Abstract
Regulation of glucocorticoid receptor (GR) levels is an important stress adaptation mechanism. Transcription factor Nfgi-a and environmentally induced Gr promoter 17 methylation have been implicated in fine-tuning the expression of Gr 17 transcripts. Here, we investigated Gr promoter 17 methylation and Gr 17 expression in adult rats exposed to either acute or chronic stress paradigms. A strong negative correlation was observed between the sum of promoter-wide methylation levels and Gr 17 transcript levels, independent of the stressor. Methylation of individual sites did not, however, correlate with transcript levels. This suggested that promoter 17 was directly regulated by promoter-wide DNA methylation. Although acute stress increased Ngfi-a expression in the hypothalamic paraventricular nucleus (PVN), Gr 17 transcript levels remained unaffected despite low methylation levels. Acute stress had little effect on these low methylation levels, except at four hippocampal CpGs. Chronic stress altered the corticosterone response to an acute stressor. In the adrenal and pituitary glands, but not in the brain, this was accompanied by an increase in methylation levels in orchestrated clusters rather than individual CpGs. PVN methylation levels, unaffected by acute or chronic stress, were significantly more variable within- than between-groups, suggesting that they were instated probably during the perinatal period and represent a pre-established trait. Thus, in addition to the known perinatal programming, the Gr 17 promoter is epigenetically regulated by chronic stress in adulthood, and retains promoter-wide tissue-specific plasticity. Differences in methylation susceptibility between the PVN in the perinatal period and the peripheral HPA axis tissues in adulthood may represent an important “trait” vs. “state” regulation of the Gr gene.
Keywords: glucocorticoid receptors, behavioral epigenetics, DNA methylation, HPA axis, adult rats
Introduction
Regulation of glucocorticoid receptor (GR) expression, particularly in the brain, is an important mechanism of adaptation to stress. The 5′ region of the GR gene (OMIM + 138040; NR3C1) consists, in humans, of nine untranslated alternative first exons (1A to 1I),1-3 each with its own promoter.4 The Gr promoter region in rats has a similar structure with 11 untranslated first exons (11 to 111). Eight of these exons (14 to 111) are highly homologous in rats and humans.2,5 The human exon 1F and its rat homolog exon 17 represent only a small fraction of all GR transcripts, but epigenetic regulation of these two exons in the brain by environmental influences has recently received much attention. Promoters 1F/17 are among the first gene promoters shown to be susceptible to environmentally induced DNA methylation, and this has been associated with a decrease in Gr transcript levels both in rodents6 and in humans,7 suggesting that it represents a more general epigenetic regulation process.
In rodents, a specific post-natal environment such as low maternal care is associated with low levels of adult Gr expression in the hippocampus and the paraventricular hypothalamic nucleus (PVN).8 Low Gr expression was associated with levels of 80–100% methylation of a fully conserved key CpG site in promoter 17 in the hippocampus(rat CpG 16; human CpG 37), thought to correspond to a binding site of the transcription factor Ngfi-a (also known as Egr-1; zif-268; Krox-24), which is believed to be implicated in 17 transcript expression.6,9,10 However, in subsequent rodent studies, such high levels of methylation have not been observed at CpGs 16 and 17 in this Ngfi-a binding site.11,12
Most of the literature on the epigenetic regulation of the glucocorticoid receptor is from the perinatal period. However, the HPA axis maintains its plasticity during adolescence and even during adulthood,13-15 with neuroendocrine changes persisting for more than 12 mo after exposure to chronic stress.14 This HPA axis plasticity is associated with significantly reduced MR and GR levels in certain hippocampus regions,13,15 possibly due to methylation of the receptor genes. Infusion of L-Methionine, a precursor of the methyl-group donor S-adenosyl-methionine, directly into the brain of rats having experienced high maternal care altered the functional and behavioral phenotype to that of the hypermethylated low maternal care group. These experimental results further confirm that the HPA axis plasticity in adulthood is associated with changes in DNA methylation and GR or MR levels and that these changes directly affect the long-term stress response.9
We hypothesized that in young adult rats HPA axis plasticity can result from a similar balance between upregulation of transcription by Ngfi-a and downregulation by promoter methylation. Since acute stress rapidly induces Ngfi-a in the PVN,16-18 and chronic stress is thought to epigenetically alter the HPA axis, we investigated Gr 17 expression and promoter methylation in adult rats after both acute and chronic stress exposure. Our data suggest that Ngfi-a does not immediately regulate 17 transcripts, and that promoter 17 methylation retains its plasticity into adulthood in the peripheral components of the HPA axis.
Results
Acute stress induces a corticosterone response and has no effect on Gr levels
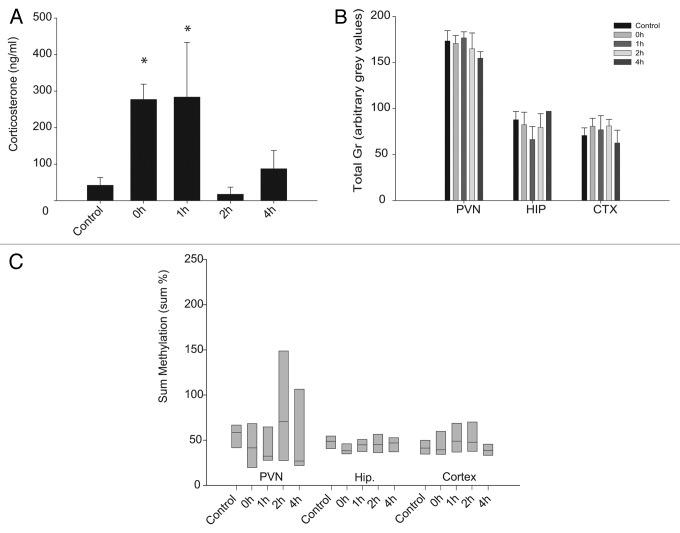
To confirm HPA axis activation after a single session of acute restraint stress, plasma corticosterone levels were measured immediately after decapitation (Fig. 1A). Animals decapitated immediately after restraining had corticosterone levels > 10-fold higher than the unrestrained controls. Animals decapitated 1 h after the onset of the stress had equally elevated corticosterone levels. Corticosterone levels returned to baseline 2 h after stress. A minor, but not significant (p > 0.05) increase in corticosterone was observed 4 h after restraint initiation, corresponding to the start of the afternoon circadian increase in corticosterone levels of these nocturnal animals.
Figure 1. Plasma corticosterone levels (A), total Gr levels (B) and sum of methylation levels (C) after exposure to a single acute stressor. Significance levels were in comparison to the no stress group. *p < 0.05. Panels A and B are mean ± SD. Panel C box plots show the 25th percentile, median and 75th percentile.
In the above animals, a single session of acute restraint stress had no significant effect on total Gr mRNA levels in any of the brain tissues investigated at least up to the 4h time point (Fig. 1B). Similarly, after a single acute stressor, there was no ANOVA group effect on the sum of methylation levels in promoter 17 of any of these tissues (Fig. 1C).
Acute stress-induced Ngfi-a does not induce Gr exon 17 expression
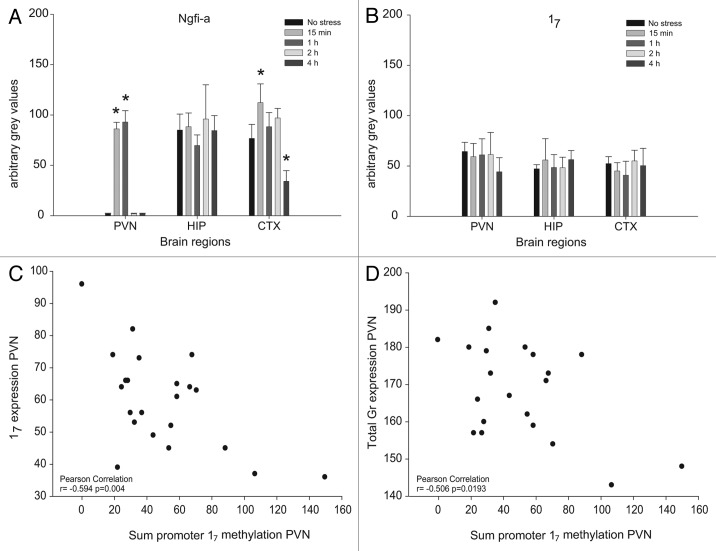
Since a single exposure to acute restraint stress is known to induce Ngfi-a expression in the PVN, in situ hybridization of both Ngfi-a and Gr17 was performed at different time points after acute stress. Restraint stress immediately induced Ngfi-a in the PVN (p < 0.05), and levels were still elevated 1h later (Fig. 2A). After 2h, Ngfi-a levels were back to pre-stress levels. Within 4h after the onset of stress, the Gr transcript 17 immediately downstream of promoter 17 (which contains an Ngfi-a recognition site),6 was not upregulated in the PVN (Fig. 2B). Also, total Gr expression was not affected within the 4 h after restraint stress (Fig. 1B). Ngfi-a and Gr 17 levels were also measured in the hippocampus and the cortex (Fig. 2A and B). Also in the cortex, Ngfi-a levels were induced by 15 min of restraint stress (p < 0.05). After 4 h in their home cage, the rats had significantly reduced Ngfi-a levels compared with the unstressed group (p < 0.05). Gr 17 and total Gr transcript levels, however, remained unchanged throughout the 4 h period. In the hippocampus, Ngfi-a, Gr 17 and total Gr levels remained unaffected by the exposure to acute restraint stress. These results from the PVN and the cortex suggest that Gr 17 transcription is independent of Ngfi-a expression within this time frame.
Figure 2. Quantification of Ngfi-a mRNA (A), 17 mRNA (B) and the correlation of 17 (C) and total Gr (D) transcripts with the sum of methylation throughout the promoter after acute stress by in situ hybridization in the paraventricular nucleus of the hypothalamus (PVN), the prefrontal cortex (CTX) and in the CA1 area, representing the hippocampus (HIP) and pyrosequencing. Panels A and B are mean +/− SD. Significance levels were in comparison to the no stress group. *p < 0.05.
Gr promoter 17 methylation levels are low in the hippocampus and PVN
Methylation levels throughout the PVN of acutely stressed rats (Fig. 3A) were below 20%, indicating that binding of Ngfi-a to promoter 17 was not inhibited. This suggests that methylation was not the reason why promoter 17 did not respond to stress-induced increases in Ngfi-a levels as described in the previous section.
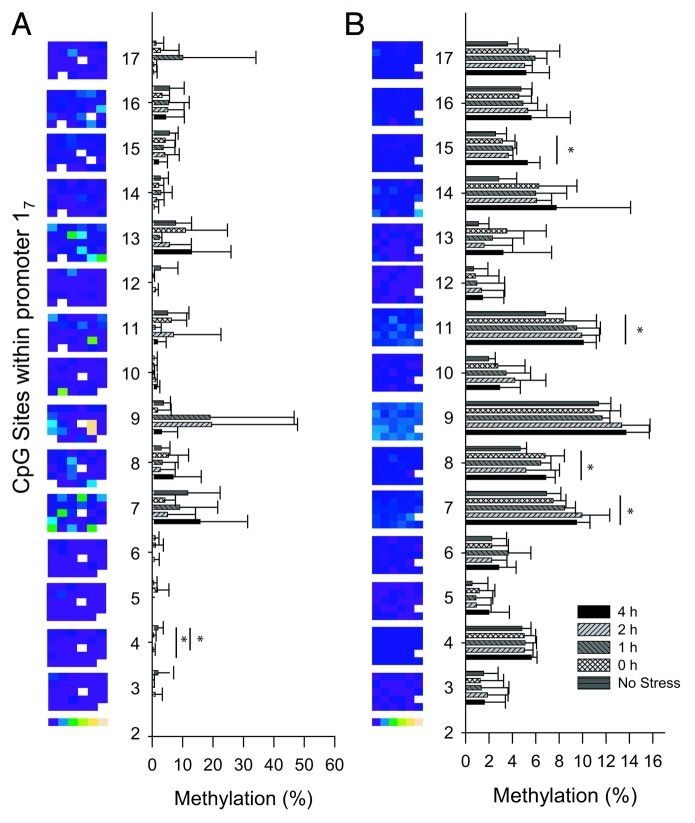
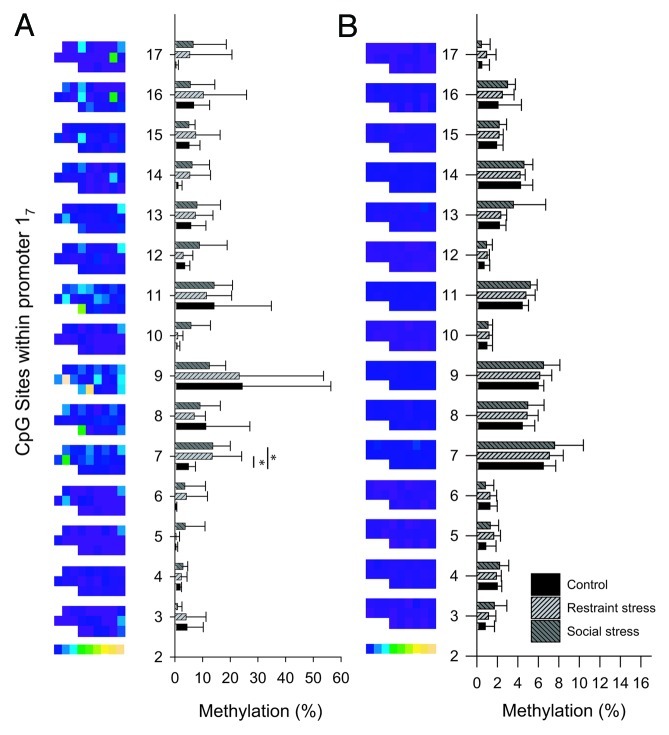
Figure 3. Methylation levels of individual CpG dinucleotides in the 17 promoter in the PVN (A) and the hippocampus (B) after a single acute stressor. Methylation levels are expressed for all animals in columns by color[0% (blue) to 75% (yellow)] and as the group mean ± SD. Significance levels were in comparison to the no stress group. *p < 0.05. CpG sites are numbered according to Weaver et al.6
While there were no group effects on the sum methylation level in the PVN or the hippocampus, several individual CpGs showed a significant response to acute stress. In the PVN, a significant demethylation of CpG 4 (p = 0.024 after post-hoc correction) 1 h and 2 h after acute stress compared with controls (Fig. 3A) was observed, although basic methylation levels were already very low (< 5%). In the hippocampus, 2 h and 4 h after stress, 4 of the 15 investigated CpG dinucleotides, CpGs 7, 8, 11 and 15, showed a significant increase (p < 0.05) in methylation levels after post-hoc corrections (Fig. 3B). One of these CpGs, CpG 11 in rats, was suggested by McGowan et al. to be part of a putative NGFI-A binding site in humans (CpG 30–32).7 In the hippocampus, this CpG increased from 6.8 ± 1.7 to 10.1 ± 1.1% (p < 0.05) after acute stress, suggesting that it was still available for binding. Thus, low and stress-modulated methylation levels do not explain the unresponsiveness of 17 transcripts to stress induced Ngfi-a expression.
Gr promoter 17 methylation levels are variable in the PVN but not the hippocampus
Methylation levels of most CpG sites in the PVN were highly variable between animals, and significantly less variable in the hippocampus (Fig. 3B). The D’Agostino and Pearson omnibus normality test revealed that the sum of methylation of all CpGs in promoter 17 showed a normal distribution in the hippocampus (K2 = 2.726; p = 0.2559) but not in the PVN (K2 = 12.78; p = 0.0017), indicating more variability in the latter region. The large intra-group differences, together with a lack of difference between the groups, suggests that the variability in PVN sum methylation levels was established prior to the acute stressor.
Promoter 17 methylation correlates with transcript expression
Both the sum and individual CpG methylation levels, as well as exon 17 transcript levels, were more variable in the PVN than in any other organ tested, and these variabilities were independent of exposure to acute stress. Therefore, the link between the methylation levels and the observed exon 17 transcript level was further investigated in this brain region. The Pearson’s correlation coefficients showed no significant link between methylation levels of individual CpG position and 17 transcript levels (r values varied between -0.396 and 0.05 and p values between 0.068 and 0.825). However, the sum of the methylation level throughout the promoter showed a significant negative correlation (Fig. 2C) with expression levels of the 17 transcript (r = 0.594; p = 0.004). This correlation was essentially independent of the first five CpG positions (CpG 3–7) including CpG 4, which was stress sensitive, as described above. This suggests that the methylation of this shorter region covering CpG 3–7 does not contribute to the regulation of 17 transcripts. The correlation of the sum of methylation from CpG 8–17 with the total Gr level was weaker than for the 17 transcript, although still significant (r = -0.503, p = 0.02; Figure 2D). In the cortex and the hippocampus of acutely stressed rats, methylation levels of neither the individual CpG sites nor the sum of all CpG sites correlated significantly with either 17 transcripts or the total Gr level. No correlation was found between PVN, hippocampus and cortex methylation levels.
Chronic stress alters growth and organ weight
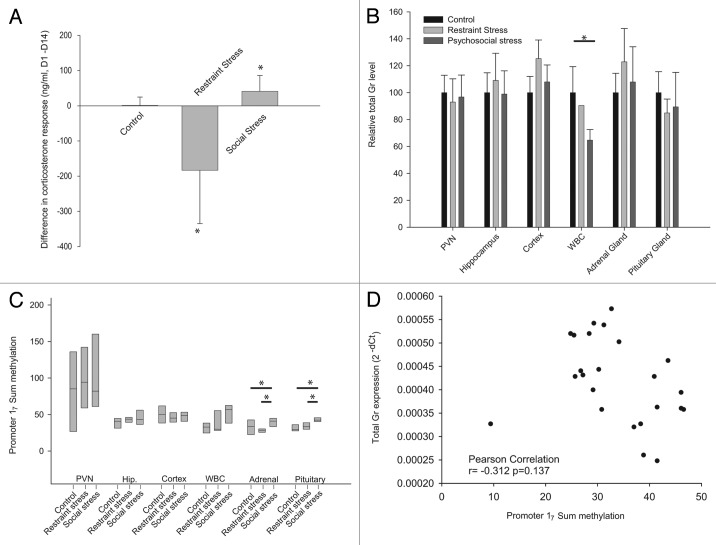
Animals were chronically stressed either by a daily restraint stress routine (chronic restraint stress) or by daily randomization of their home cage and cage mates (chronic psychosocial stress). Control animals were only weighed daily. After the 21 d of chronic stress exposure, animals with chronic restraint stress showed only 55.8 +/− 10.74 g weight gain, compared with 69.7 +/− 5.05 g for the psychosocial stress group or 77.3 +/−5.03 g for the handling control group (ANOVA p < 0.05, all pairwise comparisons significant; Fig. 4A). On day 22, the restraint stress group showed a trend toward an increase in adrenal gland weight (p = 0.08; Fig. 4B) and a trend toward a decrease in pituitary weight in animals subjected to social stress (ANOVA, p = 0.07; Fig. 4C).
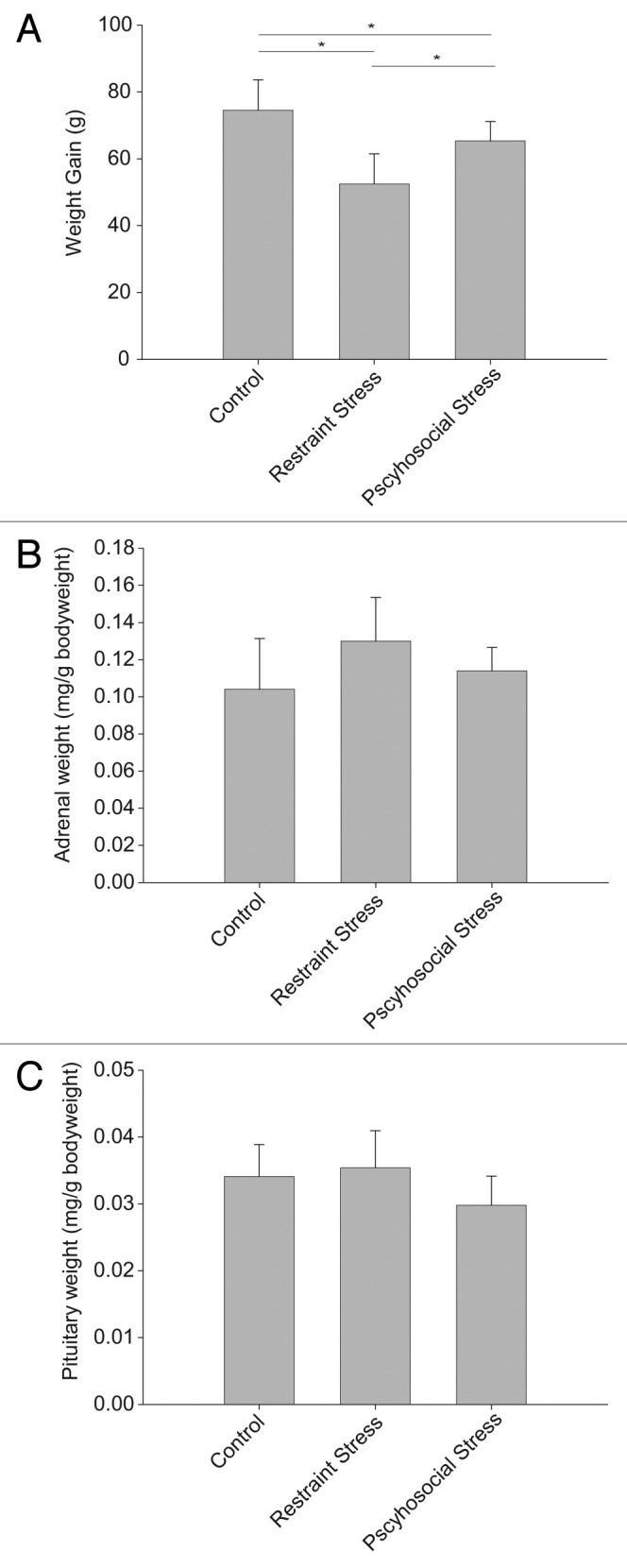
Figure 4. Exposure to 21 d chronic stress alters weight gain (A), relative adrenal gland weight (B) and relative pituitary weight (C). All panels are mean +/− SD. Significance was assessed by ANOVA, and *p < 0.05 for pairwise Tukey post-hoc comparison and correction.
Chronic stress induces an altered HPA axis
After 14 d of either chronic restraint or psychosocial stress, all animals were restrained for 1h. Corticosterone levels pre- and post-restraining showed that the control group mounted a normal HPA axis response, with a > 7 fold increase in corticosterone (t-test p < 0.01). Animals subjected to daily restraint stress had a significant corticosterone response, although levels 30 min post-stress on day 14 were significantly lower than on day 1 (mean difference -183.4 ng/ml; t-test p < 0.05). Conversely, animals subjected to chronic psychosocial stress had an increased response to restraint stress on day 14 (mean difference +44.8 ng/ml p < 0.05; Fig. 5A) compared with day 1. There was no statistical difference in the corticosterone levels pre-stress between days 1 and 14 in any of the groups (p > 0.05).
Figure 5. Change in plasma corticosterone response to a single acute stressor after 14 d exposure to chronic stress (A), total Gr mRNA levels (B) and the sum of methylation levels throughout promoter 17 (C) after chronic restraint or psychosocial stress. Panels A and B are mean ± SD. Panel C box plots show the 25th percentile, median and 75th percentile.
Chronic stress affects Gr levels only in peripheral leucocytes
After 21 d, there was no difference in total Gr mRNA levels observed in the PVN, hippocampus, cortex, adrenal glands or pituitary glands. However, lower total Gr levels were observed in the peripheral leucocytes of the psychosocial stress group (ANOVA, p < 0.05; Fig. 5B) but not in the chronically restrained group.
Chronic stress alters the sum of the methylation levels in promoter 17 in the adrenal and pituitary glands
As the sum of methylation throughout promoter 17 correlated with Gr 17 transcript levels in our acute stress paradigm, we also investigated the effect of chronic stress on this promoter. A significant ANOVA group effect on the sum of methylation was found in both the adrenal and the pituitary glands (Fig. 5C p < 0.05) after exposure to both chronic stress paradigms. In both tissues, psychosocial stress induced promoter 17 methylation; however, restraint stress decreased methylation in the adrenal gland but increased it in the pituitary. There was no effect of either stressor on methylation levels in the PVN, hippocampus or cortex. However, leucocytes showed a trend to higher methylation in both paradigms, although there was significant variability in methylation levels after both restraint and psychosocial stress.
Promoter 17 methylation correlates with transcript expression
Since there was an ANOVA group effect of chronic stress on the sum of methylation levels in the adrenal and pituitary glands, we measured their correlations with the total Gr transcript levels. In both tissues, there was a negative correlation between aggregated Gr transcript levels and the sum of the methylation, although this did not reach statistical significance (r = -0.31 p = 0.127; and r = -0.21 p = 0.11 respectively; Fig. 5D). Without the outlier in Figure 5D, the correlation becomes statistically significant in the adrenal gland (r = 0.553, p < 0.01).
PVN methylation levels are variable and independent of chronic stress
Similar to our acute stress observations above, methylation levels appeared to be much more variable in the PVN than in all other tissues (Fig. 5C). In both groups of chronically stressed rats, the D'Agostino and Pearson omnibus normality test revealed that the sum of the methylation shows a normal distribution (hippocampus: K2 = 0.068; p = 0.987, PVN: K2 = 3.572; p = 0.168). PVN methylation levels showed a greater skewedness and kurtosis than the hippocampus, although they did not deviate significantly from a normal distribution. Thus, as for acute stress exposure above, the variability in PVN methylation levels would appear to be determined prior to the stressor.
The Ngfi-a binding site remains unmethylated after chronic stress
As CpGs 16 and 17, the core Ngfi-a binding site, were poorly methylated (< 10%) and unaffected by a single acute stressor, we investigated the effect of chronic stress on their methylation levels. Although the sum of methylation, at least in the adrenal and pituitary glands, is significantly affected by chronic stress, in both chronic stress paradigms methylation of CpGs 16 and 17 was consistently < 10% in all six tissues investigated (Fig. 6; Fig. S1; p > 0.05). This is in line with previous reports from other rat stress models,11,12 but in contrast to an earlier publication.19 Methylation levels of CpG 16 increased only in the adrenal gland (p < 0.05; Fig. S1)
Figure 6. Methylation levels of individual CpG dinucleotides in the 17 promoter in the PVN (A) and the hippocampus (B) after 21 d chronic stress. Methylation levels are expressed for all animals in columns by color [0% (blue) to 75% (yellow)] and as the group mean ± SD. Significance levels were in comparison to the no stress group. *p < 0.05. CpG sites are numbered according to Weaver et al.6
Methylation levels of individual CpG positions in promoter 17 correlate after chronic stress
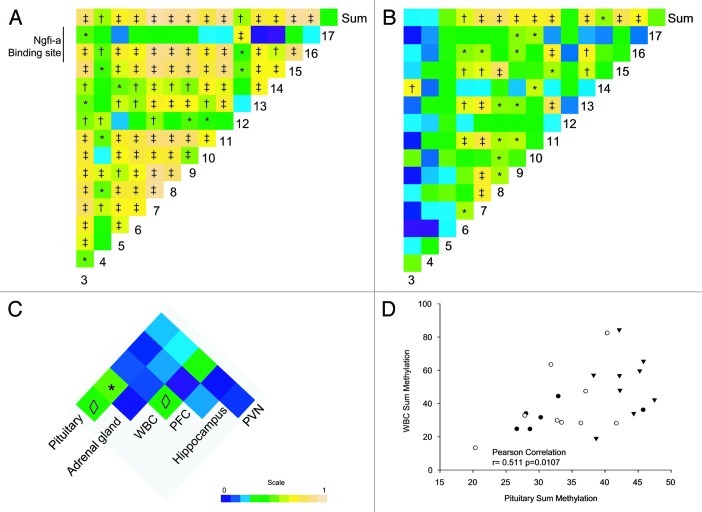
Since in chronically stressed animals ANOVA group effects on the sum of the methylation levels were only observed in the adrenal and pituitary glands, the individual CpG positions in promoter 17 of these tissues were examined in more detail. In the adrenal gland, 7 of the 15 CpGs investigated showed a significant increase in methylation levels (ANOVA p < 0.05; Fig. S1). An additional three CpGs showed a trend toward higher methylation levels. In the pituitary gland, methylation levels of promoter 17 were increased in six of the 15 CpGs, and a trend was observed in two additional CpGs. In both tissues, methylation levels were increased in the same CpGs (CpGs 7, 8, 9, 11, 13 and 15). Methylation was additionally increased at CpG 16 in the adrenal gland. Pearson correlations performed on the aggregated adrenal gland data revealed a high degree of correlation (Fig. 7A) between individual CpGs in a pairwise comparison as well as between most CpGs and the sum of methylation levels. A similar pattern was found in the pituitary gland (Fig. 7B), although CpGs 3 and 4 appeared to correlate less well with the sum of methylation levels and with the other individual CpGs within promoter 17 (p value from 0.02 to 0.38). The high correlation between individual CpGs suggests that methylation is a promoter-wide phenomenon, with levels increasing across multiple CpGs in close proximity.
Figure 7. Pearson correlation heat maps for individual CpGs in the pituitary gland (A) the adrenal gland (B), and for the sum of methylation between all tissues (C) after exposure to chronic stress. The sum of methylation levels for individual animal in (filled circles, controls; empty circles, restrain stress; triangles, psychosocial stress) (D) Pearson correlation coefficients (panels A−C) are expressed by color [0 (blue) to 1 (yellow)]. *p < 0.01, ◊p < 0.01.
Leucocyte sum methylation levels may represent a surrogate or biomarker for peripheral HPA axis tissues
Aggregating all animals irrespective of their group, the sum CpG methylation data (Fig. 7C) shows that there is a strong and significant correlation between the leucocytes and the pituitary gland methylation (r = 0.511 p = 0.011, Fig. 7D). There was, however, only a trend toward a correlation between the pituitary and adrenal gland methylation, as well as between cortex and leucocytes (r = 0.371, p = 0.075 and r = 0.368, p = 0.077, respectively; Fig. 7C).
Discussion
Gr 17 promoter methylation and Ngfi-a transcripts were measured to investigate their antagonistic roles in the production of appropriate Gr level. Exposure to a single acute stressor induced the expected corticosterone response and upregulated Ngfi-a expression in the PVN, as previously reported,16-18 as well as in the cortex but not in the hippocampus, setting the stage for investigating the subsequent regulation of the Gr 17 transcript. Upregulation of its expression confirmed that Ngfi-a is an important stress induced immediate early gene in these brain regions and that it may play a role in the subsequent stress response.17 However, upregulation of Ngfi-a had no effect on 17 transcription or on total Gr expression. Also, in previous studies, even two hours of restraint stress did not alter total Gr transcript levels in the PVN or the hippocampus.20 This suggests that the Ngfi-a binding site previously identified in the rat promoter 17 is not functional in the PVN during acute stress. In contrast, acute stress downregulated Gr in the dentate gyrus (DG) and the CA1 region of the hippocampus.21,22
Methylation of CpG 16 in the core of the Ngfi-a binding site has previously been shown to affect the regulation of Gr 17 transcripts by Ngfi-a. This CpG site was rarely methylated in the hippocampus of offspring of high caring mothers, but highly methylated (> 80%) after low maternal care.6 Therefore, binding of Ngfi-a to the Gr 17 promoter was thought to be inhibited in the maternal care paradigm by this methylation, reducing, as a result, Gr 17 expression.10 However, in our study, none of the animals in the acute stress experiments showed methylation levels > 10% at this CpG site, confirming that the Ngfi-a site was available for binding. Nevertheless, methylation of the promoter appeared to be important, especially in the PVN, since the sum of methylation levels, in particular of CpGs 8–17, correlated negatively with transcript 17 expression, indicating repression of Gr 17 transcription by methylation. Importantly, there was no correlation when only CpG 16 or 17 in the Ngfi-a binding site were considered. Thus, the absence of a response of 17 transcription to acute stress-induced Ngfi-a is not caused by methylation within the core of the Ngfi-a binding site but rather due to methylation across a much wider region of the promoter, indicative of an important role of other transcription factor binding sites and perhaps transcription factors. This suggests that Ngfi-a expression, at least within the 4 h of this study, was not sufficient to drive 17 transcription.
Our chronic stress model altered the HPA axis, as would be expected from previous reports.13,15 While the acute stressor induced a weaker corticosterone response in animals exposed to chronic restraint, it induced a stronger corticosterone response in psychosocially stressed rats. HPA axis hypo-responsiveness after chronic restraint stress was associated with a trend toward an enlarged adrenal gland, as previously reported by Schmidt et al. in a similar murine stress paradigm.15 Conversely, psychosocial stress-induced hyper-responsiveness was associated with a tendency toward a smaller pituitary gland, together with an enlarged adrenal gland, as previously reported after a similar exposure to restraint stress23 suggesting that after chronic stress the rats had not completely habituated to the stressor.24 In both of these tissues, total Gr levels were unchanged compared with control values after either restraint or psychosocial stress. Also, hippocampal Gr levels remained unaffected by either psychosocial or restraint stress. This is in contrast with the downregulation previously reported in some hippocampal subregions, e.g., CA1 or dentate gyrus, which were not independently investigated by us.22 Both of our chronic stress paradigms induced HPA axis changes reminiscent of those observed in human major depression (MDD) studies. Previously, we reported that hippocampal total GR levels were unaffected in MDD,25 although transcript 1F levels were slightly lower.26 Similarly, the adrenal glands in MDD and in suicide victims are larger, have an increased adrenal cortex, and an exaggerated cortisol response after ACTH administration.27-30 These human observations have led several authors to suggest that peripheral HPA axis elements are responsible for the observed hypercortisolemia in MDD.27,31,32 Our psychosocial stress paradigm also mirrors the increased stress reactivity of the HPA axis in MDD. Previously, such neuroendocrine changes have been shown to be prevented by concurrent treatment with antidepressants such as paroxetine.15
The peripheral organs of the HPA axis were further implicated in the altered stress response by our observation that epigenetic methylation of the Gr promoter 17 was preferentially induced by chronic stress in the adrenal gland and the pituitary. Methylation levels in the pituitary and adrenal glands were significantly increased by chronic stress, and showed a trend toward higher levels in peripheral leucocytes, but were unchanged in the PVN, hippocampus and cortex. Thus, our chronic stress models showed that epigenetic methylation of the Gr can be altered in peripheral tissues. Similar observations of Gr methylation have been made in the liver after a protein restricted diet33 in small cell lung tumors34 and in the maternal side of the placenta.35 This is compatible with the notion that DNA methylation patterns are still variable during adulthood and can be modulated by environmental challenges.36
Throughout promoter 17, irrespective of the stressor and for all tissues except the PVN, average methylation levels never exceeded 15%, measured by highly quantitative pyrosequencing.37 These low levels concur with previous reports of hippocampal 17 methylation in rats.11,12 Similar low levels of methylation were also seen in the human 1F promoter in peripheral leucocytes both in newborns38 and adults,39,40 as well as in post mortem adult brains26 and the placenta.35
While the range of methylation levels observed was small, aggregating the data from control, restraint and psychosocially stressed animals we find a high degree of correlation between methylation levels of individual CpG dinucleotides within promoter 17 in the adrenal and the pituitary glands. A similar pattern was previously found in promoter 1F of placentas from large for gestational age births.41 Methylation levels were increased in all promoter 1F CpG positions, although statistical significance was reached in only a single position after post-hoc correction for multiple testing. The importance of promoter-wide methylation is further supported both by our observation that the sum of methylation levels throughout promoter 17 correlated with Gr 17 transcript levels, and the co-regulation of CpGs in close proximity. Recently a similar pattern of increased methylation levels of CpGs in close proximity has been observed for the human GR 1H promoter in suicide completers, although the effect of distance on co-regulation was not analyzed statistically.42 Co-regulation of methylation levels at CpG dinucleotides in close proximity has previously been reported from high-throughput methylation assays of human biopsies. In genome wide studies in both normal and cancerous colon tissue, irrespective of the genomic locus, Nautiyal et al. observed a very high correlation in methylation levels that decreased as the distance between dinucleotides increased.43 Over the scale of a typical promoter, they observed a linear decrease in the Pearsons’ r from > 0.8 for dinucleotide pairs less than 50 bp apart to < 0.4 for pairs 1 kb apart. Chronic stress increased methylation levels significantly in the pituitary and adrenal glands, and in both tissues a promoter-wide regulation was observed. In contrast, only single CpG dinucleotides, such as CpG16, were reported to be altered by maternal care.6,7 We suggest that Gr 17 transcripts were regulated by promoter-wide methylation, and that the role of individual CpGs in the non-functional Ngfi-a binding site, and perhaps the role of the site itself, is more limited than previously thought.
The role of methylation throughout promoter 1F is supported by the recent observation that average methylation levels rather than individual CpG levels throughout promoters 1B and 1H correlated significantly with the expression of their corresponding exons.42 Also, in promoter 1F, mean promoter-wide methylation levels also correlated with GR expression levels in the placenta (Spearman’s rho = -0.28) although not reaching statistical significance.35
As methylation levels increased promoter-wide after chronic stress in a tissue specific manner, we investigated the correlation between the different tissues. The strong correlation between the sum of methylation levels in the pituitary gland and in leucocytes suggests that the latter may be suitable surrogates or potential biomarkers for methylation in peripheral HPA axis organs. In contrast, the hippocampus and the PVN did not correlate with any other tissue, confirming the tissue specificity of their methylation patterns. Peripheral leucocytes have been investigated in the majority of human studies so far.38,40,44,45 Methylation levels in leucocytes of CpG 37 (ortholog of the rat CpG 16), although uniformly low, were shown to be associated with both prior childhood adversity as well as maternal mood during pregnancy,38,45 and reflected functional differences in the HPA axis. In these studies increased leucocyte methylation was associated with a decreased salivary cortisol stress response.38 Since in these and our studies leucocyte methylation levels seem to reflect those in the pituitary and adrenal glands, it may be hypothesized that the functional differences in these infants were the result of altered methylation levels in peripheral HPA axis tissues.
The PVN is unique in that highly variable methylation patterns ranging from 0% to 68% were found in the different animals from both the acute and chronic stress experiments. These levels and variability were not reached in any other tissue. Since the within-group differences clearly exceeded between-group differences in both experiments, we interpret these as unrelated to the stressor, but rather as pre-existing individual differences. Thus, in these outbred rats, environmental influences that were not identified here seem to determine 17 promoter activity through methylation of CpG dinucleotides throughout the 17 promoter of the PVN. Recently, it has been shown that, for instance, the amount of maternal care received varies considerably between litter mates, and individual care levels correlated positively with hippocampal synaptic plasticity and Gr expression levels in this brain region.46 We suggest that this PVN variability may be established in the litter prior to our experiment and may represent an individual trait, in agreement with the early observations on the effects of maternal care.8 The methylation patterns in the PVN, together with the adrenal and pituitary glands, suggest that tissue-specific plasticity is retained from the perinatal period until adulthood.
In conclusion, we report that acute stress induced Ngfi-a did not affect Gr 17 transcript levels. Promoter 17 methylation, particularly in the Ngfi-a binding site, did not explain this lack of activity, although overall methylation levels throughout promoter 17 regulated exon 17 transcript levels in the PVN. Chronic stress altered the corticosterone stress response to a subsequent acute stressor; however, hippocampal and PVN methylation levels were unaffected. In contrast, methylation levels were increased in the pituitary and adrenal glands. These increased methylation levels were orchestrated in promoter-wide clusters of proximal CpGs rather than individual CpG dinucleotides in the non-functional Ngfi-a binding site. The Gr 17 promoter showed tissue-specific methylation plasticity remaining epigenetically sensitive in peripheral HPA axis tissues even in adults. In contrast, methylation levels in the PVN would appear to be unaffected by acute or chronic stress and were significantly more variable within than between groups, suggesting that they were instated before stress exposure as a pre-established trait, probably during the perinatal period.
Methods
Acute and chronic stress protocols
Ten- to twelve-week-old male Sprague Dawley rats were obtained from Harlan and acclimatized for 1 week. Animals were kept under natural light at 22 ± 2°C and 40 ± 5% relative humidity. Food and water were available ad libitum. Animals were housed in groups of 3 in 48 × 37.5 × 21 cm clear plastic cages. All animal experiments were performed in compliance with the rules of the European Communities Council Directive of 24 November 1986 (86/609/EEC), and all national ethical guidelines.
Animals subjected to the acute stress paradigm were randomly divided into 5 groups of 6 animals. Unrestrained animals served as a control group. The restraint groups were immobilized for 15 min and decapitated immediately or returned to their home cages for 1–4 h before decapitation. Isolated brains from all animals were frozen in isopentane cooled in an ethanol-dry ice bath and stored at -80°C. EDTA anti-coagulated decapitation blood was collected and plasma was stored at -20°C.
Animals subjected to the chronic stress paradigm were randomly divided into 3 groups. The control group contained six animals and both the groups exposed to chronic restraint and psychosocial stress contained nine animals. Control animals were weighed daily and returned to their home cage without further manipulation. The chronic restraint stress group was weighed daily and subsequently immobilized for 1 h before being returned to their home cage. The chronic psychosocial stress group was weighed daily, and randomly returned to one of three cages, as outlined in Table S1. All experimental and control animals were restrained for 1 h on day 1 and 14 and tail vein blood was drawn to assess corticosterone levels. All animals were sacrificed on day 21. Right hippocampus and prefrontal cortex were dissected from isolated brains, and the remainder frozen in isopentane cooled in an ethanol-dry ice bath and stored at -80°C. EDTA anti-coagulated decapitation blood was collected and plasma was stored at -20°C. Thymus, pituitary, and adrenal glands were extracted, weighed, and stored in RNAlater (Qiagen) at -20°C until analyzed.
Corticosterone ELISA
Plasma corticosterone levels were measured by ELISA (IBL) following the manufacturer’s instructions. Analyses were performed on 20 µl undiluted or 1:10 diluted plasma as necessary. Intra- and inter-assay variability were 5.2% and 6.4% respectively, and the sensitivity was < 1.63 nmol/L.
Nucleic acid extractions
Serial tissue sections (14 μm) of all brains were cut on a cryostat (Reichert-Jung Frigocut 2800) and thaw-mounted onto Superfrost Plus glass slides and stored at -80 C. One cryostat section per animal was selected for dissection at room temperature under a stereomicroscope. This section was chosen on the basis of the presence of the PVN and the hippocampus in stained adjacent sections. A surface of 3 × 3 mm including bilateral PVNs was scraped into separate RNase-free tubes for subsequent isolation of genomic DNA using the QIAamp® DNA Micro kit (Qiagen, Venlo, The Netherlands) and RNA using the RNAeasy Micro Kit (Qiagen) and stored at -20°C. Genomic DNA from hippocampal tissue was isolated using the QIAamp® DNA Mini kit (Qiagen) following the manufacturer’s instructions.
In situ hybridization
In situ hybridization was performed using 35S UTP labeled riboprobes as described previously.47 Slides were processed to measure the expression of Ngfi-a, Gr exon 17 and Gr total mRNA in the PVN, cortex and hippocampus. The 230 bp Ngfi-a probe used was a kind gift of Prof. J. Milbrandt. For total Gr we used a 500 bp fragment (exon 2, coding for N-terminus of the receptor—courtesy of Dr M. Bohn, Northwestern University) of the original full-length rat Gr clone (courtesy of Dr K. Yamamoto, University of California, San Francisco). MRNA containing exon 17 was quantified with a specific riboprobe kindly provided by Dr K. Chapman (University of Edinburgh).5 Sense probes were used as negative controls. After hybridization, slides were put in a cassette and a Biomax-MR film (Kodak) was exposed for 3 d (Gr) to 13 d (exon 17) and 14C microscales (GE healthcare) were used to calibrate the signal. Films were scanned and quantified using the Image J software. Average optical densities were corrected for film and tissue background.
Quantitative RT-PCR
First-strand (cDNA) synthesis was performed in a 40 μl reaction containing 375 mM KCl, 250 mM Tris-HCl, 15 mM MgCl2, 10 mM dithiothreitol and 500 μM deoxynucleoside triphosphates (dNTPs) at 42°C for 50 min using 200 U SuperScript II Reverse Transcriptase (Invitrogen) and 2.5 μM dT16 primer (Eurogentec).
Duplicate amplifications of cDNA by PCR were performed in 25 μl reactions containing 20 mM Tris–HCl (pH 8.4), 50 mM KCl, 200 mM deoxynucleoside triphosphates (dNTP), 2.5 U Platinum Taq DNA polymerase (Invitrogen) and 1× concentrated SYBR Green (Cambrex). Thermal cycling, (Opticon 2, BioRad) conditions were: 95°C, 2 min; followed by 45 cycles each at 95°C, 20 sec; annealing, 20 sec; 72°C, 30 sec. All PCR products were separated on a 2% agarose gel and visualized with SYBR Safe (Invitrogen) under UV illumination. Primer sequences, annealing temperatures, and MgCl2 concentrations are given in Table S2. All primers were synthesized by Eurogentec.
Methylation analysis
The EpiTect Bisulphite kit (Qiagen) was used to convert unmethylated cytosine residues to uracil, whereas methylated cytosines remained unmodified as previously described.26,40 The bisulphite-modified DNA was used to amplify promoter 17 with the primers and under the conditions shown in Table S2. PCR was performed using 20 mM TRIS-HCl (pH 8.4), 50 mM KCl, 200 mM deoxynucleoside triphosphates, 1× SYBR Green (Cambrex) and 2.5 U Diamond Taq DNA polymerase (Bioline) on an Opticon 2 thermal cycler (BioRad). Cycling conditions were as follows: 95°C for 2 min, 40 cycles, at 95°C for 20 sec, annealing temperature for 20 sec and 72°C for 25 sec. Products of first round PCRs were used as a template for nested amplifications using the primers and conditions in Table S2. The region homologous to the human GR promoter 1A was used as a positive pyrosequencing control. PCR was performed using Platinum Taq polymerase (Invitrogen) with the primers and conditions described in Table S2. Specific primer sets for rat homolog 1A and all pyrosequencing primers have been selected using the PSQ Assay Design software (Biotage). All PCR products were pyrosequenced on a Pyromark ID using Pyrogold reagents (Biotage) to allow quantitative analysis of DNA methylation patterns.
Pyrosequencing controls
As the methylation levels detected in all tissues were on average below 20%, we used the region homologous to the human GR promoter 1A as a positive methylation control in all pyrosequencing experiments. This region is outside of the CpG island and therefore highly methylated (89%).48 The bisulphite conversion efficiency was 93% and the total average methylation was 90%.
Statistical analysis
All results were analyzed using a one way analysis of variance (ANOVA) followed by Tukey post-hoc correction. The relationship between promoter methylation and 17 expression levels was analyzed using Pearson’s correlation coefficient. Normal distribution of overall methylation levels in the hippocampus and the PVN were tested using the D’Agostino-Pearson omnibus test. Statistical analyses were performed using SPSS Version 18 (SPSS Inc.) and the Sigma Stat software for Windows. Differences were considered to be significant when p < 0.05 after post-hoc correction. Sum methylation data are the sum of methylation levels at all individual CpG positions of an individual animal. Aggregated methylation and aggregated sum methylation data refer to the data of all animals combined irrespective of their experimental group.
Supplementary Material
Acknowledgments
We would like to thank Kiki Gortzak for her technical assistance, and we are grateful to Hartmut Schächinger for his initiatives within the Trier-Leiden International Research Training Group (IRTG GRK 1389/1) and the Graduate School of Psychobiology. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (GRK 1389/1) and grants from the Fonds National de la Recherche, Luxembourg (AFR grants TR-PHD BFR07-043, EXT-BFR07-043 TR).
Glossary
Abbreviations:
- GR/Gr
glucocorticoid receptor
- HPA
hypothalamus pituitary adrenal axis
- MR/Mr
mineralocorticoid receptor
- Ngfi-a
nerve growth factor-induced protein A
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/epigenetics/article/22363
Footnotes
Previously published online: www.landesbioscience.com/journals/epigenetics/article/22363
References
- 1.Breslin MB, Geng CD, Vedeckis WV. Multiple promoters exist in the human GR gene, one of which is activated by glucocorticoids. Mol Endocrinol. 2001;15:1381–95. doi: 10.1210/me.15.8.1381. [DOI] [PubMed] [Google Scholar]
- 2.Turner JD, Muller CP. Structure of the glucocorticoid receptor (NR3C1) gene 5′ untranslated region: identification, and tissue distribution of multiple new human exon 1. J Mol Endocrinol. 2005;35:283–92. doi: 10.1677/jme.1.01822. [DOI] [PubMed] [Google Scholar]
- 3.Presul E, Schmidt S, Kofler R, Helmberg A. Identification, tissue expression, and glucocorticoid responsiveness of alternative first exons of the human glucocorticoid receptor. J Mol Endocrinol. 2007;38:79–90. doi: 10.1677/jme.1.02183. [DOI] [PubMed] [Google Scholar]
- 4.Cao-Lei L, Leija SC, Kumsta R, Wüst S, Meyer J, Turner JD, et al. Transcriptional control of the human glucocorticoid receptor: identification and analysis of alternative promoter regions. Hum Genet. 2011;129:533–43. doi: 10.1007/s00439-011-0949-1. [DOI] [PubMed] [Google Scholar]
- 5.McCormick JA, Lyons V, Jacobson MD, Noble J, Diorio J, Nyirenda M, et al. 5′-heterogeneity of glucocorticoid receptor messenger RNA is tissue specific: differential regulation of variant transcripts by early-life events. Mol Endocrinol. 2000;14:506–17. doi: 10.1210/me.14.4.506. [DOI] [PubMed] [Google Scholar]
- 6.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 7.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 9.Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–54. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver IC, D’Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, et al. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. 2007;27:1756–68. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbeck YE, Gulevich RG, Amelkina OA, Plyusnina IZ, Oskina IN. Conserved methylation of the glucocorticoid receptor gene exon 1(7) promoter in rats subjected to a maternal methyl-supplemented diet. Int J Dev Neurosci. 2010;28:9–12. doi: 10.1016/j.ijdevneu.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Daniels WM, Fairbairn LR, van Tilburg G, McEvoy CR, Zigmond MJ, Russell VA, et al. Maternal separation alters nerve growth factor and corticosterone levels but not the DNA methylation status of the exon 1(7) glucocorticoid receptor promoter region. Metab Brain Dis. 2009;24:615–27. doi: 10.1007/s11011-009-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt MV, Scharf SH, Liebl C, Harbich D, Mayer B, Holsboer F, et al. A novel chronic social stress paradigm in female mice. Horm Behav. 2010;57:415–20. doi: 10.1016/j.yhbeh.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, et al. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. Horm Behav. 2008;53:386–94. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt MV, Sterlemann V, Ganea K, Liebl C, Alam S, Harbich D, et al. Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology. 2007;32:417–29. doi: 10.1016/j.psyneuen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe Y, Stone E, McEwen BS. Induction and habituation of c-fos and zif/268 by acute and repeated stressors. Neuroreport. 1994;5:1321–4. [PubMed] [Google Scholar]
- 17.Umemoto S, Kawai Y, Ueyama T, Senba E. Chronic glucocorticoid administration as well as repeated stress affects the subsequent acute immobilization stress-induced expression of immediate early genes but not that of NGFI-A. Neuroscience. 1997;80:763–73. doi: 10.1016/S0306-4522(97)00050-X. [DOI] [PubMed] [Google Scholar]
- 18.Girotti M, Weinberg MS, Spencer RL. Differential responses of hypothalamus-pituitary-adrenal axis immediate early genes to corticosterone and circadian drive. Endocrinology. 2007;148:2542–52. doi: 10.1210/en.2006-1304. [DOI] [PubMed] [Google Scholar]
- 19.Weaver IC, Diorio J, Seckl JR, Szyf M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann N Y Acad Sci. 2004;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- 20.Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–309. doi: 10.1210/en.136.8.3299. [DOI] [PubMed] [Google Scholar]
- 21.Fujikawa T, Soya H, Fukuoka H, Alam KS, Yoshizato H, McEwen BS, et al. A biphasic regulation of receptor mRNA expressions for growth hormone, glucocorticoid and mineralocorticoid in the rat dentate gyrus during acute stress. Brain Res. 2000;874:186–93. doi: 10.1016/S0006-8993(00)02576-2. [DOI] [PubMed] [Google Scholar]
- 22.Yau JL, Noble J, Seckl JR. Acute restraint stress increases 5-HT7 receptor mRNA expression in the rat hippocampus. Neurosci Lett. 2001;309:141–4. doi: 10.1016/S0304-3940(01)02054-7. [DOI] [PubMed] [Google Scholar]
- 23.Barha CK, Brummelte S, Lieblich SE, Galea LA. Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus. 2011;21:1216–27. doi: 10.1002/hipo.20829. [DOI] [PubMed] [Google Scholar]
- 24.Romeo RD, Karatsoreos IN, McEwen BS. Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Horm Behav. 2006;50:463–8. doi: 10.1016/j.yhbeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Klok MD, Alt SR, Irurzun Lafitte AJ, Turner JD, Lakke EA, Huitinga I, et al. Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. J Psychiatr Res. 2011;45:871–8. doi: 10.1016/j.jpsychires.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Alt SR, Turner JD, Klok MD, Meijer OC, Lakke EA, Derijk RH, et al. Differential expression of glucocorticoid receptor transcripts in major depressive disorder is not epigenetically programmed. Psychoneuroendocrinology. 2010;35:544–56. doi: 10.1016/j.psyneuen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Amsterdam JD, Marinelli DL, Arger P, Winokur A. Assessment of adrenal gland volume by computed tomography in depressed patients and healthy volunteers: a pilot study. Psychiatry Res. 1987;21:189–97. doi: 10.1016/0165-1781(87)90022-9. [DOI] [PubMed] [Google Scholar]
- 28.Amsterdam JD, Winokur A, Abelman E, Lucki I, Rickels K. Cosyntropin (ACTH alpha 1-24) stimulation test in depressed patients and healthy subjects. Am J Psychiatry. 1983;140:907–9. doi: 10.1176/ajp.140.7.907. [DOI] [PubMed] [Google Scholar]
- 29.Dumser T, Barocka A, Schubert E. Weight of adrenal glands may be increased in persons who commit suicide. Am J Forensic Med Pathol. 1998;19:72–6. doi: 10.1097/00000433-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Willenberg HS, Bornstein SR, Dumser T, Ehrhart-Bornstein M, Barocka A, Chrousos GP, et al. Morphological changes in adrenals from victims of suicide in relation to altered apoptosis. Endocr Res. 1998;24:963–7. doi: 10.3109/07435809809032717. [DOI] [PubMed] [Google Scholar]
- 31.Chrousos GP, Schuermeyer TH, Doppman J, Oldfield EH, Schulte HM, Gold PW, et al. NIH conference. Clinical applications of corticotropin-releasing factor. Ann Intern Med. 1985;102:344–58. doi: 10.7326/0003-4819-102-3-344. [DOI] [PubMed] [Google Scholar]
- 32.Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291:E965–73. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- 33.Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr. 2007;97:1064–73. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kay P, Schlossmacher G, Matthews L, Sommer P, Singh D, White A, et al. Loss of glucocorticoid receptor expression by DNA methylation prevents glucocorticoid induced apoptosis in human small cell lung cancer cells. PLoS One. 2011;6:e24839. doi: 10.1371/journal.pone.0024839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bromer C, Marsit CJ, Armstrong DA, Padbury JF, Lester B. Genetic and epigenetic variation of the glucocorticoid receptor (NR3C1) in placenta and infant neurobehavior. Dev Psychobiol. 2012 doi: 10.1002/dev.21061. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown SE, Weaver IC, Meaney MJ, Szyf M. Regional-specific global cytosine methylation and DNA methyltransferase expression in the adult rat hippocampus. Neurosci Lett. 2008;440:49–53. doi: 10.1016/j.neulet.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 37.Dejeux E, El abdalaoui H, Gut IG, Tost J. Identification and quantification of differentially methylated loci by the pyrosequencing technology. Methods Mol Biol. 2009;507:189–205. doi: 10.1007/978-1-59745-522-0_15. [DOI] [PubMed] [Google Scholar]
- 38.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 39.Moser D, Molitor A, Kumsta R, Tatschner T, Riederer P, Meyer J. The glucocorticoid receptor gene exon 1-F promoter is not methylated at the NGFI-A binding site in human hippocampus. World J Biol Psychiatry. 2007;8:262–8. doi: 10.1080/15622970701429862. [DOI] [PubMed] [Google Scholar]
- 40.Turner JD, Pelascini LP, Macedo JA, Muller CP. Highly individual methylation patterns of alternative glucocorticoid receptor promoters suggest individualized epigenetic regulatory mechanisms. Nucleic Acids Res. 2008;36:7207–18. doi: 10.1093/nar/gkn897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filiberto AC, Maccani MA, Koestler D, Wilhelm-Benartzi C, Avissar-Whiting M, Banister CE, et al. Birthweight is associated with DNA promoter methylation of the glucocorticoid receptor in human placenta. Epigenetics. 2011;6:566–72. doi: 10.4161/epi.6.5.15236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labonte B, Yerko V, Gross J, Mechawar N, Meaney MJ, Szyf M, et al. Differential glucocorticoid receptor exon 1(B), 1(C), and 1(H) expression and methylation in suicide completers with a history of childhood abuse. Biol Psychiatry. 2012;72:41–8. doi: 10.1016/j.biopsych.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 43.Nautiyal S, Carlton VE, Lu Y, Ireland JS, Flaucher D, Moorhead M, et al. High-throughput method for analyzing methylation of CpGs in targeted genomic regions. Proc Natl Acad Sci U S A. 2010;107:12587–92. doi: 10.1073/pnas.1005173107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Rooij SR, Costello PM, Veenendaal MV, Lillycrop KA, Gluckman PD, Hanson MA, et al. Associations between DNA methylation of a glucocorticoid receptor promoter and acute stress responses in a large healthy adult population are largely explained by lifestyle and educational differences. Psychoneuroendocrinology. 2011;37:782–8. doi: 10.1016/j.psyneuen.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS One. 2012;7:e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Hasselt FN, Cornelisse S, Zhang TY, Meaney MJ, Velzing EH, Krugers HJ, et al. Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus. 2012;22:255–66. doi: 10.1002/hipo.20892. [DOI] [PubMed] [Google Scholar]
- 47.Meijer OC, Topic B, Steenbergen PJ, Jocham G, Huston JP, Oitzl MS. Correlations between hypothalamus-pituitary-adrenal axis parameters depend on age and learning capacity. Endocrinology. 2005;146:1372–81. doi: 10.1210/en.2004-0416. [DOI] [PubMed] [Google Scholar]
- 48.Gruenbaum Y, Stein R, Cedar H, Razin A. Methylation of CpG sequences in eukaryotic DNA. FEBS Lett. 1981;124:67–71. doi: 10.1016/0014-5793(81)80055-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.