Abstract
RGMa (repulsive guidance molecule a) was the first identified molecule that possessed the necessary functional activity to repulse and steer growth cones to their target in the brain. By binding to its neogenin receptor, RGMa caused the collapse of growth cones and encouraged axons to grow along specific trajectories in vitro. Although originally characterized in 1990, RGMa was not conclusively shown to mediate axon guidance in vivo for another 12 years. Loss-of-function analysis in mice revealed that RGMa may play a more important role in neural tube morphogenesis. RGMa has now emerged as a molecule with pleiotropic roles involving cell adhesion, cell migration, cell polarity and cell differentiation which together strongly influence early morphogenetic events as well as immune responses. RGMa can be regarded as a molecule for all seasons.
Keywords: RGMa, chemorepulsion, neural tube, axon guidance, neogenin
The vertebrate embryo uses differential cell adhesion to maintain the integrity of tissues in the face of competing morphogenetic forces arising from extensive cell proliferation and cell migration occurring during early development. Major classes of adhesion include cell-cell and cell-matrix interactions, with different molecules in both systems subserving early recognition and later maturation events. While early theories proposed that specificity in interactions was achieved by limiting expression of select adhesion molecules and their ligands to different cell types and tissues, it soon became apparent that many of these molecules and/or their functional domains were shared widely in the body and specificity was instead achieved through both spatial and temporal regulation of expression patterns. In this commentary we follow the discovery and subsequent elucidation of the role of repulsive guidance molecule A (RGMa). Studies of RGMa initially revealed the importance of chemorepulsion in guiding cell interactions in the nervous system while more recently they have highlighted how a single ligand can mediate multiple functions such as repulsion, adhesion, migration and differentiation according to the context of the environment in which it is expressed.
The Discovery of RGMa, the First Neural Chemorepulsive Molecule
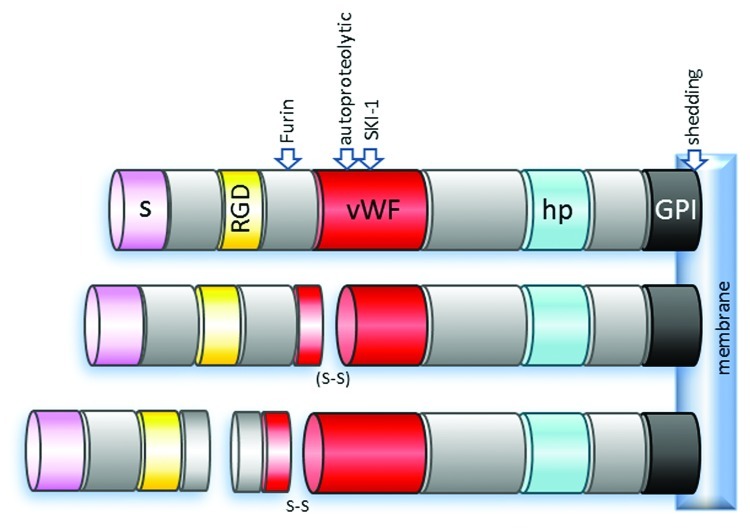
The hunt for the molecular basis of Sperry’s proposed chemoaffinity which explained cell specific connectivity between neurons in the visual system1 led to the identification of a 33 kDa glycosylphosphatidylinositol (GPI)-linked membrane glycoprotein in chick tectum.2 Rather than acting to increase the affinity between retinal axons and tectal neurons, this molecule instead appeared to induce the collapse of retinal growth cones in vitro. More than 25 years after Roger Sperry’s bold hypothesis it was becoming clear that repulsive interactions were an important contributor to the development of topographical connections in the visual system. The inhibitory activity of this 33 kDa molecule was further confirmed in bioassays using retinal axons, resulting in the founding member of chemorepulsive molecules being called repulsive guidance molecule (RGM).3 It was another eight years before RGM was cloned and found to be a 432 residue protein containing an N-terminal signal peptide, an RGD motif, a partial von Willebrand factor (vWF) type D domain, a hydrophobic region and a C-terminal GPI-anchor (Fig. 1).4 This novel molecule was expressed in HEK 293 cells and found to display growth cone collapse activity,4 confirming that RGM was indeed responsible for the repulsive interactions between retinal axons and tectal neurons that was initially observed using crude membrane fractions 12 y earlier.2
Figure 1. Structure of RGMa. The schematic represents a membrane bound full-length RGMa (top) and reported RGMa peptides generated by post-translation modifications (middle and bottom). RGMa is cleaved by proprotein convertases Furin and SKI-1 in addition to previously reported cleavage events at auto-proteolytic and the shedding cleavage sites to generate seven different membrane-bound and soluble RGMa peptides in vivo.4-6 Following autoproteolysis, two RGMa domains are either linked by disulphide bridge or cleaved. However, when RGMa is cleaved by Furin, the remaining cytoplasmic RGMa domains are linked by a disulphide bridge following autoproteolysis.6 S, signaling peptide; RGD, Arg-Gly-Asp tri amino acid motif; vWF, partial von Willebrand factor type D; hp, hydrophobic domain; GPI, glycosylphosphatidylinositol-link; SKI-1, subtilisin kexin isozyme-1; S-S, disulphide bridge; (S-S), disulfide bridge may or not be present.
Three mouse homologs of RGM were subsequently identified and referred to as RGMa, RGMb and RGMc.7,8 RGMa has the highest homology to chick RGM, sharing 80% amino acid identity, and was characterized as the true homolog of chick RGM. Both chick RGM and mouse RGMa contain putative autocatalytic cleavage sites capable of generating a soluble N-terminal peptide which contains the RGD motif and a C-terminal membrane tethered fragment.4,7 More recently it has been shown that serine proteases act together with autocatalytic cleavage to generate seven different peptides, some of which are linked via disulfide bonds.6 Interestingly, while cleavage appears essential for axon chemorepulsion we have shown that RGMa lacking the vWF type D domain, and hence lacking some of its proteolytic processing ability, continues to maintain functional activity associated with morphogenetic events.9 Thus, the significance of each of these cleaved peptides in different brain regions and in non-neural tissues in vivo remains to be clarified.
Repulsive Guidance is Not the Only Function of RGMa
It was clearly surprising when RGMa mutant mice were generated and found to display no signs of aberrant axon guidance in the visual system.7 Instead, 50% of these mice exhibited exencephaly resulting from failed cephalic neural tube closure as early as E10.5. By E14.5, the dorsal brains of these animals were severely perturbed as a result of evagination of the neural folds. While the topography of the visual pathway could not be examined in these animals due to this distorted development, the topography of retinal innervation of the superior colliculus (optic tectum) and overall neural patterning (including cell death and proliferation) of the brain within the remaining 50% of RGMa−/− mice not displaying exencephaly was completely normal. These results suggest that while RGMa exhibits strong activity in in vitro bioassays, its function is completely redundant in axon guidance in vivo, at least in the visual system. However, it is possible that animals displaying exencephaly may have also developed defects in retinotopic projections in the visual system had they not been perturbed by earlier processes in development. Clearly what is needed is conditional rather than ubiquitous deletion of RGMa in mice. By adopting Xenopus as the animal model we were subsequently able to demonstrate, for the first time, that RGMa plays a critical role in axon guidance in vivo.10 Loss of RGMa function caused perturbations to the trajectory of axons connecting the presumptive telencephalon to the ventral diencephalon during the earliest stages of axon tract formation in the embryonic brain.
RGMa is a Cell Adhesion Molecule
RGMa clearly has a critical early neurodevelopmental role in ensuring that the neural tube closes appropriately in the cephalic regions while its involvement in axon guidance may be secondary, and of lesser importance to embryonic viability. We have recently revealed that the RGMa and neogenin also act together to regulate morphogenetic processes during early embryonic development.9,11-13 Loss-of-function experiments showed that neogenin and RGMa were essential for neural tube closure, particularly in the anterior regions.11 In the absence of neogenin, the neural plate fails to elevate its lateral edges in order to form a closed tube. Neogenin-RGMa interactions also modulated apicobasal polarity and the intercalation of neuroepithelial cells,11 both of which are necessary for neural tube closure. Using unilateral loss- or gain-of-function of RGMa and neogenin in Xenopus embryos we have begun to better understand some of the cellular processes mediated by these molecules during embryogenesis.9 RGMa and neogenin were found to be acting together to modulate cell adhesion and migration leading up to and including neural tube closure. For the first time these molecules were revealed to have critical functions in early morphogenetic events.
Our initial insight into how RGMa was affecting cell behavior emerged when we mosaically overexpressed this molecule in Xenopus embryos by injecting mRNA into a single dorsal blastomere at the 8- to 16-cell stage.9 By tracing the progeny of these blastomeres within the embryonic brain we observed that RGMa caused cells to form small tightly packed clumps, rather than widely-dispersed single cells typical of control embryos injected only with reporter. This clumping of cells produced by overexpression of RGMa was reminiscent of the reduced cell mixing observed with overexpression of the cell adhesion molecule N-cadherin in Xenopus embryos.14 The idea that RGMa could be involved in adhesion may also help to explain the abnormal neural tube closure we observed when neogenin-RGMa interactions were perturbed.11 Similar phenotypes occur when the cell adhesion molecules NF-protocadherin, Nectin-2 and N-cadherin are downregulated.15,16 The abnormal apicobasal polarity we observed in the neuroepithelial cells of the neural plate11 is also consistent with the previously reported roles of adhesion molecules N-cadherin and Nectin-2 in this process.16
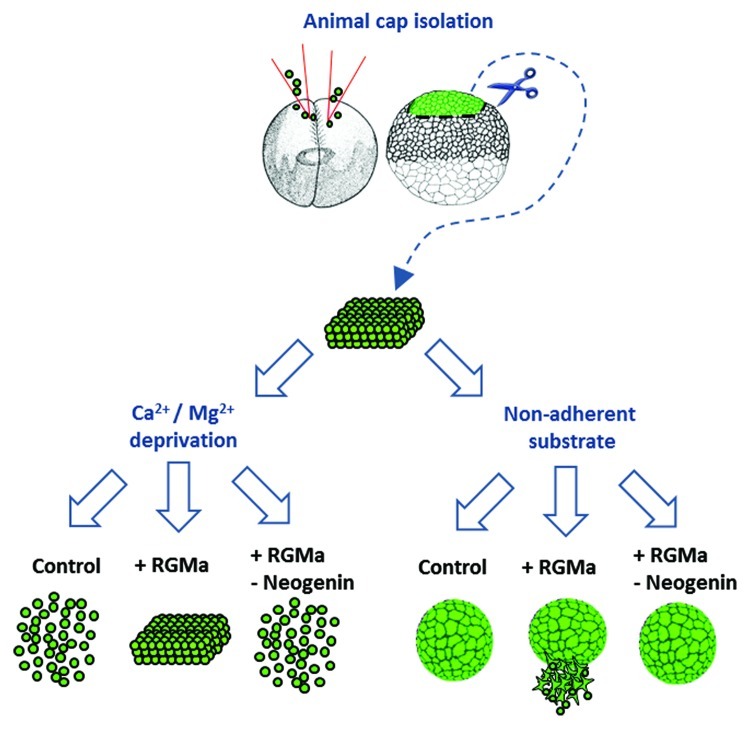
The notion that RGMa was somehow involved with cell adhesion was incongruous with the previously known chemorepulsive function of this molecule. While a novel cell adhesion role for RGMa was supported by observations that RGMa is highly expressed at the tips of the neural fold in mice and loss-of-RGMa function prevented neural tube closure we needed a direct test of the role of RGMa in cell adhesion. We turned to a classic Xenopus bioassay which involved isolating the animal cap of the early embryo (a patch of ectoderm which will subsequently form the main bulk of the animal) and culturing it ex vivo in a defined medium lacking calcium and magnesium (Fig. 2). Since the integrity of this tissue relies on the expression of calcium-dependent adhesion molecules such as cadherin, the lack of relevant bivalent ions in the medium causes the ectoderm to dissociate within 60 min. When the animal pole was obtained from embryos ubiquitously overexpressing RGMa and used in this assay the explants now retained their cohesiveness.9 This was definitive proof that RGMa was involved in cell adhesion and provided an explanation for why we observed clumps of RGMa expressing cells in the neuroepithelium of the brain, and for why the neural tube was not correctly folding. The question then arose as to whether this adhesive property of RGMa was due to interactions with neogenin, or some other receptor, or perhaps even with itself.17 To test this possibility we simultaneously overexpressed RGMa and knocked down neogenin in Xenopus embryos. Animal caps from these animals now dissociated, indicating that neogenin was mediating the adhesiveness of RGMa (Fig. 2).
Figure 2. RGMa-neogenin plays roles in adhesion and migration of Xenopus animal cap cells. Xenopus embryos were microinjected into both blastomeres at the 2-cell-stage with either: eGFP mRNA (control), RGMa mRNA + eGFP mRNA (+ RGMa) or RGMa mRNA + Neogenin translational blocking morpholino + eGFP mRNA (+RGMa-Neogenin) as previously described.9 The injected embryos were reared until they reached late blastula stage when the animal cap ectoderm was isolated. These explants were then placed either in medium lacking calcium and magnesium (Ca2+ and Mg2+ deprivation) or in complete medium on a non-adherent substrate. In the absence of divalent ions control explants dissociated whereas RGMa overexpressing explants remained intact. When RGMa was overexpressed at the same time as neogenin was downregulated explants dissociated, indicating that RGMa-neogenin interactions were mediating adhesion. When isolated animal cap explants were cultured on a non-adherent substrate RGMa overexpressing cells actively migrated from explants. This migratory behavior was neogenin dependent.
RGMa, a Link between the Immune and Nervous Systems
In an interesting twist of fate, RGMa has recently been shown to be expressed by bone marrow-derived dendritic cells and to be involved in the invasion of inflammatory cells into the central nervous system during autoimmune encephalomyelitis.18 It seems that the very same molecule that is responsible for constructing the nervous system during development is also mediating its destruction during inflammatory responses. RGMa on the dendritic cells binds to neogenin expressed by CD4+ T cells, activating these cells and causing their increased adhesion to intercellular adhesion molecule-1 (normally present on the antigen presenting dendritic cells). In this case, as opposed to what we observed in the early embryo,9 RGMa appears to be acting indirectly to modulate cell adhesion as well as other T-cell mediated immune responses such as cytokine release and proliferation.18 The underlying molecular interactions and direct involvement of neogenin in T cell adhesion remains to be determined.
From Cell Adhesion to Cell Migration
When RGMa was overexpressed in Xenopus embryos it caused severe defects in neural tube closure.9 One of these abnormalities was a ring phenotype which involved exposure of the underlying endoderm. Such phenotypes have been partially attributed to cell migration defects occurring earlier during development,19 rather than as direct result of the tips of the neural plate failing to fuse. Examination of earlier stages of development in embryos overexpressing RGMa revealed defects in morphogenetic processes that could account for some of the severe perturbations that were arising later in development. Of note was the defective blastopore closure, a phenotype that arises from aberrant ectodermal migration and disrupted gastrulation. To examine the role of RGMa in migration we analyzed isolated animal caps in culture (Fig. 2).9 Normally these caps round up and form embryoid-like balls. We found that overexpression of RGMa caused these explants to exhibit abnormal cell migration. Ectodermal cells migrated non-directionally in a radial manner away from the explants. This migratory behavior was reliant on neogenin since simultaneous knock down of this receptor rescued the phenotype and returned the explants to small spheroidal bodies. Interestingly, this non-directional migration of ectodermal cells was also observed in vivo. Clumps of migrating cells overexpressing RGMa inappropriately grew into and populated the blastocoel (internal cavity of early embryo) during gastrulation.9
To further assess the adhesive function of RGMa we have recently isolated single blastomeres from late blastula stage Xenopus embryos that were ubiquitously overexpressing RGMa (Fig. 3). Individual blastomeres were cultured on a nonadherent substrate which forced the progeny of these cells to form embryoid-like bodies as they proliferated ex vivo. RGMa overexpressing cell clones formed aggregates without undergoing cell compaction whereas wild-type or control cells clearly exhibited cell compaction similar to that observed in vivo as morula stage embryos develop tight junctions.20 The inability of RGMa expressing cells to undergo compaction may explain the abnormal migratory activity that we observed in animal cap explants. It appears that cells are not compacting and are instead able to aberrantly migrate away from the explants. While further analysis is necessary, these results have implications for understanding the role of RGMa in various cellular interactions as well as in carcinogenesis.
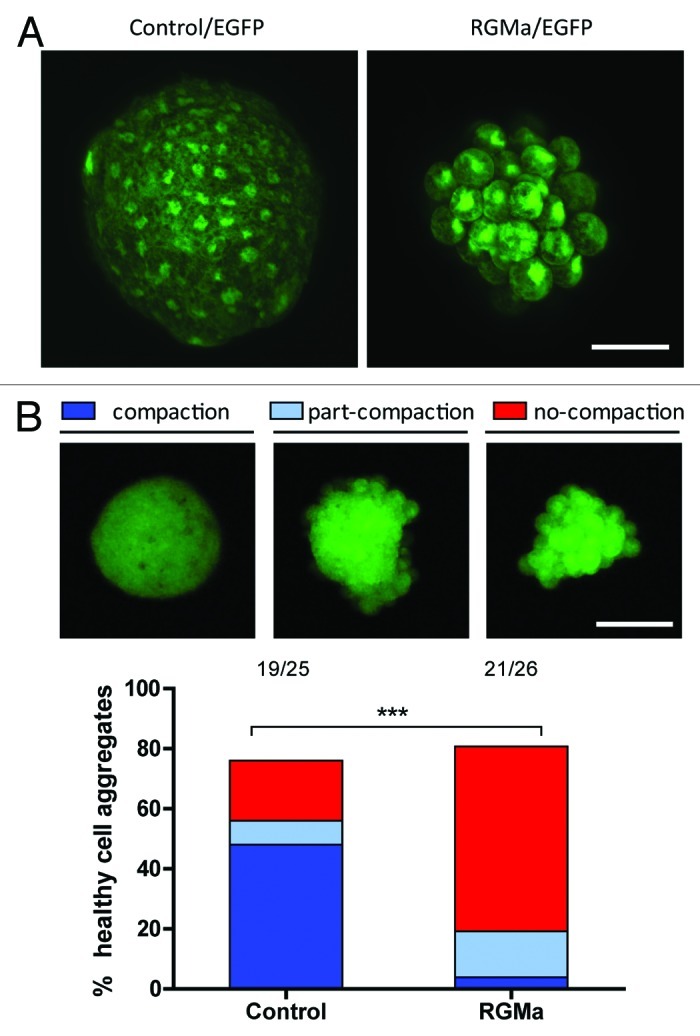
Figure 3. RGMa overexpressing animal cap stem cells showed impaired cell compaction. A single blastomere was isolated from the blastula animal cap stem cell population and cultured on a non-adherent substrate. (A) Confocal z-stacked images of a control eGFP expressing cell aggregate and an RGMa + eGFP expressing cell aggregate derived from a single animal cap cell. Unlike controls, RGMa overexpressing cell aggregates exhibited defects in cell compaction. While RGMa overexpressing cell aggregates generally showed less number of cells within the aggregate in our assay, the size of cell clusters were variable in both control and RGMa overexpressing groups. (Scale bar = 50 μm). (B) RGMa overexpressing cell aggregates showed significantly higher percentage of cell aggregates with compromised cell compaction. The cell aggregates were classified into three different groups: compaction, part-compaction and no-compaction. The representative images for each group are shown below the labels. The bar graph indicates the percentage of healthy cell aggregates within each category from control and RGMa overexpressing groups. The cell aggregates from both control and RGMa overexpressing cells showed comparable survival rate (~80%) whereas RGMa overexpressing cell aggregates showed significantly higher percentage of cell aggregates with compromised cell compaction (Chi-square test of homogeneity, ***p < 0.001). Numbers on the top of the graph indicate raw numbers of cell aggregates analyzed in the study. (Scale bar = 100 μm).
When RGMa was constitutively overexpressed in colorectal cancer cell lines it reduced colony formation and cell growth.21 Both of these phenotypes are consistent with the behavior of our single cell blastomere cultures (Fig. 3). These RGMa expressing colon cell lines also displayed slower migration, which was again consistent with the retarded blastopore closure we observed in embryos overexpressing RGMa. The blastopore is normally closed by expansion and migration of the ectoderm over the embryo. RGMa clearly slows this migratory behavior.9 A recent study has demonstrated that the role of RGMa in cell migration is not limited to development. RGMa has been shown to attenuate leukocyte migration through epithelial cells in a neogenin-dependent manner both in vitro and in vivo.22 A role of RGMa in immune responses is opening up new possibilities for understanding the molecular interactions mediating fundamental cellular processes such as cell adhesion and migration.18
In summary, we have shown that RGMa enhances cell adhesion and causes aberrant cell migration in a neogenin-dependent manner during early embryonic development. These hitherto unrecognized functions of RGMa-neogenin interactions reveal the importance of these molecules in early morphogenetic events, in axon guidance during neurodevelopment and cell migration in the immune system.
Where to Next?
In order to begin to understand the role of the principal domains in RGMa in various morphogenetic events we created mutant forms of RGMa lacking either the RGD motif or the vWF domain.9 Using the animal cap explant and blastopore closure assays we revealed that vWF domain was integral to cell adhesion while the RGD motif was involved in cell migration. These roles are not mutually exclusive, however, each domain/motif clearly has a more substantial function in either adhesion or migration. Interestingly in both cases these cell behaviors were dependent on neogenin, which raises the intriguing question about molecular interactions. Since integrin-RGD binding is necessary for gastrulaton in Xenopus,23 are integrins interacting with neogenin through RGMa or is RGD directly mediating interactions with neogenin? Is vWF domain interacting with neogenin, and if so, is it direct or are co-factors involved? Which signaling molecules downstream of neogenin are involved in RGMa mediated adhesion and migration? These are all very important questions as they will provide insight into the cell-context specific nature of RGMa function. Our most significant challenge is to understand how RGMa selectively mediates chemorepulsion, cell adhesion and cell migration at different times and places in the embryo. RGMa is without doubt a molecule for all seasons.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/20167
References
- 1.Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci U S A. 1963;50:703–10. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stahl B, Müller B, von Boxberg Y, Cox EC, Bonhoeffer F. Biochemical characterization of a putative axonal guidance molecule of the chick visual system. Neuron. 1990;5:735–43. doi: 10.1016/0896-6273(90)90227-7. [DOI] [PubMed] [Google Scholar]
- 3.Müller BK, Jay DG, Bonhoeffer F. Chromophore-assisted laser inactivation of a repulsive axonal guidance molecule. Curr Biol. 1996;6:1497–502. doi: 10.1016/S0960-9822(96)00754-3. [DOI] [PubMed] [Google Scholar]
- 4.Monnier PP, Sierra A, Macchi P, Deitinghoff L, Andersen JS, Mann M, et al. RGM is a repulsive guidance molecule for retinal axons. Nature. 2002;419:392–5. doi: 10.1038/nature01041. [DOI] [PubMed] [Google Scholar]
- 5.Hata K, Fujitani M, Yasuda Y, Doya H, Saito T, Yamagishi S, et al. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J Cell Biol. 2006;173:47–58. doi: 10.1083/jcb.200508143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tassew NG, Charish J, Seidah NG, Monnier PP. SKI-1 and Furin generate multiple RGMa fragments that regulate axonal growth. Dev Cell. 2012;22:391–402. doi: 10.1016/j.devcel.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Niederkofler V, Salie R, Sigrist M, Arber S. Repulsive guidance molecule (RGM) gene function is required for neural tube closure but not retinal topography in the mouse visual system. J Neurosci. 2004;24:808–18. doi: 10.1523/JNEUROSCI.4610-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidtmer J, Engelkamp D. Isolation and expression pattern of three mouse homologues of chick Rgm. Gene Expr Patterns. 2004;4:105–10. doi: 10.1016/S1567-133X(03)00144-3. [DOI] [PubMed] [Google Scholar]
- 9.Lah GJ, Key B. Novel roles of the chemorepellent axon guidance molecule RGMa in cell migration and adhesion. Mol Cell Biol. 2012;32:968–80. doi: 10.1128/MCB.06128-11. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson NH, Key B. Neogenin interacts with RGMa and netrin-1 to guide axons within the embryonic vertebrate forebrain. Dev Biol. 2006;296:485–98. doi: 10.1016/j.ydbio.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Kee N, Wilson NH, De Vries M, Bradford D, Key B, Cooper HM. Neogenin and RGMa control neural tube closure and neuroepithelial morphology by regulating cell polarity. J Neurosci. 2008;28:12643–53. doi: 10.1523/JNEUROSCI.4265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin GJ, Wilson NH. Overexpression of repulsive guidance molecule (RGM) a induces cell death through Neogenin in early vertebrate development. J Mol Histol. 2008;39:105–13. doi: 10.1007/s10735-007-9138-x. [DOI] [PubMed] [Google Scholar]
- 13.Lah GJ, Key B. Dual roles of the chemorepellent axon guidance molecule RGMa in establishing pioneering axon tracts and neural fate decisions in embryonic vertebrate forebrain. Dev Neurobiol. 2012 doi: 10.1002/dneu.22010. In press. [DOI] [PubMed] [Google Scholar]
- 14.Detrick RJ, Dickey D, Kintner CR. The effects of N-cadherin misexpression on morphogenesis in Xenopus embryos. Neuron. 1990;4:493–506. doi: 10.1016/0896-6273(90)90108-R. [DOI] [PubMed] [Google Scholar]
- 15.Rashid D, Newell K, Shama L, Bradley R. A requirement for NF-protocadherin and TAF1/Set in cell adhesion and neural tube formation. Dev Biol. 2006;291:170–81. doi: 10.1016/j.ydbio.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Morita H, Nandadasa S, Yamamoto TS, Terasaka-Iioka C, Wylie C, Ueno N. Nectin-2 and N-cadherin interact through extracellular domains and induce apical accumulation of F-actin in apical constriction of Xenopus neural tube morphogenesis. Development. 2010;137:1315–25. doi: 10.1242/dev.043190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samad TA, Srinivasan A, Karchewski LA, Jeong SJ, Campagna JA, Ji RR, et al. DRAGON: a member of the repulsive guidance molecule-related family of neuronal- and muscle-expressed membrane proteins is regulated by DRG11 and has neuronal adhesive properties. J Neurosci. 2004;24:2027–36. doi: 10.1523/JNEUROSCI.4115-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muramatsu R, Kubo T, Mori M, Nakamura Y, Fujita Y, Akutsu T, et al. RGMa modulates T cell responses and is involved in autoimmune encephalomyelitis. Nat Med. 2011;17:488–94. doi: 10.1038/nm.2321. [DOI] [PubMed] [Google Scholar]
- 19.Skoglund P, Rolo A, Chen X, Gumbiner BM, Keller R. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development. 2008;135:2435–44. doi: 10.1242/dev.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming TP, Sheth B, Fesenko I. Cell adhesion in the preimplantation mammalian embryo and its role in trophectoderm differentiation and blastocyst morphogenesis. Front Biosci. 2001;6:D1000–7. doi: 10.2741/Fleming. [DOI] [PubMed] [Google Scholar]
- 21.Li VSW, Yuen ST, Chan TL, Yan HHN, Law WL, Yeung BHY, et al. Frequent inactivation of axon guidance molecule RGMA in human colon cancer through genetic and epigenetic mechanisms. Gastroenterology. 2009;137:176–87. doi: 10.1053/j.gastro.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Mirakaj V, Brown S, Laucher S, Steinl C, Klein G, Köhler D, et al. Repulsive guidance molecule-A (RGM-A) inhibits leukocyte migration and mitigates inflammation. Proc Natl Acad Sci U S A. 2011;108:6555–60. doi: 10.1073/pnas.1015605108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos JW, Whittaker CA, DeSimone DW. Integrin-dependent adhesive activity is spatially controlled by inductive signals at gastrulation. Development. 1996;122:2873–83. doi: 10.1242/dev.122.9.2873. [DOI] [PubMed] [Google Scholar]