Abstract
Schistosomiasis japonica is a zoonosis with a number of mammalian species acting as reservoir hosts, including water buffaloes which can contribute up to 75% to human transmission in the People's Republic of China. Determining prevalence and intensity of Schistosoma japonicum in mammalian hosts is important for calculating transmission rates and determining environmental contamination. A new procedure, the formalin–ethyl acetate sedimentation-digestion (FEA–SD) technique, for increased visualization of S. japonicum eggs in bovine feces, is described that is an effective technique for identifying and quantifying S. japonicum eggs in fecal samples from naturally infected Chinese water buffaloes and from carabao (water buffalo) in the Philippines. The procedure involves filtration, sedimentation, potassium hydroxide digestion and centrifugation steps prior to microscopy. Bulk debris, including the dense cellulosic material present in bovine feces, often obscures schistosome eggs with the result that prevalence and infection intensity based on direct visualization cannot be made accurately. This technique removes nearly 70% of debris from the fecal samples and renders the remaining debris translucent. It allows improved microscopic visualization of S. japonicum eggs and provides an accurate quantitative method for the estimation of infection in bovines and other ruminant reservoir hosts. We show that the FEA-SD technique could be of considerable value if applied as a surveillance tool for animal reservoirs of S. japonicum, particularly in areas with low to high infection intensity, or where, following control efforts, there is suspected elimination of schistosomiasis japonica.
Author Summary
Schistosomiasis japonica, a chronic human parasitic disease in the People's Republic of China, the Philippines and areas of Indonesia, is a zoonosis with over 40 different mammals, including a number of ruminants, that can act as reservoir hosts for the infection. Precise identification of the major infection reservoirs is important for the control of Schistosoma japonicum as their targeted treatment can prevent environmental contamination and transmission of the parasite, thus reducing the risk to humans. Current copro-parasitological techniques are generally unsatisfactory for identifying and quantifying S. japonicum eggs in ruminant feces due to the large volume of cellulosic debris present. The new approach we describe here, the FEA–SD technique, removes much of this material by sieving and centrifugation with ethyl actate and renders any remaining debris transparent by use of a potassium hydroxide (KOH) digestion, providing much improved visualization of eggs, enabling the collection of more accurate data on S. japonicum infection in ruminants. This new tool will be of particular value for monitoring schistosome prevalence and intensity in animal reservoirs in areas of the People's Republic of China that are heading toward schistosomiasis elimination.
Introduction
Schistosoma japonicum, the causative agent of Asian schistosomiasis, is endemic to the People's Republic of China, the Philippines and small pockets of Indonesia [1]–[7]. Unlike African schistosomiasis, mainly caused by S. mansoni and S. haematobium, schistosomiasis japonica is a zoonosis and it is estimated that over 40 mammalian species, comprising 28 genera and 7 orders of wild and domestic animals, can act as reservoirs and harbour S. japonicum infection [8]. The range of mammalian hosts complicates schistosomiasis control efforts and, as well as the public health considerations, the disease adds to the economic burden of communities as S. japonicum infection debilitates domestic livestock that are used for food and as work animals [9], [10]. Bovines, particularly water buffaloes (Bubalus bubalis), have been shown to be major reservoir hosts for schistosomiasis japonica in the lake areas and marshlands of southern the People's Republic of China [11]–[16]. However, their role in schistosome transmission has yet to be fully determined in other endemic areas, notably the Philippines, due partly to inconsistent results obtained with the different methods used for identifying and quantifying S. japonicum eggs in mammalian hosts [17]–[20]. In particular, the presence of bulk debris, including cellulosic fibrous material, in the feces of ruminants often obscures the eggs and impairs their visualization across all current copro-parasitological methods that involve microscopy.
Here we describe a new copro-parasitological method, the formalin–ethyl acetate sedimentation-digestion (FEA–SD) technique, which eliminates much of this bulk debris and cellulose material and facilitates much improved microscopic examination of S. japonicum eggs in the feces of bovines and other ruminant hosts. We show the FEA-SD technique is an effective technique for identifying S. japonicum eggs using fecal samples from naturally infected Chinese water buffaloes (Bubalis bubalis) and confirm its reproducibility using parasite-positive samples obtained from carabao, a subspecies (Bubalus bubalis carabanesis), in the Philippines. We show that the FEA-SD method is as efficient as real-time PCR (qPCR) for determining schistosome prevalence in bovines but is less costly to implement.
Materials and Methods
Ethics Statement
The conducts and procedures involving animal experiments were approved by the Animals Ethics Committee of the Queensland Institute of Medical Research (project no. P288). This study was performed in accordance with the recommendations of the Australian code of practice for the care and use of animals for scientific purposes, 2004.
The FEA-SD Technique
Stool samples were taken intra-rectally from 13 water buffaloes, collected from Jiangxi province, People's Republic of China, shown to be naturally infected with S. japonicum, by the miracidial hatching test (MHT) [21]. The FEA–SD technique was then used on the positive samples to identify S. japonicum eggs, calculating egg recovery rates and bulk debris reduction. Full details of the procedure are as follows: First, bovine stool samples are collected rectally from the animals (approximately 500 g each) by a veterinarian or trained personnel. Each stool sample is homogenized with an applicator stick and 50 g of the stool mixture is taken and mixed to a slurry in a beaker with 300 ml of water. The slurry is then sieved, by pouring the slurry onto a 60 copper mesh (Tyler scale with a pore opening size of 250 µm) and using water to flush the smaller sediment onto a 260 copper mesh (61 µm), held below the 40–60 mesh. Sediment caught on the 260 mesh is washed with water into a conical flask and allowed to sediment naturally for 30 minutes. The excess water is removed, leaving sediment which is poured into a 50 ml tube. Approximately 50 ml of water is added to the conical flask and naturally sedimented for 30 minutes, again removing excess water and pouring sediment into the same 50 ml tube. This is repeated once more, to ensure all sediment is in the 50 ml tube. The tube is topped up to 50 ml with 10% formalin (v/v) and mixed thoroughly by vortexing, before standing at room temperature for 30 minutes to fix the eggs. The Falcon tube is then vortexed and, using a pasteur pipette, 10 ml of the suspension (equivalent to 10 g feces) placed into two 15 ml tubes (5 ml in each; equivalent to 5 g feces) labelled A and B. Ten percent (v/v) formalin solution is added to tubes A and B to take the volume up to 8 ml and the tubes mixed thoroughly by vortexing, after which 4 ml of 100% (v/v) ethyl acetate is added using a glass pipette and vigorously vortexed once more for 30 sec. With the cap of the tubes slightly loosened the tubes are centrifuged at 500 g for 10 minutes, resulting in a four-layer separation (Figure 1). It is important for this step that the tubes are spun at 500 g for stable and efficient debris removal. The ethyl acetate is removed from both tubes by gently rimming the bulk debris layer with a thin applicator stick and decanting the top three layers which are then discarded. Ethyl acetate and 10% (v/v) formalin can be added and spun again if necessary to remove further bulk debris. If the middle layer of bulk debris is very thin the tube should be shaken vigorously and the re-spun for better efficiency. Water is added to the remaining pellet to take the volume up to 5 ml and an equal volume of 10% (w/v) potassium hydroxide (KOH) is added to each tube. The tubes are mixed gently by vortexing to resuspend the pellet and the sample is digested overnight at 37°C.
Figure 1. Four layer separation of sieved fecal material using the FEA-SD technique.

The resulting four layer separation occurs after the addition of 100% ethyl acetate and centrifugation at 500 g. The top layer contains the ethyl acetate, the second layer the bulk debris to be discarded, the third layer comprises 10% (v/v) formalin and the final layer is the remaining sediment containing the eggs.
After digestion, the sample is vortexed vigorously and centrifuged at 900 g for 10 minutes. The pellet is washed once with 10–15 ml of water to remove any residual KOH by centrifuging the solution for 10 minutes at 900 g, the supernatant removed and the final pellet is resuspended in 4–6 ml of water (as the samples are now fixed) and stored at 4°C. The sample is now ready for counting the S. japonicum eggs by microscopy. Before counting, the suspension is mixed gently with a pipette and the total volume of tubes A and B counted for each sample, pipetting 200–300 µl onto each slide for microscopy. It is important that this is done as the sensitivity of the procedure increases with the amount of suspension examined. The microscopy was performed blind by two independent microscopists, although this is not essential to the completion of the procedure. Infection intensity (eggs per gram of feces, EPG) is calculated based on the total egg number in 10 g of feces (i.e., the contents of tube A plus tube B).
Reduction in Bulk Debris and Egg Recovery
In order to determine the effectiveness and reproducibility of the FEA-SD technique in reducing the bulk debris and cellulosic material present in water buffalo feces, stool samples from the 13 Chinese animals were individually processed and the initial and final volumes of debris measured and compared (Table 1). Egg recovery was measured by microscopic examination of each of the normally discarded top 3 layers (Figure 1) for the presence of eggs (Table 2). Eggs in the final sediment were counted as per the protocol described above (Table 3).
Table 1. Reduction in bulk debris of water buffalo fecal samples from the People's Republic of China by the FEA-SD method.
| Volume of sediment (ml) | ||||
| *Sample | Initial (I) | Post FEA-SD (P) | I-P (ml) | I - P/I % |
| 1A | 1.3 | 0.2 | 1.1 | 84.6 |
| 1B | 1.5 | 0.2 | 1.3 | 86.7 |
| 2A | 1.6 | 0.6 | 1.0 | 62.5 |
| 2B | 1.6 | 0.6 | 1.0 | 62.5 |
| 3A | 1.0 | 0.4 | 0.6 | 60.0 |
| 3B | 1.0 | 0.4 | 0.6 | 60.0 |
| 4A | 1.7 | 0.3 | 1.4 | 82.4 |
| 4B | 1.5 | 0.2 | 1.3 | 86.7 |
| 5A | 1.8 | 0.6 | 1.2 | 66.7 |
| 5B | 1.9 | 0.8 | 1.1 | 57.9 |
| 6A | 2.0 | 0.8 | 1.2 | 60.0 |
| 6B | 1.7 | 0.8 | 0.9 | 52.9 |
| 7A | 1.3 | 0.4 | 0.9 | 69.2 |
| 7B | 1.4 | 0.4 | 1.0 | 71.4 |
| 8A | 2.5 | 0.8 | 1.7 | 68.0 |
| 8B | 2.4 | 1.0 | 1.4 | 58.3 |
| 9A | 1.4 | 0.3 | 1.1 | 78.6 |
| 9B | 1.5 | 0.3 | 1.2 | 80.0 |
| 10A | 1.0 | 0.3 | 0.7 | 70.0 |
| 10B | 1.0 | 0.2 | 0.8 | 80.0 |
| 11A | 1.0 | 0.2 | 0.8 | 80.0 |
| 11B | 1.0 | 0.2 | 0.8 | 80.0 |
| 12A | 1.0 | 0.3 | 0.7 | 70.0 |
| 12B | 1.0 | 0.2 | 0.8 | 80.0 |
| 13A | 1.0 | 0.5 | 0.5 | 50.0 |
| 13B | 1.0 | 0.6 | 0.4 | 40.0 |
| Mean | 1.4 | 0.4 | 1.0 | 69.2 |
| SD | 12.3 | |||
Equivalent to 5 g feces/sample; n = 26.
Table 2. Reduction in bulk debris of carabao fecal samples from the Philippines by the FEA-SD method.
| Sample | Initial (I) | Post FEA-SD (P) | I-P (ml) | I - P/I % |
| 1A | 1.0 | 0.2 | 0.8 | 80.0 |
| 1B | 1.2 | 0.3 | 0.9 | 75.0 |
| 2A | 1.0 | 0.5 | 0.5 | 50.0 |
| 2B | 1.1 | 0.5 | 0.6 | 54.5 |
| 3A | 1.3 | 0.5 | 0.8 | 61.5 |
| 3B | 1.4 | 0.5 | 0.9 | 64.3 |
| 4A | 1.3 | 0.3 | 1.0 | 76.9 |
| 4B | 1.4 | 0.4 | 1.0 | 71.4 |
| 5A | 1.4 | 0.4 | 1.0 | 71.4 |
| 5B | 1.5 | 0.4 | 1.1 | 73.3 |
| 6A | 1.3 | 0.4 | 0.9 | 69.2 |
| 6B | 1.3 | 0.3 | 1.0 | 76.9 |
| 7A | 1.2 | 0.6 | 0.6 | 50.0 |
| 7B | 1.2 | 0.6 | 0.6 | 50.0 |
| 8A | 1.3 | 0.4 | 0.9 | 69.2 |
| 8B | 1.3 | 0.3 | 1.0 | 76.9 |
| 9A | 1.1 | 0.5 | 0.6 | 54.5 |
| 9B | 1.0 | 0.3 | 0.7 | 70.0 |
| 10A | 1.0 | 0.3 | 0.7 | 70.0 |
| 10B | 1.1 | 0.5 | 0.6 | 54.5 |
| 11A | 0.9 | 0.4 | 0.5 | 55.6 |
| 11B | 1.0 | 0.5 | 0.5 | 50.0 |
| 12A | 1.0 | 0.5 | 0.5 | 50.0 |
| 12B | 1.2 | 0.4 | 0.8 | 66.7 |
| 13A | 1.5 | 0.6 | 0.9 | 60.0 |
| 13B | 1.6 | 0.5 | 1.1 | 68.8 |
| 14A | 1.6 | 0.9 | 0.7 | 43.8 |
| 14B | 1.6 | 0.9 | 0.7 | 43.8 |
| 15A | 1.2 | 0.5 | 0.7 | 58.3 |
| 15B | 1.2 | 0.6 | 0.6 | 50.0 |
| 16A | 0.8 | 0.3 | 0.5 | 62.5 |
| 16B | 0.7 | 0.3 | 0.4 | 57.1 |
| 17A | 1.0 | 0.5 | 0.5 | 50.0 |
| 17B | 1.0 | 0.5 | 0.5 | 50.0 |
| 18A | 1.0 | 0.4 | 0.6 | 60.0 |
| 18B | 1.1 | 0.4 | 0.7 | 63.6 |
| 19A | 0.9 | 0.5 | 0.4 | 44.4 |
| 19B | 0.9 | 0.5 | 0.4 | 44.4 |
| 20A | 1.0 | 0.4 | 0.6 | 60.0 |
| 20B | 1.0 | 0.4 | 0.6 | 60.0 |
| 22A | 1.1 | 0.3 | 0.8 | 72.7 |
| 22B | 1.0 | 0.3 | 0.7 | 70.0 |
| 23A | 1.0 | 0.3 | 0.7 | 70.0 |
| 23B | 1.2 | 0.4 | 0.8 | 66.7 |
| 24A | 1.3 | 0.4 | 0.9 | 69.2 |
| 24B | 1.4 | 0.5 | 0.9 | 64.3 |
| 25A | 1.0 | 0.4 | 0.6 | 60.0 |
| 25B | 1.1 | 0.3 | 0.8 | 72.7 |
| Mean | 1.2 | 0.4 | 0.7 | 61.8 |
| SD | 10.2 |
Table 3. Reproducibility in recovery of S. japonicum eggs in buffalo fecal samples from the People's Republic of China using the FEA-SD technique.
| Number of eggs | ||||||
| Sample/Tube | Water supernatant (W) | Bulk debris (BD)*** | W+BD | Bottom sediment | Total | Egg recovery rate (%)**** |
| *Sample 1 | ||||||
| **Tube A1 | 0 | 1 | 1 | 50 | 51 | 98.0 |
| Tube B1 | 1 | 2 | 3 | 66 | 69 | 95.7 |
| Sample 2 | ||||||
| Tube A2 | 0 | 2 | 2 | 46 | 48 | 95.8 |
| Tube B2 | 0 | 5 | 5 | 66 | 71 | 93.0 |
| Sample 3 | ||||||
| Tube A3 | 0 | 2 | 2 | 45 | 47 | 95.7 |
| Tube B3 | 0 | 5 | 5 | 64 | 69 | 92.8 |
| Mean | 95.2 | |||||
| SD | 2.0 | |||||
Equivalent to 10 g feces.
Equivalent to 5 g feces.
BD: Sediment in the bulk debris layer (Figure 1) removed during the FEA-SD procedure.
Egg recovery rate (%) = number of eggs in the bottom sediment/total number of eggs in the sample ×100.
Application and Reproducibility of the FEA-SD Method Using Fecal Samples Collected from Philippines Carabao
Twenty-five carabao fecal samples were collected intra-rectally from S. japonicum-endemic barangays (villages) from Western Samar province, the Philippines. These were subjected to the FEA-SD procedure applied earlier in China with the debris reduction measured (Table 2) and the final egg counts from Tubes A and B for each of the 25 samples determined (Table 4). Full details of the sample collection and methods used can be found in Gordon et al. 2012 [22].
Table 4. Recovery of S. japonicum eggs in carabao fecal samples from the Philippines using the FEA-SD technique.
| Sample/Tube | Eggs recovered* | Total | Sample/Tube | Eggs recovered* | Total |
| Sample 1 | Sample 14 | ||||
| Tube A1 | 3 | Tube A14 | 1 | ||
| Tube B1 | 6 | 9 | Tube B14 | 2 | 3 |
| Sample 2 | Sample 15 | ||||
| Tube A2 | 2 | Tube A15 | 0 | ||
| Tube B2 | 1 | 3 | Tube B15 | 0 | 0 |
| Sample 3 | Sample 16 | ||||
| Tube A3 | 0 | Tube A16 | 0 | ||
| Tube B3 | 1 | 1 | Tube B16 | 2 | 2 |
| Sample 4 | Sample 17 | ||||
| Tube A4 | 0 | Tube A17 | 5 | ||
| Tube B4 | 8 | 8 | Tube B17 | 0 | 5 |
| Sample 5 | Sample 18 | ||||
| Tube A5 | 9 | Tube A18 | 5 | ||
| Tube B5 | 11 | 20 | Tube B18 | 7 | 12 |
| Sample 6 | Sample 19 | ||||
| Tube A6 | 1 | Tube A19 | 2 | ||
| Tube B6 | 1 | 2 | Tube B19 | 2 | 4 |
| Sample 7 | Sample 20 | ||||
| Tube A7 | 2 | Tube A20 | 0 | ||
| Tube B7 | 1 | 3 | Tube B20 | 1 | 1 |
| Sample 8 | Sample 21 | ||||
| Tube A8 | 0 | Tube A21 | 5 | ||
| Tube B8 | 2 | 2 | Tube B21 | 6 | 11 |
| Sample 9 | Sample 22 | ||||
| Tube A9 | 0 | Tube A22 | 1 | ||
| Tube B9 | 0 | 0 | Tube B22 | 3 | 4 |
| Sample 10 | Sample 23 | ||||
| Tube A10 | 2 | Tube A23 | 3 | ||
| Tube B10 | 7 | 9 | Tube B23 | 7 | 10 |
| Sample 11 | Sample 24 | ||||
| Tube A11 | 0 | Tube A24 | 2 | ||
| Tube B11 | 4 | 4 | Tube B24 | 6 | 8 |
| Sample 12 | Sample 25 | ||||
| Tube A12 | 2 | Tube A25 | 0 | ||
| Tube B12 | 0 | 2 | Tube B25 | 3 | 3 |
| Sample 13 | |||||
| Tube A13 | 0 | ||||
| Tube B13 | 4 | 4 |
Comparison of the FEA-SD Method with Other Fecal Examination Techniques
The FEA-SD was compared directly with other fecal examination techniques including Kato-Katz (KK), the MHT, a validated qPCR assay and conventional PCR on 44 fecal samples collected during the same survey in Western Samar province referred to above. Full details of the sample collection and methods used can be found in Gordon et al. 2012 [22].
Results
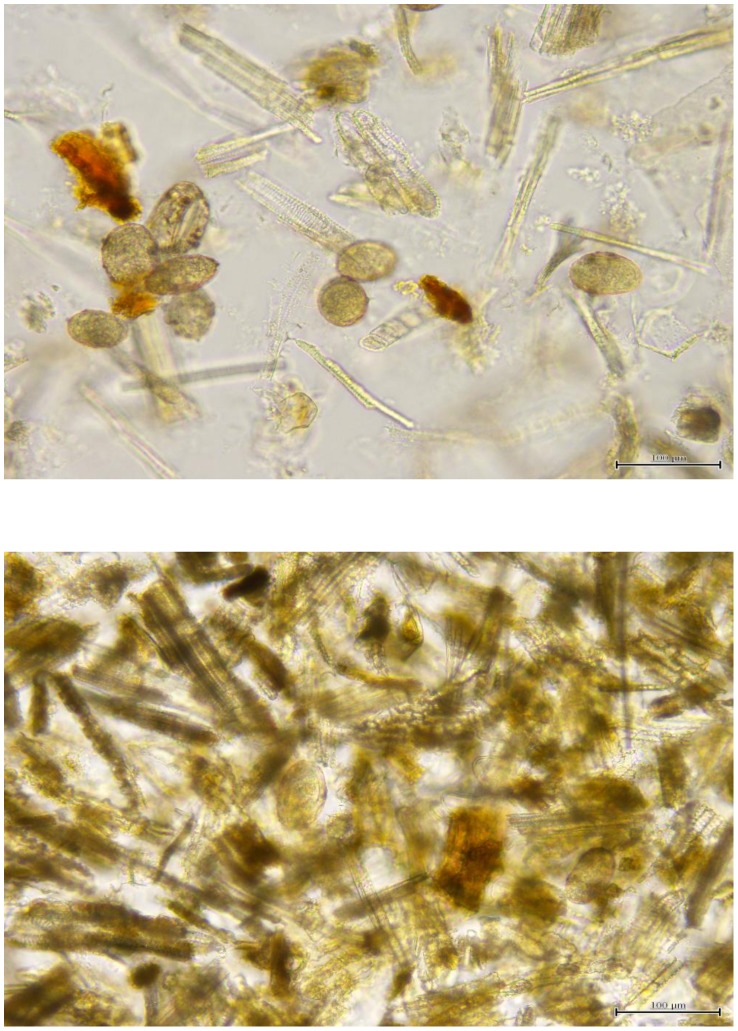
The FEA-SD technique removed an average of 61.5% — 69.2% of the bulk cellulose debris from the Philippine and Chinese, respectively, bovine stool samples prior to microscopic examination (Tables 1, 2). Any remaining debris was rendered transparent by the potassium hydroxide digestion step, so that eggs were readily observed compared with previous copro-parasitological techniques employing sieving only (Figure 2). Few eggs were present in the discarded bulk debris and supernatant, with an average of 95.2% of the total eggs recovered found in the final sedimented pellet (Table 3). Prevalence determined by the FEA-SD in the study undertaken on water buffaloes in the Philippines showed the FEA-SD (93.2%, 95% CI 85.4–100) had a similar sensitivity (90.9%, 95% CI 82.1–99.8) as the qPCR assay. By contrast the conventional PCR (31.8%, 95% CI 17.5–46.1), KK (25%, 95% CI 11.7–38.3) and MHT (19.1%, 95% CI 0.9–41.2) (Figure 3) gave much lower prevalence [21].
Figure 2. Visualization of S. japonicum eggs (circled) in water buffalo feces.
Top panel; egg visualization after sieving of feces only. Lower panel; egg visualization after feces are subjected to the FEA-SD technique.
Figure 3. The FEA-SD method compared with other diagnostic techniques (Modified from Gordon et al. [22]).
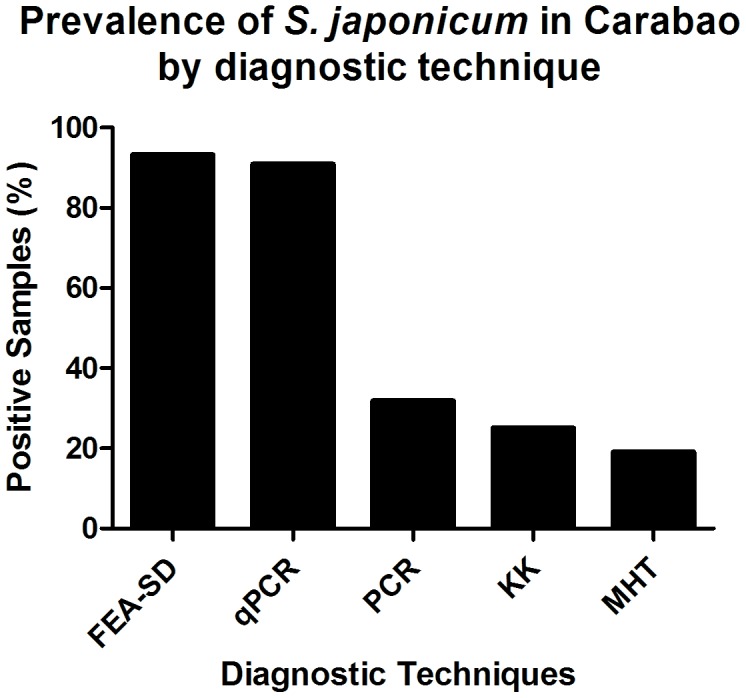
FEA-SD, formalin–ethyl acetate sedimentation-digestion technique; qPCR, quantitative PCR; PCR, conventional PCR; KK, Kato Katz method; MHT, miracidial hatching test. 95% confidence intervals are shown.
Discussion
The amount of faeces excreted in one defecation by a large animal such as a water buffalo can exceed 45 kg so it is important to determine the minimal amount of feces that can provide optimal and consistent results using the FEA-SD technique. It was found that a sample of 10 g of feces (divided into tubes A and B) was critical for accurate quantification; as a comparison, 5 g of feces resulted in very inconsistent egg counts between tubes A and B.
The direct microscopic identification of schistosome eggs is the ‘gold’ standard for the diagnosis of zoonotic schistosomiasis in both animals and humans. The current microscopic methods of choice for the identification of S. japonicum eggs in bovines and other ruminants are, however, limited in terms of sensitivity and include, among other procedures, the MHT followed by a sedimentation filtration method, and the Danish Bilharziasis Laboratory (DBL) technique [23] (Table 5). More recently developed techniques include FLOTAC and the use of magnetic beads (Helmintex test) which have been shown to detect helminth eggs in low intensity infections [24]–[29]. Immunological techniques have also been applied to diagnostics however cross reactivity and identification of past infections, rather than current infections, have been issues. A recent study looking at Thioredoxin Peroxidase-1 in an ELISA system for identification of S. japonicum in bovines has shown promising results and no cross reaction with a closely related species [30].
Table 5. Published studies of diagnostic procedures for identification of S. japonicum eggs in bovine feces.
| Location of study [Reference] | Year | Diagnostic | Bovine | Prevalence (%) | Intensity (EPG) |
| Poyang Lake, P. R. China [33] | 2002 | MHT+filtration | Cattle & water buffalo | 14 | 42 |
| Hubei, P. R. China [36] | 1994 | MHT+filtration | Water buffalo | 35.7 | 0.4* |
| Anhui, P. R. China [37] | 2010 | MHT+filtration | Water buffalo | 10.5 | 3.4 |
| Cattle | 46.5 | 2.3 | |||
| Sichuan, P. R. China [38] | 2006 | MHT+filtration | Cattle | 22.3 | - |
| Hunan and Jiangxi, P. R. China [12] | 2007 | MHT+filtration | Cattle | 21.7 | 0.5–7.2* |
| Water buffalo | 14.9 | 0.5–7.2* | |||
| Dongting Lake, P. R. China [39] | 2007 | MHT | Cattle | 6.1 | - |
| Water buffalo | 9.5 | - | |||
| Leyte, the Philippines [17] | 2010 | MHT | Water buffalo | 0 | - |
| KK | 3.7 | - | |||
| DBL Technique | 3.7 | - | |||
| qPCR | 51.5 | 2.1* | |||
| Samar, the Philippines [22] | 2012 | MHT | Water buffalo | 19.1 | - |
| KK | 25 | 4.7* | |||
| FEA-SD | 93.2 | 1.2* | |||
| qPCR | 90.9 | 6.1* | |||
| PCR | 31.3 | - | |||
| Samar and Sorsogon, the Philippines [19] | 2005 | DBL technique | Water buffalo | 6.3 | - |
| Samar, the Philippines [40] | 2007 | DBL technique | Water buffalo | 2.1 | - |
| Mindoro, the Philippines [41] | 1999 | Formalin detergent technique | Water buffalo | 0 | - |
| Leyte, the Philippines [42] | 1981 | Merthiolate iodine-formaldehyde concentration (MIFC) technique | Cattle | 0 | - |
| Water buffalo | 0.38 | - | |||
| Leyte, the Philippines [43] | 1958 | Glycerol sedimentation with egg hatching and sedimentation counting of eggs remaining | Water buffalo | 1.5 | - |
| Cattle | 3.82 | - | |||
| Leyte, the Philippines [44] | 1982 | MIFC and Circumoval Precipitin Test (COPT) | Cattle | 1 (MIFC) | - |
| Cattle | 0 (COPT) | - | |||
| Water buffalo | 9 (MIFC) | - | |||
| Water buffalo | 1 (COPT) | - | |||
| Cagayan, The Philippines [30] | 2012 | COPT ELISA | Water buffalo | 34 (COPT) | - |
| 36 (ELISA) | - |
Geometric mean eggs per gram (EPG). All other values are arithmetic mean EPG.
MHT, miracidial hatching test; DBL, Danish Bilharziasis Laboratory method; KK, Kato-Katz technique; qPCR, quantitative real time PCR.
The MHT has been used extensively in the People's Republic of China for the identification of S. japonicum in bovine feces [11]. The MHT – a qualitative diagnostic test – involves the concentration of ova from saline using fresh feces through a nylon tissue bag and suspension in distilled water. Miracidia are visualized macroscopically and their presence is diagnostic of infection; three hatches (50 g feces per hatch) are routinely carried out. The MHT is preferred to the Kato Katz technique (KK) (recommended for diagnosis of intestinal schistosomiasis in humans) [26], [31], [32] and the other microscopic methods, due to the large volume of feces produced by bovines, and the fact that, as discussed, bovine feces contain considerable amounts of cellulosic material. This obscures the microscopic visualization of schistosome eggs making slide reading difficult and hindering diagnosis. A drawback of the MHT is that it has fairly rigid requirements for suitable pH, temperature and water quality, which cannot always be met under field conditions. Furthermore, it does not, on its own, provide infection intensity information and, like the KK, its sensitivity decreases as infection intensity decreases. In order to obtain intensity of infection estimates, additional microscopic visualization of eggs is performed on MHT-positive samples following a filtration sedimentation procedure whereby 50 g of feces are passed through 30 (595 µm) and 150 (90–105 µm) sieves and the flow through suspended in a nylon bag to capture the sediment which is then resuspended and the eggs present counted [33]. This is similar to the DBL technique [21] and the same problem of the presence of cellulosic material is common to both procedures.
The differences in sensitivity of these different techniques for examining ruminant feces for the presence of S. japonicum eggs makes it difficult to compare historical data for prevalence and incidence and infection intensity and to evaluate the involvement of potential reservoir hosts, particularly bovines in schistosomiasis transmission. Table 5 reviews the published studies of diagnostic procedures for the identification of S. japonicum in bovines and the inconsistent data obtained for prevalence and intensity. Telling examples are our recent pilot survey of S. japonicum infection in carabao from Western Samar [22] and the results of another recently published (2010) study on carabao from Leyte, the Philippines, which showed very low S. japonicum prevalence by KK (3.7%), the DBL technique (3.7%) and the MHT (0%) but a high prevalence (51.5%) using qPCR on the same fecal samples [17]. These two studies clearly highlighted the requirement for a more accurate microscopic technique, exemplified by the FEA-SD method, if only to validate diagnosis by qPCR.
The FEA-SD technique, including sieving, sedimentation, centrifugation and digestion, takes approximately 1.5 hours to complete. The length of time taken for subsequent slide reading depends on the skill and experience of the technicians involved, but two well trained and experienced microscopists are able to read one sample in 20 minutes. This procedure is relatively straight forward and only requires a centrifuge. Bovine feces comprise a large mass containing primarily cellulosic fibres and a direct count is the only way to get infection intensity but the debris obscure eggs to a large extent and the FEA-SD is the only currently available technique which clears a large proportion of the debris and renders remaining debris transparent, thereby increasing egg visualisation (Figure 2). Based on cost of reagents only, the FEA-SD technique is far less expensive ($US0.65) to perform than qPCR ($US9.2) although both approaches provide a very similar level of diagnostic accuracy [22]. We are currently using the FEA-SD method to determine the prevalence and intensity of S. japonicum in large animal cohorts as part of extensive epidemiological and surveillance studies we are undertaking in both the People's Republic of China and the Philippines.
In summary, the FEA-SD method is an improved tool that can be used to visualize schistosome eggs and to determine the prevalence and intensity of infection of S. japonicum in bovines. The increased visibility of eggs in the final sediment (Figure 2) compared with the DBL, MHT (+filtration) and KK techniques, makes the FEA-SD an important new technique applicable for epidemiological studies where bovines and other ruminants, such as goats, are potentially important reservoir hosts for S. japonicum [11]–[13], [34], [35]. In addition to S. japonicum the FEA-SD method can also be used to identify and quantify eggs of other helminths, such as Fasciola sp. in naturally infected animals. The FEA-SD also has the benefit of costing less than qPCR, which increases its potential as a surveillance tool for evaluating control programs, including in areas where control has led to the suspected elimination of schistosomiasis japonica.
Funding Statement
This work was partially supported by the following grants: The National High Technology Research and Development Program of China (grant No. 2007AA02Z153), and National Science and Technology Major Program (grant Nos. 2009ZX10004-302, 2008ZX10004-011). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Garjito TA, Sudomo M, Abdullah, Dahlan M, Nurwidayati A (2008) Schistosomiasis in Indonesia: past and present. Parasitol Int 57: 277–280. [DOI] [PubMed] [Google Scholar]
- 2. Izhar A, Sinaga RM, Sudomo M, Wardiyo ND (2002) Recent situation of schistosomiasis in Indonesia. Acta Trop 82: 283–288. [DOI] [PubMed] [Google Scholar]
- 3. Utzinger J, Zhou X, Chen M, Bergquist R (2005) Conquering schistosomiasis in China: the long march. Acta Trop 96: 69–96. [DOI] [PubMed] [Google Scholar]
- 4. Wang L, Chen H, Guo J, Zeng X, Hong X, et al. (2009) A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med 360: 121–128. [DOI] [PubMed] [Google Scholar]
- 5. Blas BL, Rosales MI, Lipayon IL, Yasuraoka K, Matsuda H, et al. (2004) The schistosomiasis problem in the Philippines: a review. Parasitol Int 53: 127–134. [DOI] [PubMed] [Google Scholar]
- 6. Leonardo LR, Rivera P, Saniel O, Villacorte E, Crisostomo B, et al. (2008) Prevalence survey of schistosomiasis in Mindanao and the Visayas, the Philippines. Parasitol Int 57: 246–251. [DOI] [PubMed] [Google Scholar]
- 7. McGarvey ST, Zhou XN, Willingham AL III, Feng Z, Olveda R (1998) The epidemiology and host-parasite relationships of Schistosoma japonicum in definitve hosts. Parasitol Today 15: 214–215. [DOI] [PubMed] [Google Scholar]
- 8. He Y, Salafsky B, Ramaswamy K (2001) Host-parasite relationships of Schistosoma japonicum in mammalian hosts. Trends Parasitol 17: 320–324. [DOI] [PubMed] [Google Scholar]
- 9. Blas BL, Lipayon IL, Tormis LC, Portillo LA, Hayashi M, et al. (2006) An attempt to study the economic loss arising from Schistosoma japonicum infection and the benefits derived from treatment. Southeast Asian J Trop Med Public Health 37: 26–32. [PubMed] [Google Scholar]
- 10. Ross AGP, Sleigh AC, Li Y, Davis GM, Williams G, et al. (2001) Schistosomiasis in the People's Republic of China: prospects and challenges for the 21st century. Clin Microbiol Rev 14: 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gray DJ, Williams GM, Li Y, Chen H, Forsyth SJ, et al. (2009) A cluster-randomised intervention trial against Schistosoma japonicum in the Peoples' Republic of China: bovine and human transmission. PLoS ONE 4: e5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gray DJ, Williams GM, Li Y, Chen H, Li RS, et al. (2007) A cluster-randomized bovine intervention trial against Schistosoma japonicum in the People's Republic of China: design and baseline results. Am J Trop Med Hyg 77: 866–874. [PMC free article] [PubMed] [Google Scholar]
- 13. Gray DJ, Williams GM, Li Y, McManus DP (2008) Transmission dynamics of Schistosoma japonicum in the lakes and marshlands of China. PLoS ONE 3: e4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gray DJ, Williams GM, Li YS, Chen HG, Forsyth S, et al. (2009) The role of bovines in human Schistosoma japonicum infection in the People's Republic China. Am J Trop Med Hyg 81: 1046. [Google Scholar]
- 15. Guo J, Li Y, Gray DJ, Hu G, Chen H, et al. (2006) A drug-based intervention study on the importance of buffaloes for human Schistosoma japonicum infection around Poyang Lake, People's Republic of China. Am J Trop Med Hyg 74: 335–341. [PubMed] [Google Scholar]
- 16. Guo J, Ross AG, Lin D, Williams GM, Chen H, et al. (2001) A baseline study on the importance of bovines for human Schistosoma japonicum infection around Poyang Lake, China. Am J Trop Med Hyg 65: 272–278. [DOI] [PubMed] [Google Scholar]
- 17. Wu H, Qin Y, Chu K, Meng R, Liu Y, et al. (2010) High prevalence of Schistosoma japonicum infection in water buffaloes in the Philippines assessed by real-time polymerase chain reaction. Am J Trop Med Hyg 82: 646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cabrera BD (1976) Schistosomiasis japonica in field rats in Leyte, Philippines. Southeast Asian J Trop Med Public Health 7: 50–55. [PubMed] [Google Scholar]
- 19. Carabin H, Balolong E, Joseph L, McGarvey ST, Johansen MV, et al. (2005) Estimating sensitivity and specificity of a faecal examination method for Schistosoma japonicum infection in cats, dogs, water buffaloes, pigs, and rats in Western Samar and Sorsogon Provinces, The Philippines. Int J Parasitol 35: 1517–1524. [DOI] [PubMed] [Google Scholar]
- 20. McGarvey ST, Carabin H, Balolong E Jr, Bélisle P, Fernandez T, et al. (2006) Cross-sectional associations between intensity of animal and human infection with Schistosoma japonicum in Western Samar province, Philippines. Bull World Health Organ 84: 446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu JM, de Vlas SJ, Jiang QW, Gryseels B (2007) Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol Int 56: 45–49. [DOI] [PubMed] [Google Scholar]
- 22. Gordon CA, Acosta LP, Gray DJ, Olveda R, Jarilla B, et al. (2012) High prevalence of Schistosoma japonicum infection in carabao from Samar province, the Philippines: implications for transmission and control. PLoS Negl Trop Dis doi: 10.1371/journal.pntd.0001778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Willingham AL, Johansen MV, Barnes EH (1998) A new technique for counting Schistosoma japonicum eggs in pig faeces. Southeast Asian J Trop Med Public Health 29: 128–130. [PubMed] [Google Scholar]
- 24. Cringoli G (2006) FLOTAC, a novel apparatus for a multivalent faecal egg count technique. Parassitologica 48: 381–384. [PubMed] [Google Scholar]
- 25. Cringoli G, Rinaldi L, Maurelli MP, Utzinger J (2010) FLOTAC: new multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat Protoc 5: 503–515. [DOI] [PubMed] [Google Scholar]
- 26. Glinz D, Silué KD, Knopp S, Lohouringnon LK, Yao KP, et al. (2010) Comparing diagnostic accuraccy of Kato-Katz, koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soli-transmitted helminths. PLoS Negl Trop Dis 4: e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knopp S, Glinz D, Rinaldi L, Mohammed KA, N'Goran EK, et al. (2009) FLOTAC: a promising technique for detecting helminth eggs in human faeces. Trans R Soc Trop Med Hyg 103: 1190–1194. [DOI] [PubMed] [Google Scholar]
- 28. Jones MK, Balen J (2007) Magnetic beads for schistosomiasis diagnosis. PLoS Negl Trop Dis 1: e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Teixeira CF, Neuhauss E, Ben R, Romanzini J, Graeff-Teixeira C (2007) Detection of Schistosoma mansoni eggs in feces through their interaction with paramagnetic beads in a magnetic field. PLoS Negl Trop Dis 1: e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Angeles JM, Goto Y, Kirinoki M, Asada M, Leonardo LR, et al. (2012) Utilization of ELISA using thioredoxin peroxidase-1 and tandem repeat proteins for diagnosis of Schistosoma japonicum infection among water buffaloes. PLoS Negl Trop Dis 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Katz N, Chaves A, Pellegrino J (1972) A simple device for quantitative stool thick smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 14: 397–400. [PubMed] [Google Scholar]
- 32. Zhang Y-Y, Luo J-P, Liu Y-M, Wang Q-Z, Chen J-H, et al. (2009) Evaluation of Kato-Katz examination method in three areas with low-level endemicity of schistosomiasis japonica in China: a Bayesian modeling approach. Acta Trop 112: 16–22. [DOI] [PubMed] [Google Scholar]
- 33. Davis GM, Wu W, Chen H, Liu H, Guo J, et al. (2002) A baseline study of the importance of bovines for human Schistosoma japonicum infections around Poyang Lake, China: Villages studied and snail sampling strategy. Am J Trop Med Hyg 66: 359–371. [DOI] [PubMed] [Google Scholar]
- 34. Wang T, Johansen MV, Zhang S, Wang F, Wu W, et al. (2005) Transmission of Schistosoma japonicum by humans and domestic animals in the Yangtze River valley, Anhui province, China. Acta Trop 96: 198–204. [DOI] [PubMed] [Google Scholar]
- 35. Zou FC, Dong GD, Yang JF, Xie YJ, Zhang YG, et al. (2010) Prevalences of Schistosoma japonicum infection in reservoir hosts in south-western China. Ann Trop Med Parasitol 104: 181–185. [DOI] [PubMed] [Google Scholar]
- 36. Su ZW, Hu CQ, Fu Y, Cheng W, Huang XB (1994) Role of several hosts in transmission of Schistosomiasis japonica in lake region. Chinese Journal of Parasitology and Parasitic Diseases 12: 48–51. [PubMed] [Google Scholar]
- 37. Lu D, Wang T, Rudge JW, Donnely CA, Fang G, et al. (2010) Contrasting reservoirs for Schistosoma japonicum between marshland and hilly regions in Anhui, China – a two-year longitudinal parasitological survey. Parasitology 137: 99–110. [DOI] [PubMed] [Google Scholar]
- 38. Liang S, Yang C, Zhong B, Qiu D (2006) Re-emerging schistosomiasis in hilly and mountainout areas of Sichuan, China. Bull World Health Organ 84: 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Balen J, Zhao ZY, Williams GM, McManus DP, Raso G, et al. (2007) Prevalence, intensity and associated morbidity of Schistosoma japonicum infection in the Dongting Lake region, China. Bull World Health Organ 85: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fernandez TJ, Tarafder MR, Balolong E, Joseph L, Willingham AL III, et al. (2007) Prevalence of Schistosoma japonicum infection among animals in fifty villages of Samar Province, the Philippines. Vector Borne Zoonotic Dis 7: 147–155. [DOI] [PubMed] [Google Scholar]
- 41. Matsumoto J, Kirinoki M, Kawai S, Chigusa Y (1999) Prevalence of schistosomiasis japonica among school children and animal reservoirs in Oriental Mindoro, Philippines. Japanese Journal of Tropical Medicine and Hygiene 27: 175–180. [Google Scholar]
- 42. Dumag PU (1981) Epidemiology of animal schistosomiasis in the Philippines. Phil J Anim Indust 36: 1–23. [Google Scholar]
- 43. Pesigan TP, Farooq M, Hairston NG, Jauregui JJ, Garcia EG, et al. (1958) Studies on Schistosoma japonicum infection in the Philippines. 1. General considerations and epidemiology. Bull World Health Organ 18: 345–455. [PMC free article] [PubMed] [Google Scholar]
- 44. Fernandez TJ Jr, Petilla T, Banez B (1982) An epidemiological study on Schistosoma japonicum in domestic animals in Leyte, Philippines. Southeast Asian J Trop Med Public Health 13: 575–579. [PubMed] [Google Scholar]