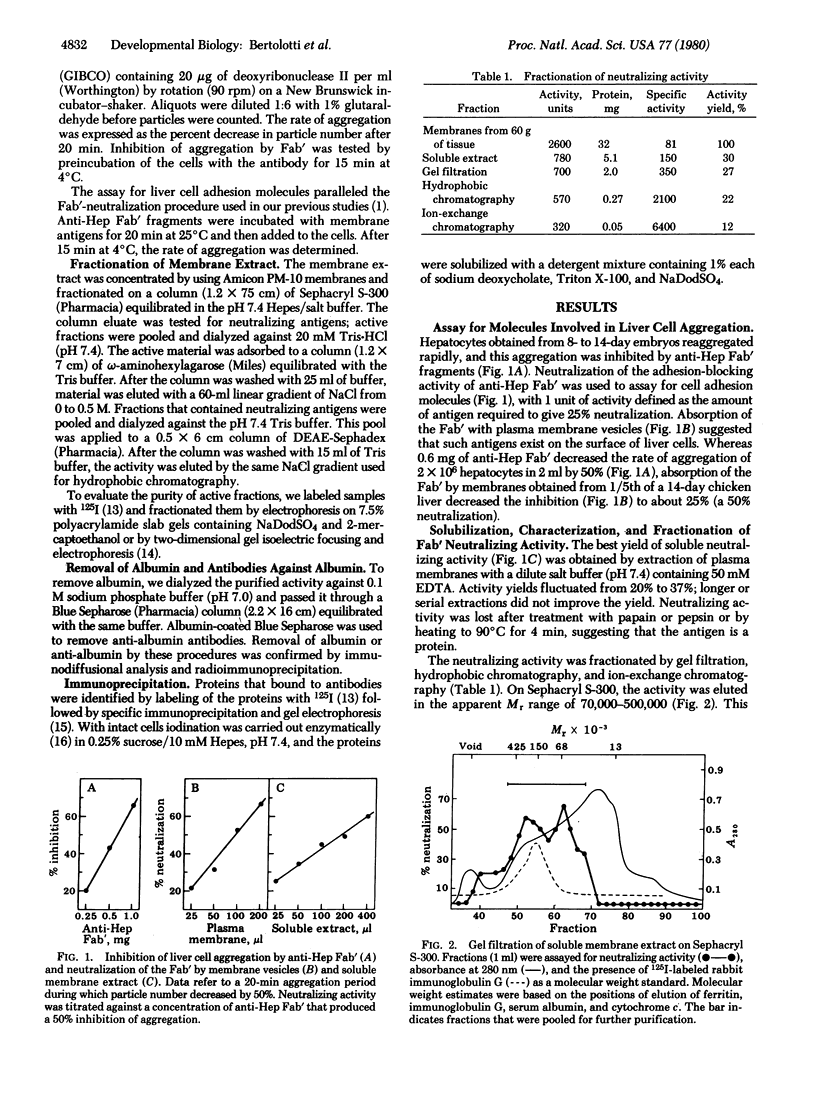
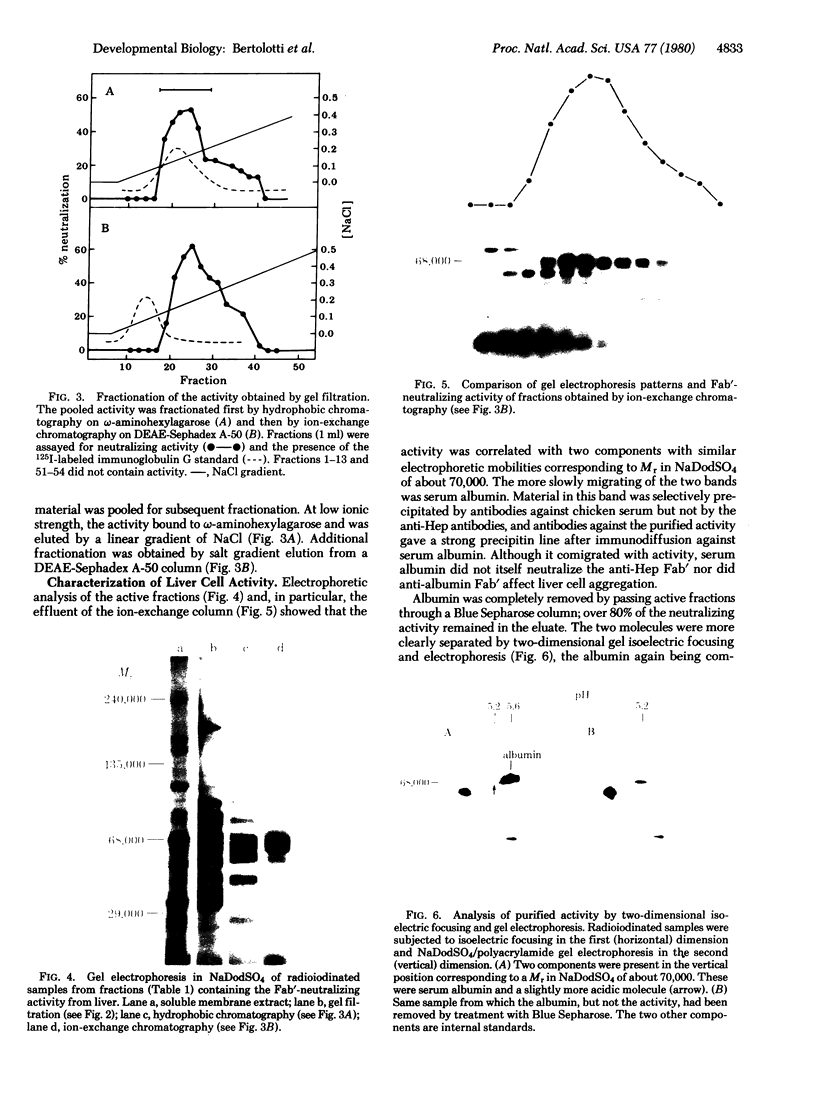
Abstract
Aggragation of chicken enbryo hepatocytes can be inhibited by Fab' fragments of antibodies prepared against the cells. An aqueous extract of liver cell membranes contained antigens that neutralized the adhesion-blocking properties of the Fab' fragments. This neutralization activity was associated with a polypeptide of Mr68,000 in NaDodSO4; the polypeptide was distinct from serum albumin. Specific antibodies prepared against the 80-fold purified active fraction inhibited liver cell adhesion and immunoprecipitated the 68,000 Mr polypeptide from active fractions as well as from a detergent extract of liver cell membranes. In hepatocyte cultures, Fab' fragments of antibodies against the liver molecule prevented both colony formation and appearance of histotypic patterns. Liver cell adhesion was compared at the cellular and molecular levels to that of embryonic neural retina cells. Antibodies against the cell adhesion molecule from neural tissue inhibited retinal but not liver cell aggregation; conversely, antibodies against the liver polypeptide inhibited liver but not retinal cell aggregation. By means of antibody absorption and immunoprecipitation, it was confirmed that the two cell adhesion molecules are antigenically unrelated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brackenbury R., Thiery J. P., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell-cell binding. J Biol Chem. 1977 Oct 10;252(19):6835–6840. [PubMed] [Google Scholar]
- Brown J. L., Kato K., Silver J., Nathenson S. G. Notable diversity in peptide composition of murine H-2K and H-2D alloantigens. Biochemistry. 1974 Jul 16;13(15):3174–3178. doi: 10.1021/bi00712a027. [DOI] [PubMed] [Google Scholar]
- Buskirk D. R., Thiery J. P., Rutishauser U., Edelman G. M. Antibodies to a neural cell adhesion molecule disrupt histogenesis in cultured chick retinae. Nature. 1980 Jun 12;285(5765):488–489. doi: 10.1038/285488a0. [DOI] [PubMed] [Google Scholar]
- Coon H. G. Clonal stability and phenotypic expression of chick cartilage cells in vitro. Proc Natl Acad Sci U S A. 1966 Jan;55(1):66–73. doi: 10.1073/pnas.55.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huesgen A., Gerisch G. Solubilized contact sites a from cell membranes of Dictyostelium discoideum. FEBS Lett. 1975 Aug 1;56(1):46–49. doi: 10.1016/0014-5793(75)80108-6. [DOI] [PubMed] [Google Scholar]
- Lambiotte M., Vorbrodt A., Benedetti E. L. Apparition de canalicules biliares dans le foie foetal de rat en culture cellulaire sous l'influence de glucocorticoïdes. C R Acad Sci Hebd Seances Acad Sci D. 1972 Nov 27;275(22):2539–2542. [PubMed] [Google Scholar]
- Lindgren J., Vaheri A., Ruoslahti E. Identification and isolation of a foetoprotein in the chicken. Differentiation. 1974 Aug;2(4):233–236. doi: 10.1111/j.1432-0436.1974.tb00356.x. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- McGuire E. J. Intercellular adhesive selectivity. II. Properties of embryonic chick liver cell-cell adhesion. J Cell Biol. 1976 Jan;68(1):90–100. doi: 10.1083/jcb.68.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrink B., Ocklind C. Cell surface component(s) involved in rat hepatocyte intercellular adhesion. Biochem Biophys Res Commun. 1978 Nov 29;85(2):837–843. doi: 10.1016/0006-291x(78)91238-x. [DOI] [PubMed] [Google Scholar]
- Piperno G., Huang B., Luck D. J. Two-dimensional analysis of flagellar proteins from wild-type and paralyzed mutants of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1600–1604. doi: 10.1073/pnas.74.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. K. A modified method for the isolation of the plasma membrane from rat liver. Biochim Biophys Acta. 1970 Jan 6;196(1):1–9. doi: 10.1016/0005-2736(70)90159-8. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Gall W. E., Edelman G. M. Adhesion among neural cells of the chick embryo. IV. Role of the cell surface molecule CAM in the formation of neurite bundles in cultures of spinal ganglia. J Cell Biol. 1978 Nov;79(2 Pt 1):382–393. doi: 10.1083/jcb.79.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Thiery J. P., Brackenbury R., Edelman G. M. Adhesion among neural cells of the chick embryo. III. Relationship of the surface molecule CAM to cell adhesion and the development of histotypic patterns. J Cell Biol. 1978 Nov;79(2 Pt 1):371–381. doi: 10.1083/jcb.79.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J. P., Brackenbury R., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. II. Purification and characterization of a cell adhesion molecule from neural retina. J Biol Chem. 1977 Oct 10;252(19):6841–6845. [PubMed] [Google Scholar]
- Umbreit J., Roseman S. A requirement for reversible binding between aggregating embryonic cells before stable adhesion. J Biol Chem. 1975 Dec 25;250(24):9360–9368. [PubMed] [Google Scholar]









