Abstract
Historically, a hallmark of tumorigenesis was the ability to grow in an anchorage-independent manner. Hence, tumors were thought to proliferate and survive independently of integrin attachment to the substratum. However, recent data suggest that integrins regulate not only tumor cell proliferation, survival and migration, but may also influence their response to anti-cancer agents. Interestingly, these influences are largely masked by growth of tumor cells in the standard, yet artificial, environment of 2D cell culture, but are readily apparent under 3D in vitro culture conditions and in tumor growth in vivo. We, and others, have recently demonstrated that the β1 integrin subunit controls the growth and invasion of prostate tumor cells in 3D culture conditions. Recently, the importance of integrins has also been demonstrated using tissue specific conditional knockout strategies in transgenic mouse tumor models, where they control primary tumor growth and dictate the site of metastatic spread. Furthermore, integrin-extracellular matrix interactions may modulate the response of tumors to standard chemotherapy agents or radiation. Taken together, these results highlight the important role of integrins in regulating tumor growth and metastasis; however, point out that the evaluation of their contribution to these processes requires appropriate contextual modeling.
Keywords: 3D growth, drug resistance, fibronectin, invasion, matrix metalloproteinase, tumor, β1 integrin
Integrins
Integrins are heterodimeric cell surface molecules that link the internal signaling components of the cytoskeleton to the extracellular proteinacious microenvironment. There are 18 α and 8 β subunits, comprising 24 unique integrin receptor heterodimers with varied affinities for binding different extracellular matrix (ECM) proteins.1,2 Integrins are capable of mediating signal transduction through the cell membrane in both directions: binding of integrins to ECM ligands results in cell signals that have effects on proliferation, survival, migration and gene expression (termed outside-in signaling) and signals from within the cell, as a result of, for example growth factor stimulation, can act to regulate integrin ligand-binding affinity and cell adhesion (termed inside-out signaling).1,3 Signals from the microenvironment are transmitted through integrins with the aid of a variety of signaling partners such as adaptor proteins and intracellular protein kinases including focal adhesion kinase (FAK)4-8 and integrin-linked kinase (ILK).9-13
By far the most commonly found subchain in integrin heterodimers is β1 integrin, which has been shown to pair with a variety of different α subchains to form 12 different known integrins.1,2 Importantly, the integrin heterodimers that predominantly bind the ECM proteins that are upregulated in tumors contain the β1 subchain.14,15 A number of studies have demonstrated that the ECM composition in tumors is vastly different than that of its normal tissue counterparts, with generally decreased levels of the basement membrane ECM proteins laminin and collagen IV, and increased levels of ECM proteins associated with remodeling tissues such as fibronectin, collagen I and tenascin-C.16-28 As we have an ongoing interest in prostate cancer progression and metastasis, we became interested in the potential regulation of prostate tumor growth by ECM-β1 integrin interactions. Similar to other tumor types, prostate tumors have been shown to have decreased expression of collagen VII and increased expression of fibronectin.29-31 Although β1 integrin has been reported to be expressed in normal prostate epithelium,32 its expression is increased in prostate tumor cells33,34 and is correlated with worse overall outcomes in prostate carcinoma patients.35 β1 integrin has also been shown to be the predominant β integrin expressed in prostate cancer cell lines.36 Given these reported associations, we were prompted to further examine the role of β1 integrin in prostate tumor growth and metastasis using relevant preclinical models.
β1 Integrin Regulates Tumorigenesis in 3D Contexts
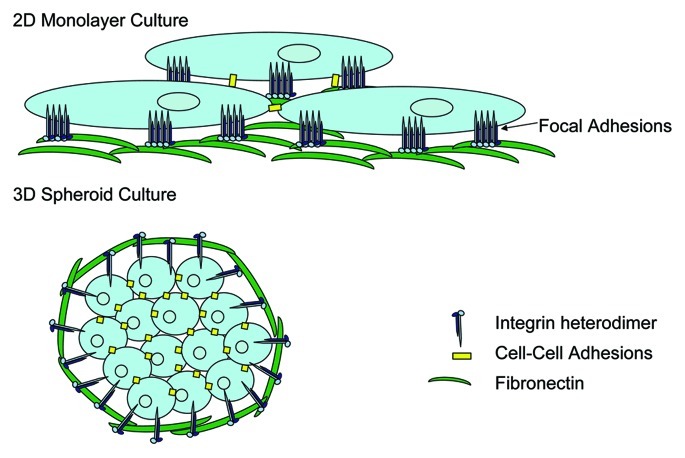
Traditionally, analysis of tumor cell growth and phenotype has been performed using in vitro models based on 2D adherent cell growth, likely as a result of the relative ease of this technique. In reality, growth in a 2D monolayer does not take into account environmental stimuli that tumor cells likely experience in vivo, and cells are known to form focal adhesions whereby integrins and their signal transduction partners are clustered (Fig. 1). Additionally, stromal-derived signals, including those from other tumor resident cells or stromally produced ECM proteins would be absent under these conditions. While the most appropriate 3D tumor growth modeling would be using in vivo xenograft or orthotopic tumor growth in animal models, the value of assessing tumor growth using 3D in vitro modeling techniques has been recently discussed.37,38 This is primarily performed in the context of artificial microenvironments that enforce 3D growth of cells, such as soft agarose, or more recently using more relevant basement membrane ECM extracts produced by tumor cells (e.g., matrigel). Although not without its limitations, in vitro 3D tumor cell growth does allow for more appropriate assessment of the contribution of tumor microenvironmental factors such as ECM and integrin signaling to tumor growth and invasion. In this context, cells predominantly grow as spheroids with numerous cell-cell contacts in place and no evidence of focal adhesions but instead sites of focal contacts (Fig. 1). It is likely that ECM-integrin engagement plays a significant role in the prevention of detachment-mediated death (termed anoikis) in cells grown in these contexts.
Figure 1. Tumor cell growth in 2D vs. 3D results in differential integrin sublocalization. Cells grown in 2D tissue culture form monolayers which result in fewer cell-cell contact points, and the clustering of integrins and their associated signal transduction molecules at sites of focal adhesion contacts between the cells and the culture surface. In contrast, growth in 3D promotes cell growth in clusters or spheroids whereby cell-cell contacts are increased, and integrins are not clustered at sites of focal contacts, but may be more dispersed across the cell membrane in association with ECM proteins at a multitude of points. This lack of integrin clustering likely leads to different signal transduction events in cells grown in 3D as compared with those grown in 2D and hence may render the cell more dependent on ECM engagement by integrins to overcome anoikis.
Our group has recently published that depletion of β1 integrin in the PC3 prostate carcinoma cell line, abolished the ability of these tumor cells to grow in 3D anchorage-independent growth assays in soft agarose and impaired their 3D growth in matrigel.39 We further observed that inhibition of fibronectin-β1 integrin interactions following use of neutralizing antibodies to fibronectin resulted in a similar inhibition of anchorage-independent growth, suggesting that this ECM-integrin interaction plays an important role in this process. Interestingly, we saw no difference in the growth or survival of β1-depleted tumor cells [including prostate,39 lung and neuroblastoma tumor lines (unpublished personal findings)], following growth in 2D culture conditions, possibly as a result of other β-subunit containing integrins compensating for lack of β1 under these conditions Alternatively, the fact that 2D culture promotes clustering of integrins and their signaling partners at sites of focal adhesions may result in cell signaling that significantly differs than that which occurs in 3D growth.
Goel et al. have recently published findings that also suggest a role for β1 integrin in regulating 3D growth of prostate tumor cells.40 They also observed reduced colony formation in 3D matrigel by β1 integrin-depleted prostate tumor cells.40 However in contrast to our findings, they observed that depletion of β1 integrin resulted in equal numbers of tumor cell colonies, but with reduced colony size in their 3D matrigel assays. The reduced colony size was attributed to a proliferative defect resulting from lack of Gli1 expression (a transcription factor that functions as an effector of hedgehog signaling to modulate cell proliferation and apoptosis), in β1 integrin-depleted cells. Interestingly, we did not observe proliferative defects in our 3D growth model assays (which used growth factor depleted matrigel), suggesting that differences in the composition of the microenvironment, for example, the presence of various growth factors, may also influence the observed β1 integrin-controlled phenotypes.
When considering differences between 2D culture conditions and 3D culture systems such as soft agar or matrigel, one should note that these environments are not necessarily identical in terms of the rigidity of the surfaces that are in contact with the cells. Cells cultured in 3D environments in vitro are in contact with substrate that is conceivably less rigid than that encountered when cells are directly plated onto a tissue culture plate, be it uncoated or even coated with ECM. Thus the elasticity of the growth environment may also be considered as a contributing factor to phenotypic differences observed in 2D vs. 3D assay conditions. Indeed, the importance of ECM elasticity has recently been noted for a variety of processes including transcription and replication,41 and for the self-renewal and differentiation of stem cell populations in culture,42-44 among others. Interestingly, the study by Kocgozlu et al.41 indicated that substrate rigidity affected both integrin and FAK activation, with a small window of optimal substrate elasticity for the activation of FAK to occur. A similar phenomenon was also observed by Wei and colleagues, who saw an inhibition of β1-integrin activation and decreased phosphorylation of FAK on soft substrate.45 In contrast, the study by Du et al.44 indicated that the reliance on specific ECM elasticity for cell lineage specificity was due to increased activation and internalization of β1 integrin on soft ECM substrates. The differences seen in integrin activation in these studies suggests that integrin regulation of the observed phenotypes may be cell type-dependent in addition to being influenced by elasticity and hence these factors should also be considered when examining the role of integrin signaling in vitro.
β1 Integrin Control of Tumor Growth in 3D In Vivo Models
Tumor growth can be regulated at three different levels: tumor initiation usually resulting from deregulated cell proliferation following acquisition of genetic mutations; tumor progression, including the ability to induce angiogenesis; and tumor invasion whereby tumor cells gain enhanced migratory and invasive abilities to access the circulation and intravasate into new sites of metastatic tumor growth. Recently, a significant role for β1 integrin in tumor initiation has been demonstrated in transgenic mouse models of breast cancer. In this system, disruption of β1 integrin specifically in the mammary epithelium essentially blocked the polyomavirus middle T antigen (PyV MT) oncogene-driven tumorigenic process.46 Importantly, in this highly tumorigenic background (PyV MT oncogene), not only was initiation of tumors prevented, but there was also no evidence of hyperplasia in β1-depleted mammary epithelium. Similar studies in the PyV MT background in which the downstream integrin associated kinase FAK was specifically deleted in mammary epithelium, also resulted in inhibition of breast tumor progression; however, in these animals, evidence of pre-neoplastic lesions was present.47 This suggests that although FAK contributes downstream of β1 integrin in modulating initiation and progression of breast tumors, β1 integrin has additional roles that appear to be independent of FAK in mediating tumor initiation. Our data also suggested that β1 integrin played a significant role in the initiation of colony formation in the 3D assays; however, future work is required to elucidate the mechanisms by which β1 integrin controls the process of tumor initiation.
Interestingly, in contrast to the results observed in transgenic mouse mammary tumor models driven by PyV MT, mammary-specific deletion of β1 integrin in activated ErbB2 oncogene driven mammary tumors readily formed tumors with only a one month delay in onset.48 The β1 integrin-deleted ErbB2 tumors did however have significant defects in tumor progression, with significantly smaller, less angiogenic tumors developing compared with β1 integrin-expressing control tumors. These findings support the notion that the contribution of β1 integrin to tumor initiation and progression is modulated by other important factors, such as growth factor stimulation, or the type of oncogenic tumor transformation.
β1 Integrin Regulates Tumor Cell Invasion
As tumors progress, they acquire increased invasion capabilities, in part via their ability to induce degradation of their surrounding extracellular environment. This is primarily mediated through their ability to regulate matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) expression, thereby facilitating ECM degradation and tumor cell migration.49-51 In addition to modulation of anchorage-independent growth, we also identified an important role for β1 integrin in regulating tumor cell invasion through 3D ECM gels.39 We elucidated a putative mechanism whereby β1 integrin-depleted prostate tumor cells expressed decreased levels of MMP-9 with concomitant increased expression of TIMP2 as compared with control cells following culture on fibronectin. This suggests that β1-containing integrins, likely α5β1 which is one of the primary fibronectin receptors in tumor cells, are responsible for the upregulation of MMP-9. Previous studies have shown that fibronectin can induce MMP-9 expression in a α5 integrin-dependent manner in both breast and laryngeal carcinoma cells supporting this hypothesis.52,53 In conjunction with suppressing the expression of the endogenous MMP inhibitor TIMP-2, this would result in an overall enhancement of protease activity and invasive capabilities. Although we were unable to find published evidence that β1 integrin-fibronectin interaction controls TIMP-2 expression in tumor cells, this has been shown to be the case in T-cells where fibronectin engagement significantly inhibited TIMP-2 expression.54 Interestingly, we did not observe β1 integrin-regulated suppression of TIMP-2 expression when the shRNA transduced PC3 cell clones were cultured on plastic as similar levels of TIMP-2 expression was observed in control and β1 integrin depleted PC3 cells (unpublished personal data). This further highlights the importance of β1 integrin in regulating tumor invasion particularly in the context of tumor-associated ECM proteins such as fibronectin. The fibronectin-β1 integrin regulation of MMP-9 (or lack of it in the case of β1-integrin depleted cells) may also be directly contributing to the colony formation in soft agar, as other studies have shown that MMP-9 is required for STAT3C-induced transformation (a constitutively active form of the transcription factor STAT3 which promotes growth in 3D conditions) and anchorage-independent growth of normal mammary epithelial cells.55 These observations support the contention that regulation of these proteins is specific to integrin-fibronectin interactions in our system.
Important roles for other β1-containing integrins, namely α1β1 and α2β1 in modulating tumor cell invasion have also been demonstrated in hepatocellular carcinoma cells;56 however, no direct link to regulation of MMP activity by integrin-ECM engagement was investigated in these studies. Additional evidence for β1-subunit containing integrin control of tumor cell invasion has also been demonstrated in melanoma,57 osteosarcoma,58 glioma,59,60 ovarian carcinoma61 and hepatocellular carcinoma.62 More recently, a direct interaction between the β1 integrin cytoplasmic tail and the GTPase Rab25 has been demonstrated.63 Rab25 has also been linked to tumor aggressiveness and metastasis and can promote directional migration on 3D matrices by promoting localization of vesicles that deliver integrins to the plasma membrane at the cell front. Interestingly, this Rab25-driven tumor-cell invasion is strongly dependent on ligation of fibronectin by α5β1 integrin and the capacity of Rab25 to interact with β1 integrin, again supporting our contention that fibronectin-β1 integrin interactions may be important in prostate tumor cell invasion.
β1 Integrin in Metastasis
In addition to a putative role for β1 integrin in tumor initiation and progression, there is also increasing evidence that β1 integrin may regulate tumor metastasis in vivo. For example, in transgenic mouse mammary tumor models driven by the activated ErbB2 oncogene, mammary-specific deletion of β1 integrin resulted in a significant reduction in the number of lung metastases that spontaneously arose in the β1 integrin-deleted ErbB2 expressing animals.48 β1 integrin is also upregulated in a number of human tumor cells,64 and its overexpression appears to correlate with more aggressive phenotypes. For example, in a study evaluating expression of the integrin heterodimer α3β1 in paired primary and metastatic breast cancer biopsies, there was a significant increase in expression in the metastatic lesions compared with their counterpart primary tumors.65 Increased β1 integrin expression has also been found in ovarian carcinoma cells isolated from pleural effusions as compared with primary ovarian tumor cells.66 The β1 integrin-fibronectin interaction has also been suggested to be important in determining metastatic potential, as a study looking at breast cancer cells with varying degrees of metastatic ability showed a fibronectin-dependent, β1 integrin-mediated control over the ability of metastatic cells to sense the rigidity of the microenvironment;67 an effect that could contribute to the increased metastatic ability of cells expressing higher levels of β1 integrin.
There is also experimental support for a role of specific integrin subunits in regulating the sites of tumor metastasis. For example, α4β1 positive melanoma cells were found to establish bone metastases, while α4β1 negative cells only readily formed pulmonary metastases.68 Similarly, α2β1 or α3β1 overexpression was correlated with the ability of gastric carcinoma cells to spread specifically to the peritoneum.69 The interaction of overexpressed α5β1 with fibronectin also facilitated the metastasis of Chinese hamster ovary cells to the kidney in mouse models, while the counterpart parental cells did not metastasize to the kidneys.70 The results of these and other studies suggest targeted inhibition of β1 integrins may limit the not only the metastatic spread of certain tumor types, but also their spread to particular organ sites.
β1 Integrin-ECM Engagement May Influence Response to Therapy
There is increasing evidence that not only does β1 integrin modulate tumor initiation and progression, but it may also regulate tumor cell response to chemo- and radiation therapy. β1 integrin-fibronectin interactions have been shown to confer resistance of multiple myeloma cells to a number of chemotherapy agents including doxorubicin, melphalan and etoposide.71 β1 integrin-fibronectin induced resistance to etoposide was also observed in Burkitt lymphoma,72 and in small cell lung carcinoma (SCLC).73 SCLC was also found to be resistant to doxorubicin, etoposide, cisplatinum and cyclophosphamide following similar engagement of β1 integrins by fibronectin.73 At least with respect to etoposide treatment, β1 integrin engagement of fibronectin inhibited caspase-3 induced apoptosis of SCLC.74 Ligation of β1 integrins by ECM ligand also significantly inhibited the apoptosis induced by the microtubule-directed chemotherapy drugs paclitaxel and vincristine in two different breast cancer cell lines.75 The β1 integrin-mediated inhibition of apoptosis in these cases was mediated via inhibition of a PI3K-dependent cytochrome c release from the mitochondria in response to drug treatment.
β1 integrin engagement has also been associated with increased resistance to radiation treatment. In lung cancer cell lines, radiation treatment was found to induce increased expression of β1 integrin and its downstream signaling partner ILK,76 which in turn resulted in modulation of GSK-3β and Akt activities to enhance cell survival post irradiation.77 Similar observations were made in glioma cells, where β1 integrin was found to confer enhanced survival in response to radiation via its ability to activate PI3K and Akt.78 The β1 integrin induced activation of PI3K was also observed as a mechanism of resistance to radiation and etoposide in SCLC cells by overriding the G2/M checkpoint induced following DNA damage by the agents hence facilitating continued proliferation of these cells in the presence of this damage.79 Interestingly, in a breast cancer xenograft, targeted inhibition of β1 integrin with an inhibitory antibody post-radiation, effectively enhanced tumor growth inhibition, with tumor cells exhibiting decreased Akt activity following combination treatment.80 This enhanced inhibitory activity following blockade of β1 integrin allowed for a lower efficacious dose of radiation to be used with similar levels of tumor growth inhibition being achieved.80 Importantly, the influence of 2D vs. 3D assay systems on the tumor cell response to radiation has also been recently discussed.81 Taken together, these results highlight the importance of ECM-integrin engagement in response to standard anti-cancer treatments and suggest that evaluation of the role of these mechanisms in cancer patients is warranted.
As more pre-clinical data supporting the important role of β1 integrin in modulating tumor growth, progression and response to therapies is unveiled, it is not surprising that novel approaches targeting integrins or their signaling pathway are beginning to be evaluated as targeted anti-cancer agents. Targeting of ILK,82-85 or more recently FAK,86-91 with small molecule tyrosine kinase inhibitors effectively inhibits tumor growth in a variety of xenograft models. Specific inhibitors to the α5β1 integrin heterodimer have also been shown to attenuate glioma growth and invasion in organ slice cultures92 and impair colorectal cancer metastases in xenograft models.93 However, clinical evaluation of these agents appears to still be in its early stages, so we will have to await the outcomes reporting their ability to act as effective anti-cancer agents.
Summary
Given the increasing evidence supporting the role of β1 integrins in tumorigenesis and response to therapy, future work elucidating the specific mechanisms of these responses is warranted. As clearly indicated by our results and those of others, however, the experimental conditions under which the influence of β1 integrin is evaluated can affect the observed outcome. Culture in 2D creates a rather artificial environment whereby cells grow in a monolayer attached to substratum by integrins that are clustered, along with their signaling partners, in focal adhesions with fewer cell-cell contacts being formed. Growth in 3D however, creates a situation whereby significant increases in cell-cell contact points are created, and integrins likely engage ECM ligands in order to overcome anoikis-mediated apoptosis signals and survive in this context (Fig. 1). Furthermore, given the possible influences of various microenvironmental factors such as the varied composition and elasticity of ECM, along with the presence of various growth factors, definitive analysis of specific mechanisms of β1 integrin regulation of tumorigenic processes will require modeling in the most appropriate contextual environments that best mimics each tumor type or treatment setting if we are to truly understand its role in tumor growth and metastasis in patients.
Acknowledgments
This work was supported by a research grant awarded to C.L.A. from the Canadian Institutes of Health Research (MOP-84369). G.A.H. is a recipient of an award from the Ontario Graduate Scholarships in Science and Technology (OGSST).
Glossary
Abbreviations:
- 2D
two dimensional
- 3D
three dimensional
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- GTPase
guanosine triphosphate hydrolase
- GSK
glycogen synthase kinase
- ILK
integrin linked kinase
- MMP
matrix metalloproteinase
- PI3K
phosphotidyl inositol 3′ kinase
- PyV MT
polyoma virus middle T antigen
- SCLC
small cell lung cancer
- shRNA
short hairpin ribonucleic acid
- STAT
signal transducer and activator of transcription
- TIMP
tissue inhibitor of metalloproteinases
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/20077
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Fu G, Wang W, Luo BH. Overview: structural biology of integrins. Methods Mol Biol. 2012;757:81–99. doi: 10.1007/978-1-61779-166-6_7. [DOI] [PubMed] [Google Scholar]
- 3.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-S. [DOI] [PubMed] [Google Scholar]
- 4.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–78. doi: 10.1016/S0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 5.Chen LM, Bailey D, Fernandez-Valle C. Association of beta 1 integrin with focal adhesion kinase and paxillin in differentiating Schwann cells. J Neurosci. 2000;20:3776–84. doi: 10.1523/JNEUROSCI.20-10-03776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons JT, Parsons SJ. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol. 1997;9:187–92. doi: 10.1016/S0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- 7.Guan JL. Integrin signaling through FAK in the regulation of mammary stem cells and breast cancer. IUBMB Life. 2010;62:268–76. doi: 10.1002/iub.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–23. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, et al. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91–6. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 10.Hannigan GE, McDonald PC, Walsh MP, Dedhar S. Integrin-linked kinase: not so ‘pseudo’ after all. Oncogene. 2011;30:4375–85. doi: 10.1038/onc.2011.177. [DOI] [PubMed] [Google Scholar]
- 11.Wu C. Integrin-linked kinase and PINCH: partners in regulation of cell-extracellular matrix interaction and signal transduction. J Cell Sci. 1999;112:4485–9. doi: 10.1242/jcs.112.24.4485. [DOI] [PubMed] [Google Scholar]
- 12.Tu Y, Li F, Goicoechea S, Wu C. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol. 1999;19:2425–34. doi: 10.1128/mcb.19.3.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P. Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer. 2010;10:858–70. doi: 10.1038/nrc2967. [DOI] [PubMed] [Google Scholar]
- 14.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–62. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 16.Schedin P, Keely PJ. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb Perspect Biol. 2011;3:a003228. doi: 10.1101/cshperspect.a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watt FM, Fujiwara H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol. 2011;3:a005124. doi: 10.1101/cshperspect.a005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagedorn HG, Bachmeier BE, Nerlich AG. Synthesis and degradation of basement membranes and extracellular matrix and their regulation by TGF-beta in invasive carcinomas (Review) Int J Oncol. 2001;18:669–81. doi: 10.3892/ijo.18.4.669. [Review] [DOI] [PubMed] [Google Scholar]
- 19.Lai KK, Shang S, Lohia N, Booth GC, Masse DJ, Fausto N, et al. Extracellular matrix dynamics in hepatocarcinogenesis: a comparative proteomics study of PDGFC transgenic and Pten null mouse models. PLoS Genet. 2011;7:e1002147. doi: 10.1371/journal.pgen.1002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lochter A, Bissell MJ. Involvement of extracellular matrix constituents in breast cancer. Semin Cancer Biol. 1995;6:165–73. doi: 10.1006/scbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- 21.Noel A, Kebers F, Maquoi E, Foidart JM. Cell-cell and cell-matrix interactions during breast cancer progression. Curr Top Pathol. 1999;93:183–93. doi: 10.1007/978-3-642-58456-5_19. [DOI] [PubMed] [Google Scholar]
- 22.Jang YC, Arumugam S, Ferguson M, Gibran NS, Isik FF. Changes in matrix composition during the growth and regression of human hemangiomas. J Surg Res. 1998;80:9–15. doi: 10.1006/jsre.1998.5355. [DOI] [PubMed] [Google Scholar]
- 23.Nair SA, Nair MB, Jayaprakash PG, Rajalekshmy TN, Nair MK, Pillai MR. The basement membrane and tumor progression in the uterine cervix. Gen Diagn Pathol. 1997;142:297–303. [PubMed] [Google Scholar]
- 24.Tuominen H, Kallioinen M. Increased tenascin expression in melanocytic tumors. J Cutan Pathol. 1994;21:424–9. doi: 10.1111/j.1600-0560.1994.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 25.Ramos DM, Chen B, Regezi J, Zardi L, Pytela R. Tenascin-C matrix assembly in oral squamous cell carcinoma. Int J Cancer. 1998;75:680–7. doi: 10.1002/(SICI)1097-0215(19980302)75:5<680::AID-IJC4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 26.Zeng ZS, Cohen AM, Guillem JG. Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during human colorectal tumorigenesis. Carcinogenesis. 1999;20:749–55. doi: 10.1093/carcin/20.5.749. [DOI] [PubMed] [Google Scholar]
- 27.Kannan S, Balaram P, Chandran GJ, Pillai MR, Mathew B, Nalinakumari KR, et al. Alterations in expression of basement membrane proteins during tumour progression in oral mucosa. Histopathology. 1994;24:531–7. doi: 10.1111/j.1365-2559.1994.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 28.Tosios KI, Kapranos N, Papanicolaou SI. Loss of basement membrane components laminin and type IV collagen parallels the progression of oral epithelial neoplasia. Histopathology. 1998;33:261–8. doi: 10.1046/j.1365-2559.1998.00452.x. [DOI] [PubMed] [Google Scholar]
- 29.Albrecht M, Renneberg H, Wennemuth G, Möschler O, Janssen M, Aumüller G, et al. Fibronectin in human prostatic cells in vivo and in vitro: expression, distribution, and pathological significance. Histochem Cell Biol. 1999;112:51–61. doi: 10.1007/s004180050391. [DOI] [PubMed] [Google Scholar]
- 30.Nagle RB, Hao J, Knox JD, Dalkin BL, Clark V, Cress AE. Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am J Pathol. 1995;146:1498–507. [PMC free article] [PubMed] [Google Scholar]
- 31.Nagle RB. Role of the extracellular matrix in prostate carcinogenesis. J Cell Biochem. 2004;91:36–40. doi: 10.1002/jcb.10692. [DOI] [PubMed] [Google Scholar]
- 32.Knox JD, Cress AE, Clark V, Manriquez L, Affinito KS, Dalkin BL, et al. Differential expression of extracellular matrix molecules and the alpha 6-integrins in the normal and neoplastic prostate. Am J Pathol. 1994;145:167–74. [PMC free article] [PubMed] [Google Scholar]
- 33.Cress AE, Rabinovitz I, Zhu W, Nagle RB. The alpha 6 beta 1 and alpha 6 beta 4 integrins in human prostate cancer progression. Cancer Metastasis Rev. 1995;14:219–28. doi: 10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- 34.Murant SJ, Handley J, Stower M, Reid N, Cussenot O, Maitland NJ. Co-ordinated changes in expression of cell adhesion molecules in prostate cancer. Eur J Cancer. 1997;33:263–71. doi: 10.1016/S0959-8049(96)00418-2. [DOI] [PubMed] [Google Scholar]
- 35.Pontes-Júnior J, Reis ST, de Oliveira LC, Sant’anna AC, Dall’oglio MF, Antunes AA, et al. Association between integrin expression and prognosis in localized prostate cancer. Prostate. 2010;70:1189–95. doi: 10.1002/pros.21153. [DOI] [PubMed] [Google Scholar]
- 36.Witkowski CM, Rabinovitz I, Nagle RB, Affinito KS, Cress AE. Characterization of integrin subunits, cellular adhesion and tumorgenicity of four human prostate cell lines. J Cancer Res Clin Oncol. 1993;119:637–44. doi: 10.1007/BF01215981. [DOI] [PubMed] [Google Scholar]
- 37.Nyga A, Cheema U, Loizidou M. 3D tumour models: novel in vitro approaches to cancer studies. J Cell Commun Signal. 2011;5:239–48. doi: 10.1007/s12079-011-0132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–65. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schooley AM, Andrews NM, Zhao H, Addison CL. β1 integrin is required for anchorage-independent growth and invasion of tumor cells in a context dependent manner. Cancer Lett. 2012;316:157–67. doi: 10.1016/j.canlet.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 40.Goel HL, Underwood JM, Nickerson JA, Hsieh CC, Languino LR. Beta1 integrins mediate cell proliferation in three-dimensional cultures by regulating expression of the sonic hedgehog effector protein, GLI1. J Cell Physiol. 2010;224:210–7. doi: 10.1002/jcp.22116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kocgozlu L, Lavalle P, Koenig G, Senger B, Haikel Y, Schaaf P, et al. Selective and uncoupled role of substrate elasticity in the regulation of replication and transcription in epithelial cells. J Cell Sci. 2010;123:29–39. doi: 10.1242/jcs.053520. [DOI] [PubMed] [Google Scholar]
- 42.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–81. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, et al. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat Biotechnol. 2010;28:1123–8. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- 44.Du J, Chen X, Liang X, Zhang G, Xu J, He L, et al. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc Natl Acad Sci U S A. 2011;108:9466–71. doi: 10.1073/pnas.1106467108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei WC, Lin HH, Shen MR, Tang MJ. Mechanosensing machinery for cells under low substratum rigidity. Am J Physiol Cell Physiol. 2008;295:C1579–89. doi: 10.1152/ajpcell.00223.2008. [DOI] [PubMed] [Google Scholar]
- 46.White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, et al. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–70. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 47.Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC, et al. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci U S A. 2007;104:20302–7. doi: 10.1073/pnas.0710091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huck L, Pontier SM, Zuo DM, Muller WJ. beta1-integrin is dispensable for the induction of ErbB2 mammary tumors but plays a critical role in the metastatic phase of tumor progression. Proc Natl Acad Sci U S A. 2010;107:15559–64. doi: 10.1073/pnas.1003034107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noël A, Jost M, Maquoi E. Matrix metalloproteinases at cancer tumor-host interface. Semin Cell Dev Biol. 2008;19:52–60. doi: 10.1016/j.semcdb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20:161–8. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cruz-Munoz W, Khokha R. The role of tissue inhibitors of metalloproteinases in tumorigenesis and metastasis. Crit Rev Clin Lab Sci. 2008;45:291–338. doi: 10.1080/10408360801973244. [DOI] [PubMed] [Google Scholar]
- 52.Maity G, Choudhury PR, Sen T, Ganguly KK, Sil H, Chatterjee A. Culture of human breast cancer cell line (MDA-MB-231) on fibronectin-coated surface induces pro-matrix metalloproteinase-9 expression and activity. Tumour Biol. 2011;32:129–38. doi: 10.1007/s13277-010-0106-9. [DOI] [PubMed] [Google Scholar]
- 53.Sen T, Dutta A, Maity G, Chatterjee A. Fibronectin induces matrix metalloproteinase-9 (MMP-9) in human laryngeal carcinoma cells by involving multiple signaling pathways. Biochimie. 2010;92:1422–34. doi: 10.1016/j.biochi.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Esparza J, Vilardell C, Calvo J, Juan M, Vives J, Urbano-Márquez A, et al. Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signaling pathways. Blood. 1999;94:2754–66. [PubMed] [Google Scholar]
- 55.Dechow TN, Pedranzini L, Leitch A, Leslie K, Gerald WL, Linkov I, et al. Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc Natl Acad Sci U S A. 2004;101:10602–7. doi: 10.1073/pnas.0404100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang C, Zeisberg M, Lively JC, Nyberg P, Afdhal N, Kalluri R. Integrin alpha1beta1 and alpha2beta1 are the key regulators of hepatocarcinoma cell invasion across the fibrotic matrix microenvironment. Cancer Res. 2003;63:8312–7. [PubMed] [Google Scholar]
- 57.Knutson JR, Iida J, Fields GB, McCarthy JB. CD44/chondroitin sulfate proteoglycan and alpha 2 beta 1 integrin mediate human melanoma cell migration on type IV collagen and invasion of basement membranes. Mol Biol Cell. 1996;7:383–96. doi: 10.1091/mbc.7.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vihinen P, Riikonen T, Laine A, Heino J. Integrin alpha 2 beta 1 in tumorigenic human osteosarcoma cell lines regulates cell adhesion, migration, and invasion by nteraction with type I collagen. Cell Growth Differ. 1996;7:439–47. [PubMed] [Google Scholar]
- 59.Tysnes BB, Larsen LF, Ness GO, Mahesparan R, Edvardsen K, Garcia-Cabrera I, et al. Stimulation of glioma-cell migration by laminin and inhibition by anti-alpha3 and anti-beta1 integrin antibodies. Int J Cancer. 1996;67:777–84. doi: 10.1002/(SICI)1097-0215(19960917)67:6<777::AID-IJC5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 60.Deryugina EI, Bourdon MA. Tenascin mediates human glioma cell migration and modulates cell migration on fibronectin. J Cell Sci. 1996;109:643–52. doi: 10.1242/jcs.109.3.643. [DOI] [PubMed] [Google Scholar]
- 61.Fishman DA, Kearns A, Chilukuri K, Bafetti LM, O’Toole EA, Georgacopoulos J, et al. Metastatic dissemination of human ovarian epithelial carcinoma is promoted by alpha2beta1-integrin-mediated interaction with type I collagen. Invasion Metastasis. 1998;18:15–26. doi: 10.1159/000024495. [DOI] [PubMed] [Google Scholar]
- 62.Masumoto A, Arao S, Otsuki M. Role of beta1 integrins in adhesion and invasion of hepatocellular carcinoma cells. Hepatology. 1999;29:68–74. doi: 10.1002/hep.510290146. [DOI] [PubMed] [Google Scholar]
- 63.Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, et al. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Juliano RL. The role of beta 1 integrins in tumors. Semin Cancer Biol. 1993;4:277–83. [PubMed] [Google Scholar]
- 65.Morini M, Mottolese M, Ferrari N, Ghiorzo F, Buglioni S, Mortarini R, et al. The alpha 3 beta 1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP-9) activity. Int J Cancer. 2000;87:336–42. doi: 10.1002/1097-0215(20000801)87:3<336::AID-IJC5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 66.Davidson B, Goldberg I, Reich R, Tell L, Dong HP, Trope’ CG, et al. AlphaV- and beta1-integrin subunits are commonly expressed in malignant effusions from ovarian carcinoma patients. Gynecol Oncol. 2003;90:248–57. doi: 10.1016/S0090-8258(03)00321-4. [DOI] [PubMed] [Google Scholar]
- 67.Indra I, Beningo KA. An in vitro correlation of metastatic capacity, substrate rigidity, and ECM composition. J Cell Biochem. 2011;112:3151–8. doi: 10.1002/jcb.23241. [DOI] [PubMed] [Google Scholar]
- 68.Matsuura N, Puzon-McLaughlin W, Irie A, Morikawa Y, Kakudo K, Takada Y. Induction of experimental bone metastasis in mice by transfection of integrin alpha 4 beta 1 into tumor cells. Am J Pathol. 1996;148:55–61. [PMC free article] [PubMed] [Google Scholar]
- 69.Nishimura S, Chung YS, Yashiro M, Inoue T, Sowa M. Role of alpha 2 beta 1- and alpha 3 beta 1-integrin in the peritoneal implantation of scirrhous gastric carcinoma. Br J Cancer. 1996;74:1406–12. doi: 10.1038/bjc.1996.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tani N, Higashiyama S, Kawaguchi N, Madarame J, Ota I, Ito Y, et al. Expression level of integrin alpha 5 on tumour cells affects the rate of metastasis to the kidney. Br J Cancer. 2003;88:327–33. doi: 10.1038/sj.bjc.6600710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hazlehurst LA, Valkov N, Wisner L, Storey JA, Boulware D, Sullivan DM, et al. Reduction in drug-induced DNA double-strand breaks associated with beta1 integrin-mediated adhesion correlates with drug resistance in U937 cells. Blood. 2001;98:1897–903. doi: 10.1182/blood.V98.6.1897. [DOI] [PubMed] [Google Scholar]
- 72.Taylor ST, Hickman JA, Dive C. Epigenetic determinants of resistance to etoposide regulation of Bcl-X(L) and Bax by tumor microenvironmental factors. J Natl Cancer Inst. 2000;92:18–23. doi: 10.1093/jnci/92.1.18. [DOI] [PubMed] [Google Scholar]
- 73.Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5:662–8. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 74.Rintoul RC, Sethi T. Extracellular matrix regulation of drug resistance in small-cell lung cancer. Clin Sci (Lond) 2002;102:417–24. doi: 10.1042/CS20010216. [DOI] [PubMed] [Google Scholar]
- 75.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 76.Cordes N, Blaese MA, Meineke V, Van Beuningen D. Ionizing radiation induces up-regulation of functional beta1-integrin in human lung tumour cell lines in vitro. Int J Radiat Biol. 2002;78:347–57. doi: 10.1080/09553000110117340. [DOI] [PubMed] [Google Scholar]
- 77.Cordes N, van Beuningen D. Cell adhesion to the extracellular matrix protein fibronectin modulates radiation-dependent G2 phase arrest involving integrin-linked kinase (ILK) and glycogen synthase kinase-3beta (GSK-3beta) in vitro. Br J Cancer. 2003;88:1470–9. doi: 10.1038/sj.bjc.6600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cordes N, Seidler J, Durzok R, Geinitz H, Brakebusch C. beta1-integrin-mediated signaling essentially contributes to cell survival after radiation-induced genotoxic injury. Oncogene. 2006;25:1378–90. doi: 10.1038/sj.onc.1209164. [DOI] [PubMed] [Google Scholar]
- 79.Hodkinson PS, Elliott T, Wong WS, Rintoul RC, Mackinnon AC, Haslett C, et al. ECM overrides DNA damage-induced cell cycle arrest and apoptosis in small-cell lung cancer cells through beta1 integrin-dependent activation of PI3-kinase. Cell Death Differ. 2006;13:1776–88. doi: 10.1038/sj.cdd.4401849. [DOI] [PubMed] [Google Scholar]
- 80.Park CC, Zhang HJ, Yao ES, Park CJ, Bissell MJ. Beta1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 2008;68:4398–405. doi: 10.1158/0008-5472.CAN-07-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eke I, Cordes N. Radiobiology goes 3D: how ECM and cell morphology impact on cell survival after irradiation. Radiother Oncol. 2011;99:271–8. doi: 10.1016/j.radonc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Yau CY, Wheeler JJ, Sutton KL, Hedley DW. Inhibition of integrin-linked kinase by a selective small molecule inhibitor, QLT0254, inhibits the PI3K/PKB/mTOR, Stat3, and FKHR pathways and tumor growth, and enhances gemcitabine-induced apoptosis in human orthotopic primary pancreatic cancer xenografts. Cancer Res. 2005;65:1497–504. doi: 10.1158/0008-5472.CAN-04-2940. [DOI] [PubMed] [Google Scholar]
- 83.Wong RP, Ng P, Dedhar S, Li G. The role of integrin-linked kinase in melanoma cell migration, invasion, and tumor growth. Mol Cancer Ther. 2007;6:1692–700. doi: 10.1158/1535-7163.MCT-07-0134. [DOI] [PubMed] [Google Scholar]
- 84.Edwards LA, Woo J, Huxham LA, Verreault M, Dragowska WH, Chiu G, et al. Suppression of VEGF secretion and changes in glioblastoma multiforme microenvironment by inhibition of integrin-linked kinase (ILK) Mol Cancer Ther. 2008;7:59–70. doi: 10.1158/1535-7163.MCT-07-0329. [DOI] [PubMed] [Google Scholar]
- 85.Troussard AA, McDonald PC, Wederell ED, Mawji NM, Filipenko NR, Gelmon KA, et al. Preferential dependence of breast cancer cells versus normal cells on integrin-linked kinase for protein kinase B/Akt activation and cell survival. Cancer Res. 2006;66:393–403. doi: 10.1158/0008-5472.CAN-05-2304. [DOI] [PubMed] [Google Scholar]
- 86.Walsh C, Tanjoni I, Uryu S, Tomar A, Nam JO, Luo H, et al. Oral delivery of PND-1186 FAK inhibitor decreases tumor growth and spontaneous breast to lung metastasis in pre-clinical models. Cancer Biol Ther. 2010;9:778–90. doi: 10.4161/cbt.9.10.11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Golubovskaya VM, Nyberg C, Zheng M, Kweh F, Magis A, Ostrov D, et al. A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth. J Med Chem. 2008;51:7405–16. doi: 10.1021/jm800483v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68:1935–44. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 89.Shi Q, Hjelmeland AB, Keir ST, Song L, Wickman S, Jackson D, et al. A novel low-molecular weight inhibitor of focal adhesion kinase, TAE226, inhibits glioma growth. Mol Carcinog. 2007;46:488–96. doi: 10.1002/mc.20297. [DOI] [PubMed] [Google Scholar]
- 90.Halder J, Lin YG, Merritt WM, Spannuth WA, Nick AM, Honda T, et al. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. 2007;67:10976–83. doi: 10.1158/0008-5472.CAN-07-2667. [DOI] [PubMed] [Google Scholar]
- 91.Hochwald SN, Nyberg C, Zheng M, Zheng D, Wood C, Massoll NA, et al. A novel small molecule inhibitor of FAK decreases growth of human pancreatic cancer. Cell Cycle. 2009;8:2435–43. doi: 10.4161/cc.8.15.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Färber K, Synowitz M, Zahn G, Vossmeyer D, Stragies R, van Rooijen N, et al. An alpha5beta1 integrin inhibitor attenuates glioma growth. Mol Cell Neurosci. 2008;39:579–85. doi: 10.1016/j.mcn.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 93.Stoeltzing O, Liu W, Reinmuth N, Fan F, Parry GC, Parikh AA, et al. Inhibition of integrin alpha5beta1 function with a small peptide (ATN-161) plus continuous 5-FU infusion reduces colorectal liver metastases and improves survival in mice. Int J Cancer. 2003;104:496–503. doi: 10.1002/ijc.10958. [DOI] [PubMed] [Google Scholar]