transcription factors c-Fos (encoded by Fos) and nuclear factor of activated T-cells c1 (NFATc1) in macrophagic precursors.32,33 These mononuclear precursor cells fuse to form multinucleated osteoclasts. In addition to bone cells, endothelial cells and neurons in bones and in the bone marrow also express ephrins and Ephs. Therefore, diverse cell-cell interactions mediated by ephrins and Ephs occur in bone.34
Expression of Ephrins and Ephs in Bone Cells
Eph receptors and ephrin ligands are also found in chondrocytes, osteoclasts and osteoblasts and osteocytes. EphA4 is expressed in mouse growth plate cartilage, and its expression increases during endochondral ossification. EphA4 is also expressed in human chondrocytic cell lines.35 Human articular cartilage cells express ephrinB2 and EphB4.36 During differentiation of cultured osteoclasts induced by RANKL, ephrinA2, B1, and B2, and receptors EphA1, A2, A4 are dynamically expressed as revealed by RT-PCR (Fig. 2).37-39 Since expression of EphB receptors is not detectable in osteoclasts, ephrinB1 and B2 expressed on osteoclasts likely stimulate EphB receptors on non-osteoclastic cells such as osteoblasts. Cells in osteoblast cultures express most ephrinAs, ephrinBs, EphAs and EphB2, and their expression levels are generally uniform (Fig. 2).37,38,40 Osteocytes also express ephrins and Ephs (see below).
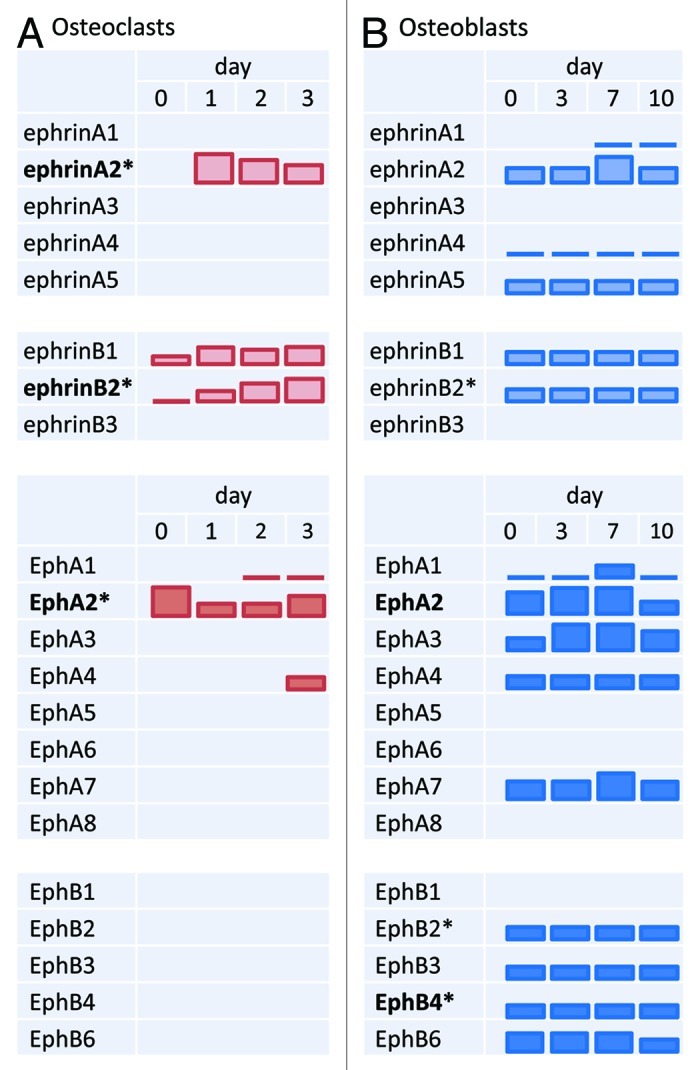
Figure 2. Expression of ephrins and Ephs during bone cell differentiation. (A) Osteoclast differentiation is induced by RANKL and M-CSF in vitro and then ephrin/Eph mRNA expression is monitored on the days indicated. (B) Osteoblast differentiation is induced by ascorbic acid and β-glycerophosphate in vitro and ephrin/Eph mRNA expression was similarly analyzed. Bar height represents changes in relative expression levels of each gene, based mainly on RT-PCR data.37,38 Levels can be compared only within a gene, not across genes. ephrin and Eph names in bold are discussed in detail in this review. Asterisks (*) indicate that mRNA expression levels are accompanied by corresponding changes in protein expression.37,38,40
Endochondral Ossification and Ephrin/Eph
Several flat bones including calvarial bone form through the process of membranous ossification. Other bones, such as basal skull, long bones and vertebrae form through endochondral ossification.29 During development, mesenchymal cells condense and differentiate into chondrocytes to form avascular cartilage models of future bones. Chondrocytes within cartilage terminally differentiate into hypertrophic chondrocytes, which produce vascular endothelial growth factor (VEGF) to stimulate angiogenesis. In the vasculature, ephrinB2 is expressed in arterial endothelial cells, while one of its cognate receptors, EphB4, is predominantly expressed in venous endothelial cells.41 Although formation of the primary blood capillary plexus reportedly requires signaling mediated by ephrinB2 and its Eph receptors,42-44 it is unclear whether ephrinB2 functions in the blood capillary invasion into hypertrophic chondrocytes at the growth plate.
Concomitant with capillary invasion, osteoclast and osteoblast precursors enter into cartilage and replace it with bone.45 Since atrial endothelial cells express ephrinB2 on their luminal side,46,47 interaction between ephrinB2-expressing endothelial cells and EphB-receptor-expressing monocytes47 or MSCs/osteoblast precursors likely promotes adhesion and transmigration of these cells, which may serve as a mechanism to deliver osteoclast and osteoblast precursors to hypertrophic chondrocytes, where they are exposed to respective differentiation cues including RANKL48,49 and bone morphogenetic proteins (BMPs). Curiously, ephrinB2 controls VEGF-induced angiogenesis50,51 and osteoclasts express ephrinB2.37 Thus it is tempting to speculate that osteoclasts contribute to angiogenesis into the growth plate near the tips of capillaries, as do a group of osteoclasts at the tip of the osteon, which drill into cortical bone followed by capillary penetration and osteoblastic bone formation around the capillary or the central canal. Consistently, in the absence of osteoclasts, parts of bone, which normally ossifies, remain avascular and cartilaginous.52
Bidirectional Osteoclast-Osteoblast Communication
Mice lacking the transcription factor c-Fos (Fos) lack osteoclasts and develop osteopetrosis.53 These mice also fail to express other Fos family proteins, Fra-1, Fra-2 and FosB, in osteoclast lineage cells, and overexpression of any Fos protein in Fos−/− hematopoietic precursors rescues osteoclastogenesis.54 Expression of Fos family proteins is essential to induce expression of the transcription factor NFATc1, the master regulator of osteoclast differentiation, which rescues osteoclastogenesis of Fos−/− hematopoietic precursors.32,33 Several osteoclast-specific genes including tartrate-resistant acid phosphatase (TRAP, Acp5), cathepsin K (Ctsk), chloride channel 7 (Clcn7) and matrix metallopeptidase 9 (Mmp9) are transcriptional targets of NFATc1.55 ephrinB2 was identified as an NFATc1 target gene in microarray screening for genes upregulated following expression of a constitutively active form of an NFAT protein in Fos−/− hematopoietic precursors cultured in the presence of RANKL and M-CSF.37 Both ephrinB2 mRNA and protein are significantly upregulated during osteoclastogenesis in vitro, and immunohistochemistry indicates high ephrinB2 expression in TRAP-positive osteoclasts in vivo.37 Addition of EphB4-Fc to osteoclastogenic cultures to stimulate reverse signaling suppresses osteoclast differentiation via suppression of Fos and therefore Nfatc1 transcription, while cultured hematopoietic precursors lacking ephrinB2 differentiate more efficiently than do wild-type controls. Therefore, ephrinB2 is a negative regulator of bone resorption (Fig. 3). The ephrinB2 C-terminal PDZ interaction site is indispensable for suppression of osteoclast differentiation.37 A requirement for ephrinB2 interaction with PDZ domain effectors has also been reported for lymphatic development.56 Dishevelled 2 (Dvl2) is a candidate PDZ domain effector that interacts with eprhinB2 during osteoclast differentiation.57
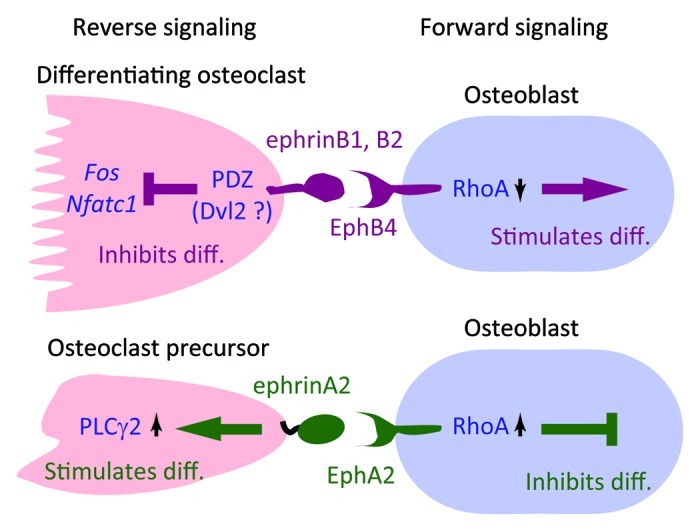
Figure 3. Osteoclast-osteoblast interactions through ephrins/Ephs. ephrinB2 is expressed in differentiating and mature osteoclasts, while ephrinA2 is expressed in early differentiating osteoclasts.37,38 Reverse signaling through ephrinB2, which may be mediated by PDZ domain proteins such as Dishevelled 2 (Dvl2),57 suppresses transcription of Fos and Nfatc1, thereby inhibiting osteoclast differentiation. Reverse signaling through ephrinA2 is mediated by activation of phospholipase Cγ2 (PLCγ2).38 Forward signaling through EphB4 suppresses RhoA activity and thereby stimulates osteoblast differentiation, while signaling through EphA2 likely enhances RhoA activity and inhibits osteoblast differentiation.37,38 How RhoA activity is differentially regulated by EphB4 and EphA2 signaling is unclear.
Conditional KO mice with myeloid lineage-specific deletion of ephrinB1 (Efnb1floxed/floxed, LysM-cre) show increased osteoclast formation and reduced bone mass, indicating that ephrinB1 in osteoclasts negatively regulates bone resorption as does ephrinB2.39 Similar conditional ephrinB2 KO mice generated using the same LysMcre mice do not show reduced bone mass,37 suggesting that ephrinB1 can compensate ephrinB2 functions, but not vice versa.
Forward signaling through EphB4 into osteoblasts enhances osteoblast differentiation both in osteoblastogenic cultures and in α1 (I) collagen promoter-EphB4 transgenic mice via inhibition of the small GTPase RhoA.37 Therefore, bidirectional signaling between osteoclasts and osteoblasts is likely mediated by ephrinB1/B2 and EphB receptors including EphB4 (Fig. 3). On the other hand, osteoblasts also upregulate ephrinB2 in response to parathyroid hormone (PTH) or parathyroid hormone-related protein (PTHrP) signaling, and blockade of ephrinB2/EphB4 interaction in osteoblasts inhibits mineralization.40 In mice lacking the cytoplasmic adaptor protein β-arrestin2, PTH significantly downregulates expression of ephrins B1 and B2 and Eph B2, B3, B4, A3 and A4 relative to levels seen in wild-type mice, consistent with enhanced PTH-stimulated osteoclastogenesis seen in these KO mice.58
EphrinB as a “Coupling Stimulator”
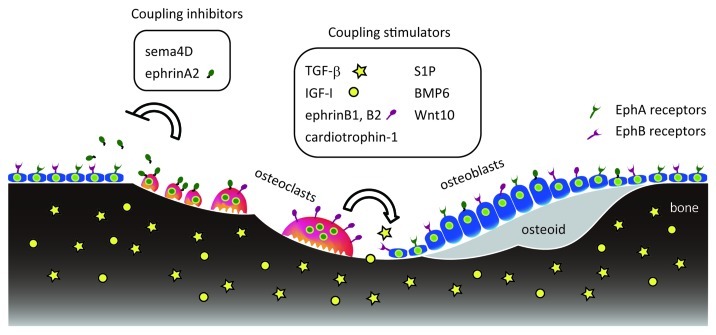
The “coupling” concept was postulated in the 1960s to explain the balance in bone resorption and formation observed at whole body and microscopic levels. Using a radiocalcium kinetics technique, investigators observed a positive correlation or “coupling” between whole body rates of bone resorption and formation.59 Microscopic examination showed sequential bone remodeling in which bone resorption was followed by an equivalent amount of bone formation at each basic multicellular unit (BMU).60-62 “Coupling factors” have been defined as osteoclast-derived molecules that induce osteoblastic bone formation at the BMU level by either recruiting osteoprogenitors and osteoblasts or promoting their differentiation and activation. Proposed coupling factors, such as tansforming growth factor β (TGF- β) and insulin-like growth factor-I (IGF-1), are released from the extracellular matrix by bone resorption, while factors such as cardiotrophin-1, sphingosine-1-phosphate, BMP6 and Wnt10b are secreted from osteoclasts.63-67 By contrast, ephrinB1 and ephrinB2 are membrane-bound and must function locally, unless released by shedding,68 to facilitate transition from bone resorption to bone formation phase at the BMU.37,69 In rat alveolar bone remodeling models, Baron and colleagues demonstrated that mononuclear phagocytic cells occupy resorption lacunae (the reversal phase) for 2.5 to 4 d between resorption and formation.70,71 These mononuclear cells are “bone lining cells” that remove collagen left by osteoclasts in resorption lacunae.72 Both bone lining cells and EphB4-positive pre-osteoblasts can directly interact with osteoclasts, allowing potential osteoblastic activation through ephrinB/EphB4.72-74 Since osteoclasts produce factors that not only stimulate but inhibit coupling, the above-mentioned factors may be considered “coupling stimulators” (Fig. 4).
Figure 4. Coupling stimulators and inhibitors during bone remodeling. Bone matrix contains TGF-β (yellow stars) and IGF-I (yellow circles), which are released by osteoclastic bone resorption to stimulate coupling. Cells in the osteoclast lineage (red) produce various coupling stimulators and inhibitors that act on osteoblasts or their progenitors (blue).
EphrinA2 as a “Coupling Inhibitor”
Osteoclast-derived factors also negatively regulate osteoblasts and antagonize coupling. Osteoclast-derived ephrinA2, which is expressed early during osteoclastogenesis (Fig. 2), may be such a negative regulator. In addition to ephrinA2 anchored on osteoclast membranes, soluble ephrinA2 (and ephrinB2) generated by proteolytic cleavage (ectodomain shedding) may act at a distance on osteoblasts.38,68 In osteoblastogenic cultures, forward signaling through the EphA2 receptor expressed on osteoblasts inhibit both differentiation via activation of RhoA,38 and mineralization (Irie et al., our unpublished data). We propose that ephrinA2 and other osteoclast-efferent factors that negatively regulate bone formation be designated “coupling inhibitors” (Fig. 4). Furthermore, reverse signaling through ephrinA2 into osteoclasts enhances osteoclastogenesis most likely via phospholipase Cγ2 activation (Fig. 3).38 In addition to osteoclast-osteoblast interactions, osteoclast-osteoclast or osteoblast-osteoblast interactions through ephrinA2 and EphA2 (EphA4) can also occur (Fig. 2).
Coupling inhibitors other than ephrinA2 have been identified. Semaphorin 4D, an axon guidance molecule, is expressed in osteoclasts and binds to its receptor, Plexin-B1, in osteoblasts. This interaction suppresses bone formation through activation of RhoA.75 Mice lacking BMP receptor type1A (Bmpr1a) specifically in osteoclasts show enhanced bone formation, suggesting that BMP receptor signaling in osteoclasts suppresses bone formation perhaps through production of a coupling inhibitor that interacts with osteoblasts.76
Arthritis and Ephrin/Eph
Osteoarthritis (OA) is a common condition that affects joints, causing pain and stiffness, cartilage degradation and synovial membrane inflammation. Cartilage pathologies may be associated with changes in subchondral bone. A subpopulation of osteoblasts derived from a human OA patients produces low levels of prostaglandin E2 (PGE2) show upregulation of EphB4 by PGE2 and interleukin (IL)-17.77 Moreover, osteoclastogenic activity of OA-derived osteoblasts is reduced by stimulating EphB4 forward signaling by ephrinB2.77 In vivo studies are necessary to determine whether manipulation of ephrins/Ephs alters OA progression. In addition, treatment of human OA chondrocytes with ephrinB2 inhibits expression of IL-1β, IL-6, matrix metalloproteinase-1 (MMP1), MMP9 and MMP13, among other catabolic factors, suggesting that ephrinB2 treatment may increase anabolic activity.36
Rheumatoid arthritis (RA) is a chronic, systemic inflammatory disease that affects joints and many other tissues and organs. Kitamura et al. reported that ephrinB1 expression is significantly higher in synovial fibroblasts and exudate lymphocytes in patients with RA compared with those in OA.78 They also showed that a recombinant ephrinB1-Fc protein stimulates enhanced migration of normal peripheral blood lymphocytes, increases their TNF-α production, and induces RA synovial cells to produce IL-6.78 High expression of ephrinBs and EphB kinases might be correlated with RA pathogenesis.79
Bone-Related Tumors and Ephrin/Eph
Multiple myeloma (MM) is a plasma cell malignancy often accompanied by bone osteolytic lesions.80,81 MM patients suffer pain, fractures and hypercalcemia. Osteoclastic bone resorption is elevated but osteoblastic bone formation is suppressed in MM patients, leading to osteolytic lesions. Pennisi et al. reported that ephrinB2/EphB4 expression in myelomatous bones is reduced.82 Myeloma cells suppress osteoblast differentiation by secreting Wnt signaling inhibitors such as dickkopf 1 (DKK1), sclerostin and soluble frizzled receptor-like proteins (sFRPs),83-85 as well as BMP2 signaling inhibitors, such activin A.86 Since EphB4 is a target of Wnt signaling in some tumors,87 decreased Wnt signaling in myeloma bone may suppress osteoblast differentiation, at least in part, by reducing EphB4 expression.
Osteosarcoma is a malignant bone tumor that usually develops in adolescence as rapid growth occurs. Genome-wide microarray analysis of patient osteosarcoma samples has revealed increased expression of the EPHA2 receptor and its ligand EFNA1.88 Other studies suggest that EFNB1 expression by osteosarcoma cells is a marker of poor prognosis.89 A staining pattern indicative of cytoplasmic ephrinA4 in primary osteosarcoma is associated with both progression and poor prognosis, while cytoplasmic and nuclear staining is associated with favorable prognosis.90 However, the roles of these ephrins and Ephs in osteosarcoma development and dissemination are as yet undefined. Curiously, ephrinA5 is downregulated in chondrosarcomas compared with normal cartilage.91
Concluding Remarks
In this review, we have discussed ephrins and Ephs expressed in bone cells, in particular, osteoclasts, osteoblasts and bone-associated tumor cells. Although little is known about function of ephrins and Ephs in osteocytes, these abundant bone cells do express ephrins/Ephs such as ephrinB1, ephrinB2, and EphB4,6,40,77 and blockade of ephrinB2/EphB4 interaction results in decreased expression of sclerostin, a potent inhibitor of osteoblastogenesis.92 Therefore, osteocytes may communicate bidirectionally with osteoclasts or osteoblasts in response to various stimuli through ephrins/Ephs. Moreover, ephrin/Eph interaction in non-skeletal organs might contribute to bone phenotypes, given the connection of bone with organs such as kidney, brain and gut.93 Bidirectional exchange of findings relevant to ephrins and Ephs between multiple fields could shed new light on common mechanisms governing modeling and remodeling of tissues.
Acknowledgments
We thank Yumiko Saga, Yoshiko Takahashi, Naoko Irie, Yasunari Takada, Yukiko Kuroda and Elise Lamar for valuable comments. This work was supported by KAKENHI (21390425).
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/20888
References
- 1.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Pitulescu ME, Adams RH. Eph/ephrin molecules--a hub for signaling and endocytosis. Genes Dev. 2010;24:2480–92. doi: 10.1101/gad.1973910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17:230–8. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Davy A, Soriano P. Ephrin signaling in vivo: look both ways. Dev Dyn. 2005;232:1–10. doi: 10.1002/dvdy.20200. [DOI] [PubMed] [Google Scholar]
- 5.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165–80. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arthur A, Zannettino A, Panagopoulos R, Koblar SA, Sims NA, Stylianou C, et al. EphB/ephrin-B interactions mediate human MSC attachment, migration and osteochondral differentiation. Bone. 2011;48:533–42. doi: 10.1016/j.bone.2010.10.180. [DOI] [PubMed] [Google Scholar]
- 7.Stokowski A, Shi S, Sun T, Bartold PM, Koblar SA, Gronthos S. EphB/ephrin-B interaction mediates adult stem cell attachment, spreading, and migration: implications for dental tissue repair. Stem Cells. 2007;25:156–64. doi: 10.1634/stemcells.2006-0373. [DOI] [PubMed] [Google Scholar]
- 8.Chal J, Pourquié O. Patterning and Differentiation of the Vertebrate Spine. In: Pourquié O, ed. The Skeletal System New York: Cold Spring Harbor Laboratory Press, 2009:41-116. [Google Scholar]
- 9.Yasuhiko Y, Haraguchi S, Kitajima S, Takahashi Y, Kanno J, Saga Y. Tbx6-mediated Notch signaling controls somite-specific Mesp2 expression. Proc Natl Acad Sci U S A. 2006;103:3651–6. doi: 10.1073/pnas.0508238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morimoto M, Takahashi Y, Endo M, Saga Y. The Mesp2 transcription factor establishes segmental borders by suppressing Notch activity. Nature. 2005;435:354–9. doi: 10.1038/nature03591. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima Y, Morimoto M, Takahashi Y, Koseki H, Saga Y. Identification of Epha4 enhancer required for segmental expression and the regulation by Mesp2. Development. 2006;133:2517–25. doi: 10.1242/dev.02422. [DOI] [PubMed] [Google Scholar]
- 12.Barrios A, Poole RJ, Durbin L, Brennan C, Holder N, Wilson SW. Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr Biol. 2003;13:1571–82. doi: 10.1016/j.cub.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe T, Sato Y, Saito D, Tadokoro R, Takahashi Y. EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite segmentation. Proc Natl Acad Sci U S A. 2009;106:7467–72. doi: 10.1073/pnas.0902859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kullander K, Mather NK, Diella F, Dottori M, Boyd AW, Klein R. Kinase-dependent and kinase-independent functions of EphA4 receptors in major axon tract formation in vivo. Neuron. 2001;29:73–84. doi: 10.1016/S0896-6273(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 15.Twigg SR, Kan R, Babbs C, Bochukova EG, Robertson SP, Wall SA, et al. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc Natl Acad Sci U S A. 2004;101:8652–7. doi: 10.1073/pnas.0402819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieland I, Jakubiczka S, Muschke P, Cohen M, Thiele H, Gerlach KL, et al. Mutations of the ephrin-B1 gene cause craniofrontonasal syndrome. Am J Hum Genet. 2004;74:1209–15. doi: 10.1086/421532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zafeiriou DI, Pavlidou EL, Vargìami E. Diverse clinical and genetic aspects of craniofrontonasal syndrome. Pediatr Neurol. 2011;44:83–7. doi: 10.1016/j.pediatrneurol.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Compagni A, Logan M, Klein R, Adams RH. Control of skeletal patterning by ephrinB1-EphB interactions. Dev Cell. 2003;5:217–30. doi: 10.1016/S1534-5807(03)00198-9. [DOI] [PubMed] [Google Scholar]
- 19.Orioli D, Henkemeyer M, Lemke G, Klein R, Pawson T. Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. EMBO J. 1996;15:6035–49. [PMC free article] [PubMed] [Google Scholar]
- 20.Risley M, Garrod D, Henkemeyer M, McLean W. EphB2 and EphB3 forward signalling are required for palate development. Mech Dev. 2009;126:230–9. doi: 10.1016/j.mod.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Bush JO, Soriano P. Ephrin-B1 forward signaling regulates craniofacial morphogenesis by controlling cell proliferation across Eph-ephrin boundaries. Genes Dev. 2010;24:2068–80. doi: 10.1101/gad.1963210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson D, Wilkie AO. Craniosynostosis. Eur J Hum Genet. 2011;19:369–76. doi: 10.1038/ejhg.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wieacker P, Wieland I. Clinical and genetic aspects of craniofrontonasal syndrome: towards resolving a genetic paradox. Mol Genet Metab. 2005;86:110–6. doi: 10.1016/j.ymgme.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4:e315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xing W, Kim J, Wergedal J, Chen ST, Mohan S. Ephrin B1 regulates bone marrow stromal cell differentiation and bone formation by influencing TAZ transactivation via complex formation with NHERF1. Mol Cell Biol. 2010;30:711–21. doi: 10.1128/MCB.00610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004;18:572–83. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stadler HS, Higgins KM, Capecchi MR. Loss of Eph-receptor expression correlates with loss of cell adhesion and chondrogenic capacity in Hoxa13 mutant limbs. Development. 2001;128:4177–88. doi: 10.1242/dev.128.21.4177. [DOI] [PubMed] [Google Scholar]
- 28.Patel K, Nittenberg R, D’Souza D, Irving C, Burt D, Wilkinson DG, et al. Expression and regulation of Cek-8, a cell to cell signalling receptor in developing chick limb buds. Development. 1996;122:1147–55. doi: 10.1242/dev.122.4.1147. [DOI] [PubMed] [Google Scholar]
- 29.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2012;13:27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 30.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards JR, Mundy GR. Advances in osteoclast biology: old findings and new insights from mouse models. Nat Rev Rheumatol. 2011;7:235–43. doi: 10.1038/nrrheum.2011.23. [DOI] [PubMed] [Google Scholar]
- 32.Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem. 2004;279:26475–80. doi: 10.1074/jbc.M313973200. [DOI] [PubMed] [Google Scholar]
- 33.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 34.Sims NA. EPHs and Ephrins: Many Pathways to Regulate Osteoblasts and Osteoclasts. IBMS BoneKEy. 2010;7:304–13. doi: 10.1138/20100463. [DOI] [Google Scholar]
- 35.Kuroda C, Kubota S, Kawata K, Aoyama E, Sumiyoshi K, Oka M, et al. Distribution, gene expression, and functional role of EphA4 during ossification. Biochem Biophys Res Commun. 2008;374:22–7. doi: 10.1016/j.bbrc.2008.06.089. [DOI] [PubMed] [Google Scholar]
- 36.Kwan Tat S, Pelletier JP, Amiable N, Boileau C, Lavigne M, Martel-Pelletier J. Treatment with ephrin B2 positively impacts the abnormal metabolism of human osteoarthritic chondrocytes. Arthritis Res Ther. 2009;11:R119. doi: 10.1186/ar2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–21. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Irie N, Takada Y, Watanabe Y, Matsuzaki Y, Naruse C, Asano M, et al. Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J Biol Chem. 2009;284:14637–44. doi: 10.1074/jbc.M807598200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng S, Zhao SL, Nelson B, Kesavan C, Qin X, Wergedal J, et al. Targeted disruption of ephrin B1 in cells of myeloid lineage increases osteoclast differentiation and bone resorption in mice. PLoS One. 2012;7:e32887. doi: 10.1371/journal.pone.0032887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allan EH, Häusler KD, Wei T, Gooi JH, Quinn JM, Crimeen-Irwin B, et al. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res. 2008;23:1170–81. doi: 10.1359/jbmr.080324. [DOI] [PubMed] [Google Scholar]
- 41.Lamont RE, Childs S. MAPping out arteries and veins. Sci STKE 2006; 2006:pe39. [DOI] [PubMed]
- 42.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–53. doi: 10.1016/S0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 43.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4:403–14. doi: 10.1016/S1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- 45.Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–44. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korff T, Dandekar G, Pfaff D, Füller T, Goettsch W, Morawietz H, et al. Endothelial ephrinB2 is controlled by microenvironmental determinants and associates context-dependently with CD31. Arterioscler Thromb Vasc Biol. 2006;26:468–74. doi: 10.1161/01.ATV.0000200081.42064.e7. [DOI] [PubMed] [Google Scholar]
- 47.Pfaff D, Héroult M, Riedel M, Reiss Y, Kirmse R, Ludwig T, et al. Involvement of endothelial ephrin-B2 in adhesion and transmigration of EphB-receptor-expressing monocytes. J Cell Sci. 2008;121:3842–50. doi: 10.1242/jcs.030627. [DOI] [PubMed] [Google Scholar]
- 48.Kartsogiannis V, Zhou H, Horwood NJ, Thomas RJ, Hards DK, Quinn JM, et al. Localization of RANKL (receptor activator of NF κ B ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone. 1999;25:525–34. doi: 10.1016/S8756-3282(99)00214-8. [DOI] [PubMed] [Google Scholar]
- 49.Silvestrini G, Ballanti P, Patacchioli F, Leopizzi M, Gualtieri N, Monnazzi P, et al. Detection of osteoprotegerin (OPG) and its ligand (RANKL) mRNA and protein in femur and tibia of the rat. J Mol Histol. 2005;36:59–67. doi: 10.1007/s10735-004-3839-1. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–6. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 51.Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, et al. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–91. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 52.Kanzaki S, Takada Y, Niida S, Takeda Y, Udagawa N, Ogawa K, et al. Impaired vibration of auditory ossicles in osteopetrotic mice. Am J Pathol. 2011;178:1270–8. doi: 10.1016/j.ajpath.2010.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grigoriadis AE, Schellander K, Wang ZQ, Wagner EF. Osteoblasts are target cells for transformation in c-fos transgenic mice. J Cell Biol. 1993;122:685–701. doi: 10.1083/jcb.122.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsuo K, Owens JM, Tonko M, Elliott C, Chambers TJ, Wagner EF. Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat Genet. 2000;24:184–7. doi: 10.1038/72855. [DOI] [PubMed] [Google Scholar]
- 55.Song I, Kim JH, Kim K, Jin HM, Youn BU, Kim N. Regulatory mechanism of NFATc1 in RANKL-induced osteoclast activation. FEBS Lett. 2009;583:2435–40. doi: 10.1016/j.febslet.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 56.Mäkinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mao Y, Huang X, Zhao J, Gu Z. Preliminary identification of potential PDZ-domain proteins downstream of ephrin B2 during osteoclast differentiation of RAW264.7 cells. Int J Mol Med. 2011;27:669–77. doi: 10.3892/ijmm.2011.639. [DOI] [PubMed] [Google Scholar]
- 58.Pierroz DD, Rufo A, Bianchi EN, Glatt V, Capulli M, Rucci N, et al. β-Arrestin2 regulates RANKL and ephrins gene expression in response to bone remodeling in mice. J Bone Miner Res. 2009;24:775–84. doi: 10.1359/jbmr.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harris WH, Heaney RP. Skeletal renewal and metabolic bone disease. N Engl J Med. 1969;280:253–9. doi: 10.1056/NEJM196901302800507. [DOI] [PubMed] [Google Scholar]
- 60.Hattner R, Epker BN, Frost HM. Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature. 1965;206:489–90. doi: 10.1038/206489a0. [DOI] [PubMed] [Google Scholar]
- 61.Eriksen EF. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord. 2010;11:219–27. doi: 10.1007/s11154-010-9153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin TJ, Sims NA, Quinn JMW. Interactions among osteoblasts, osteoclasts, and other cells in bone. In: Lorenzo J, Choi Y, Horowitz M, Takayanagi H, eds. Osteoimmunology: Interactions of the immune and skelatal systems: Elsevier, 2011:227-67. [Google Scholar]
- 63.Sims NA, Gooi JH. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19:444–51. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 64.Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone. 2009;44:1026–33. doi: 10.1016/j.bone.2009.03.671. [DOI] [PubMed] [Google Scholar]
- 65.Walker EC, McGregor NE, Poulton IJ, Pompolo S, Allan EH, Quinn JM, et al. Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J Bone Miner Res. 2008;23:2025–32. doi: 10.1359/jbmr.080706. [DOI] [PubMed] [Google Scholar]
- 66.Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A. 2008;105:20764–9. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, et al. TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–65. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guaiquil VH, Swendeman S, Zhou W, Guaiquil P, Weskamp G, Bartsch JW, et al. ADAM8 is a negative regulator of retinal neovascularization and of the growth of heterotopically injected tumor cells in mice. J Mol Med (Berl) 2010;88:497–505. doi: 10.1007/s00109-010-0591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mundy GR, Elefteriou F. Boning up on ephrin signaling. Cell. 2006;126:441–3. doi: 10.1016/j.cell.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 70.Tran Van PT, Vignery A, Baron R. Cellular kinetics of the bone remodeling sequence in the rat. Anat Rec. 1982;202:445–51. doi: 10.1002/ar.1092020403. [DOI] [PubMed] [Google Scholar]
- 71.Vignery A, Baron R. Dynamic histomorphometry of alveolar bone remodeling in the adult rat. Anat Rec. 1980;196:191–200. doi: 10.1002/ar.1091960210. [DOI] [PubMed] [Google Scholar]
- 72.Everts V, Delaissé JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P, et al. The bone lining cell: its role in cleaning Howship’s lacunae and initiating bone formation. J Bone Miner Res. 2002;17:77–90. doi: 10.1359/jbmr.2002.17.1.77. [DOI] [PubMed] [Google Scholar]
- 73.Luiz de Freitas PH, Li M, Ninomiya T, Nakamura M, Ubaidus S, Oda K, et al. Intermittent PTH administration stimulates pre-osteoblastic proliferation without leading to enhanced bone formation in osteoclast-less c-fos(-/-) mice. J Bone Miner Res. 2009;24:1586–97. doi: 10.1359/jbmr.090413. [DOI] [PubMed] [Google Scholar]
- 74.Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473:201–9. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 75.Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17:1473–80. doi: 10.1038/nm.2489. [DOI] [PubMed] [Google Scholar]
- 76.Okamoto M, Murai J, Imai Y, Ikegami D, Kamiya N, Kato S, et al. Conditional deletion of Bmpr1a in differentiated osteoclasts increases osteoblastic bone formation, increasing volume of remodeling bone in mice. J Bone Miner Res. 2011;26:2511–22. doi: 10.1002/jbmr.477. [DOI] [PubMed] [Google Scholar]
- 77.Kwan Tat S, Pelletier JP, Amiable N, Boileau C, Lajeunesse D, Duval N, et al. Activation of the receptor EphB4 by its specific ligand ephrin B2 in human osteoarthritic subchondral bone osteoblasts. Arthritis Rheum. 2008;58:3820–30. doi: 10.1002/art.24029. [DOI] [PubMed] [Google Scholar]
- 78.Kitamura T, Kabuyama Y, Kamataki A, Homma MK, Kobayashi H, Aota S, et al. Enhancement of lymphocyte migration and cytokine production by ephrinB1 system in rheumatoid arthritis. Am J Physiol Cell Physiol. 2008;294:C189–96. doi: 10.1152/ajpcell.00314.2007. [DOI] [PubMed] [Google Scholar]
- 79.Romanovsky AA, Ivanov AI, Petersen SR. Microsomal prostaglandin E synthase-1, ephrins, and ephrin kinases as suspected therapeutic targets in arthritis: exposed by “criminal profiling”. Ann N Y Acad Sci. 2006;1069:183–94. doi: 10.1196/annals.1351.016. [DOI] [PubMed] [Google Scholar]
- 80.Edwards CM, Zhuang J, Mundy GR. The pathogenesis of the bone disease of multiple myeloma. Bone. 2008;42:1007–13. doi: 10.1016/j.bone.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roodman GD. Osteoblast function in myeloma. Bone. 2011;48:135–40. doi: 10.1016/j.bone.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 82.Pennisi A, Ling W, Li X, Khan S, Shaughnessy JD, Jr., Barlogie B, et al. The ephrinB2/EphB4 axis is dysregulated in osteoprogenitors from myeloma patients and its activation affects myeloma bone disease and tumor growth. Blood. 2009;114:1803–12. doi: 10.1182/blood-2009-01-201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–94. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 84.Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, et al. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106:3160–5. doi: 10.1182/blood-2004-12-4940. [DOI] [PubMed] [Google Scholar]
- 85.Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J, et al. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008;112:196–207. doi: 10.1182/blood-2008-01-132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vallet S, Mukherjee S, Vaghela N, Hideshima T, Fulciniti M, Pozzi S, et al. Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proc Natl Acad Sci U S A. 2010;107:5124–9. doi: 10.1073/pnas.0911929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar SR, Scehnet JS, Ley EJ, Singh J, Krasnoperov V, Liu R, et al. Preferential induction of EphB4 over EphB2 and its implication in colorectal cancer progression. Cancer Res. 2009;69:3736–45. doi: 10.1158/0008-5472.CAN-08-3232. [DOI] [PubMed] [Google Scholar]
- 88.Fritsche-Guenther R, Noske A, Ungethüm U, Kuban RJ, Schlag PM, Tunn PU, et al. De novo expression of EphA2 in osteosarcoma modulates activation of the mitogenic signalling pathway. Histopathology. 2010;57:836–50. doi: 10.1111/j.1365-2559.2010.03713.x. [DOI] [PubMed] [Google Scholar]
- 89.Varelias A, Koblar SA, Cowled PA, Carter CD, Clayer M. Human osteosarcoma expresses specific ephrin profiles: implications for tumorigenicity and prognosis. Cancer. 2002;95:862–9. doi: 10.1002/cncr.10749. [DOI] [PubMed] [Google Scholar]
- 90.Abdou AG, Abd el-Wahed MM, Asaad NY, Samaka RM, Abdallaha R. Ephrin A4 expression in osteosarcoma, impact on prognosis, and patient outcome. Indian J Cancer. 2010;47:46–52. doi: 10.4103/0019-509X.58859. [DOI] [PubMed] [Google Scholar]
- 91.Kalinski T, Röpke A, Sel S, Kouznetsova I, Röpke M, Roessner A. Down-regulation of ephrin-A5, a gene product of normal cartilage, in chondrosarcoma. Hum Pathol. 2009;40:1679–85. doi: 10.1016/j.humpath.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 92.Martin TJ, Allan EH, Ho PW, Gooi JH, Quinn JM, Gillespie MT, et al. Communication between ephrinB2 and EphB4 within the osteoblast lineage. Adv Exp Med Biol. 2010;658:51–60. doi: 10.1007/978-1-4419-1050-9_6. [DOI] [PubMed] [Google Scholar]
- 93.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–20. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]