Abstract
Purpose
Among the various stoma complications, the parastomal hernia (PSH) is the most common. Prevention of PSH is very important to improve the quality of life and to prevent further serious complications. The aim of this study was to analyze the incidence and the risk factors of PSH.
Methods
From January 2002 and October 2008, we retrospectively reviewed 165 patients who underwent an end colostomy. As a routine oncologic follow-up, abdomino-pelvic computed tomography was used to examine the occurrence of the PSH. The associations of age, sex, body mass index (BMI), history of steroid use and comorbidities to the development of the PSH were analyzed. The median duration of the follow-up was 36 months (0 to 99 months).
Results
During follow-up, 50 patients developed a PSH and the 5-year cumulative incidence rate of a PSH, obtained by using the Kaplan-Meier method, was 37.8%. In the multivariate COX analysis, female gender (hazard ratio [HR], 3.29; 95% confidence interval [CI], 1.77 to 6.11; P < 0.0001), age over 60 years (HR, 2.37; 95% CI, 1.26 to 4.46; P = 0.01), BMI more than 25 kg/m2 (HR, 1.8; 95% CI, 1.02 to 3.16; P = 0.04), and hypertension (HR, 2.08; 95% CI, 1.14 to 3.81; P = 0.02) were all independent risk factors for the development of a PSH.
Conclusion
The 5-year incidence rate of a PSH was 37.8%. The significant risk factors of a PSH were as follows: female gender, age over 60 years, BMI more than 25 kg/m2, and hypertension. Using a prophylactic mesh during colostomy formation might be advisable when the patients have these factors.
Keywords: Colostomy, Hernia, Incidence, Risk factors, Complication
INTRODUCTION
Many stoma-related complications, such as parastomal hernias (PSHs), prolapses, stenosis, skin rashes, and problems of adaptation, exist. Among them, the PSH is the most common [1-3]. The prevalence of a PSH ranges widely according to the follow-up time [4] and the type of colostomy. In the end colostomy, it varies from 4 to 50% [5], and in the loop colostomy, between 0% and 30.8% was reported [6]. Although, many PSHs are asymptomatic, they may create problems ranging from discomfort to life-threatening complications, such as perforation, occlusion, or strangulation [7]. As PSH repair techniques, local tissue repair, stoma relocation, and mesh repair have been used. However, the results of PSH repair have been disappointing, with reported recurrence rates of 30 to 76% after local aponeurotic repair, stoma relocation, and laparoscopic repair [8-15]. Due to the frequency of PSHs and the limited success of repair, attention has been focused on preventing PSHs at the outset when a stoma is fashioned. Recently, mesh reinforcement at the time of stoma formation has further decreased the incidence of PSHs [16, 17]. However, mesh insertion may also present potential complications in the form of adhesions and intestinal obstruction. Therefore, it is not suitable for all patients of colostomy [18]. For this reason, we conducted this retrospective study to investigate the annual incidence of PSHs and the risk factors for development of a PSH in order to select patients who would most benefit from prophylactic mesh insertion.
METHODS
Between January 2002 and October 2008, we collected 165 consecutive patients who underwent end-colostomy formation after resection of malignancies located in the distal colon or rectum without a history of previous laparotomy at the Korea Cancer Center Hospital. All end-colostomies were formed at an intra-operatively-calibrated site through the rectus muscle in a trans-peritoneal manner under open surgical fields. Clinical data, age at the time of stoma creation, sex, comorbidities, uses of steroids as premedication for chemotherapy, body mass index (BMI, kg/m2), and indication of colostomy, were all retrieved from a computerized database and medical records. Differences in frequency of those clinical variables were checked between the groups with a PSH (PSH+ group) and without a PSH (PSH- group). This study was approved by our Institutional Review Board for Human Research.
To check the development of the PSH, we assessed the results from abdomino-pelvic computed tomography (APCT), which was performed every 6 to 12 months for oncologic follow-up. A PSH was defined as a protrusion of any abdominal content other than the stomal loop through a widened opening of posterior rectus sheaths at APCT.
To calculate the cumulative annual incidence rate of PSHs, we used the Kaplan-Meier estimate analysis. The follow-up period was calculated from the date of stoma formation to the date of PSH detection or to the last date of the practice of APCT in patients with no PSH. To compare the cumulative incidence rate according to the clinical variables, we performed log rank tests. To investigate the contribution of clinical factors, such as age, sex, comorbidities, uses of steroids as premedication for chemotherapy, BMI, to the development of the PSH, we used the uni- and multivariate Cox proportional hazard model and the backward stepwise elimination method. All variables considered in regression models were reported with hazard ratios (HRs) and 95% confidence intervals (CIs). All statistical analyses were performed using SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA). Differences between the groups were assessed by using the chi-square test and the Fisher's exact test for categorical variable and the independent sample t-test for continuous variables. A P-value of < 0.05 was considered statistically significant.
RESULTS
The mean age of all patients was 57.3 years (standard deviation [SD], 10.8 years), and males (58.8%) were predominant. All patients had neoplastic primary disease. One hundred fifty-nine patients (96.4%) had colorectal cancer, 3 patients (1.8%) had anal cancer, and the other 3 patients had, respectively, a gastrointestinal stromal tumor (0.6%), a malignant melanoma (0.6%), and ovarian cancer (0.6%). An end-colostomy was formed in 159 cases after abdomino-perineal resection (96.4%) and in 6 cases after a Hartmann's operation (3.6%).
During an overall median follow-up of 36 months (range, 1 to 99 months), 50 patients (30.3%) developed a PSH after a median of 14 months (range, 1 to 60 months) from the date of stoma formation. Six patients with a PSH (12.0%) had hernia-related symptoms; four patients complained of subcutaneous bulging around the stoma, 1 patient had a stomal prolapse, and the other patient had symptoms of bowel obstruction. Including patients with bowel obstruction and stomal prolapse, five patients received a hernia repair; four patients received local tissue repair, and one patient received mesh reinforcement. However, the PSH reappeared in four patients after hernia repair.
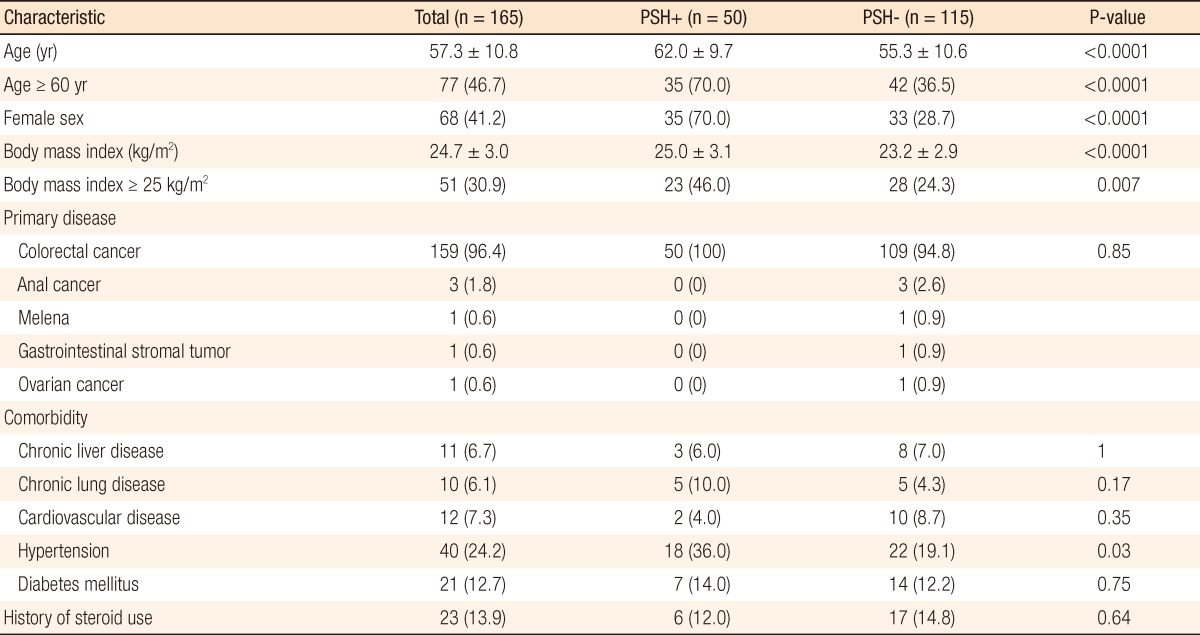
When the clinical variables were compared according to the presence of a PSH, the mean age and the frequency of age more than 60 years were significantly higher in the PSH+ group (70.0% vs. 36.5%, P < 0.0001). Also, female gender was significantly more frequent in the PSH+ group (70.0% vs. 28.7%, P < 0.0001). The mean value of the BMI of the PSH+ group was 25.0 kg/m2 (SD, 3.0), which was significantly higher than that of the PSH- group (mean, 23.2 kg/m2; SD, 2.9; P < 0.0001). The frequency of hypertension in the PSH+ group was significantly higher (36.0% vs. 19.1%, P = 0.03). The distribution of primary disease, as well as the frequencies of comorbidities such as chronic liver disease, chronic lung disease, cardiovascular disease, and diabetes mellitus, was similar between the groups. Steroid use for premedication for chemotherapy was not significantly associated with the presentation of a PSH (Table 1).
Table 1.
Patients characteristics
Values are presented as mean ± SD or number (%).
PSH, parastomal hernia.
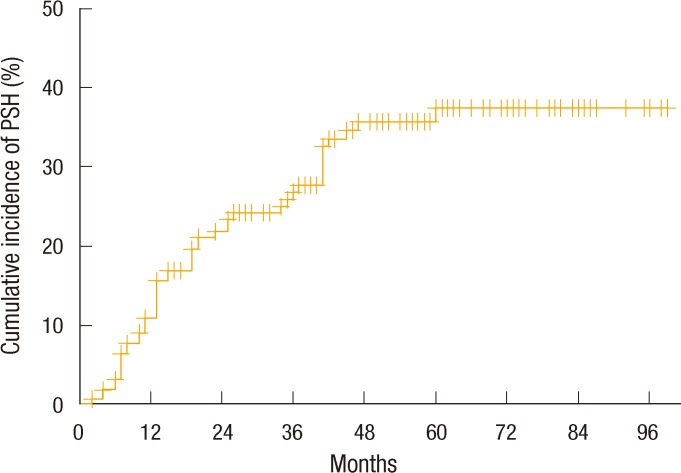
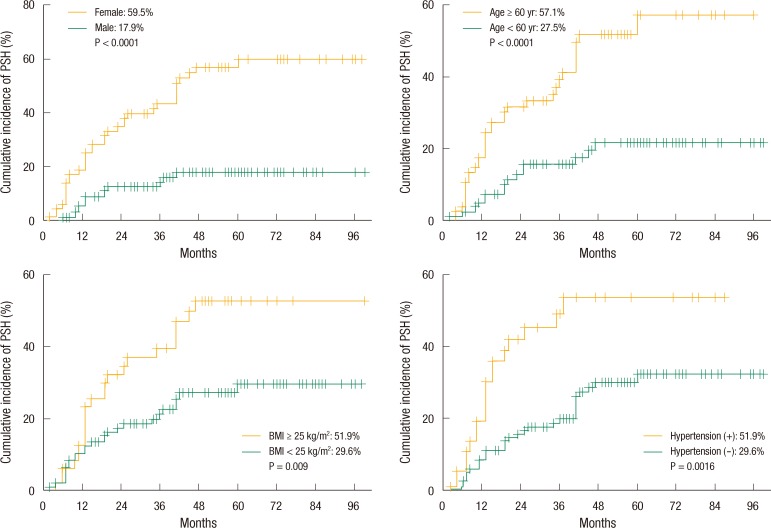
A Kaplan-Meier analysis showed that the annual cumulative incidence rates of PSH were 10.9% at 1 year, 21.7% at 2 years, 26.4% at 3 years, 35.6% at 4 years, and 37.3% at 5 years. However, no further development of a PSH was noted for follow-up periods of more than 5 years (Fig. 1). When the 5-year cumulative incidence rates of PSH were compared according to clinical variables, female patients showed a significantly higher incidence rate than male patients (59.5% vs. 17.9%, P < 0.0001). Also, the PSH incidence rate of patients aged more than 60 years was significantly higher than that younger patients (57.1% vs. 27.5%, P < 0.0001). The 5-year cumulative incidence rate of patients with a BMI of more than 25 kg/m2 was 51.9%, which was significantly higher than that of patients with a BMI of under 25 kg/m2 (29.6%, P = 0.009). Further, patients with hypertension showed a significantly higher incidence rate of PSH (51.9% vs. 29.6%, P = 0.002) (Fig. 2).
Fig. 1.
Cumulative incidence rate of a parastomal hernia (PSH) in all patients.
Fig. 2.
Cumulative incidence rate of a parastomal hernia (PSH) according to sex, age, body mass index (BMI) and hypertension.
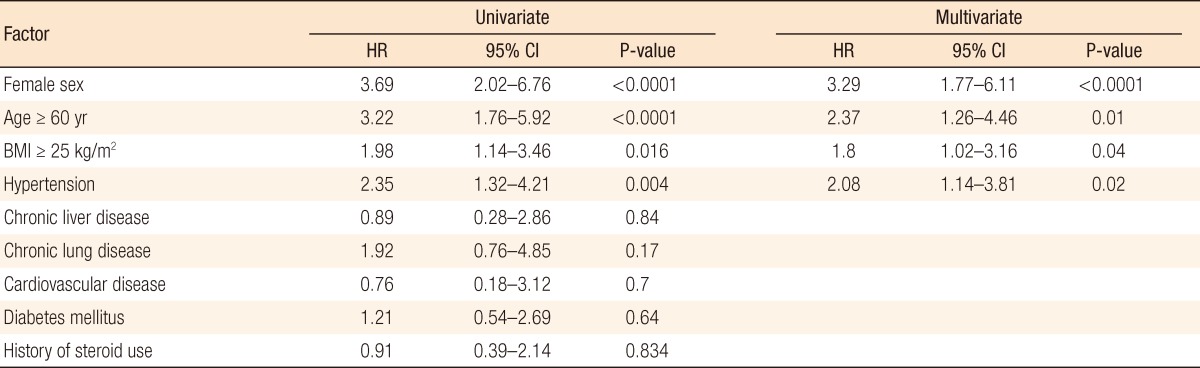
In the multivariate analysis, female gender (HR, 3.29; 95% CI, 1.77 to 6.11; P < 0.0001), age more than 60 years (HR, 2.37; 95% CI, 1.26 to 4.46; P = 0.01), BMI of more than 25 kg/m2 (HR, 1.8; 95% CI, 1.02 to 3.16; P = 0.04), and hypertension (HR, 2.08; 95% CI, 1.14 to 3.81; P = 0.02) were independent risk factors for the development of a PSH. Other factors, such as use of steroids, chronic liver disease, chronic pulmonary disease, and diabetes mellitus, were not associated with the development of a PSH (Table 2).
Table 2.
Factors associated with the occurrences of a parastomal hernia
HR, hazard ratio; CI, confidence interval; BMI, body mass index.
DISCUSSION
Although, the PSH is the most frequent complication among the various stoma-related problems according to many reports [1-3], the true rate of PSHs has been very difficult to estimate. In an end colostomy, the prevalence of PSHs has a very wide range from 4 to 50% [5]. The reason for the range of the PSH incidence rate being so wide is the lack of a consistent definition of a PSH. A PSH is a kind of incisional hernia related to an abdominal wall stoma [5]. In some clinical reports, a PSH was defined as a palpable defect or a bulge adjacent to the stoma [4, 19, 20]. In another study, herniation was defined as a palpable "cough impulse" at the ostomy site [15]. In another study, a CT scan result was added to a radiological definition of any intraabdominal content protruding along the ostomy [17, 21]. The use of CT scans may have contributed to the high hernia rates reported during the last decade, as they allow the detection of small PSHs [15, 21, 22]. Indeed, the estimated PSH incidence rate of our study, using a definition of PSH by CT scan, was 37.8% at 5 years, which was relatively higher than those in other studies using a clinical definition of a PSH [4, 20]. Diagnosis via a CT scan, due to its higher sensitivity, may result in a diagnosis of asymptomatic and clinically insignificant PSHs [23]. However, to compare the prevalence between studies and to obtain objective information, we believe a CT scan will be a better alternative because it defines the PSH with greater precision than can be achieved using only a clinical diagnosis. A recent study showed significant differences for clinical diagnoses among various experienced examiners on the same surgical service [24].
Another important factor to estimate the true PSH rate is the follow-up time after stoma creation. Similar to a ventral incisional hernia, reports regarding this incidence of PSHs, with a life-table analysis, showed an increasing rate of PSHs with increasing follow-up time [4, 25]. Our population also showed an increasing rate of PSHs with increasing follow-up time. In a comprehensive review in 2008 [26], the follow-up times were seen to vary considerably in clinical reports, and only a few studies had followed patients for at least 1 year or longer. In this review, reported PSH rates were 11 to 50%. Because of the inconsistent definition of a PSH, the variable follow-up period after the index operation in the reported cases also makes it difficult to compare rates of PSHs between different series and to estimate a true rate of PSHs, but the rate is most likely between 30% and 50% in general surgical practice. In this study, the cumulative incidence rate of PSHs increased annually to 37.8% after 5 years. However, after a 5-year follow-up, we did not observe any further occurrence of a PSH. The reason the incidence of PSHs after 5 years showed a plateau is uncertain. However, we assumed that all stomas enrolled in this study were created after surgeries for neoplastic disease and that the majority of them were colorectal cancer. Thus, the follow-up may be restricted by patients' deaths due to disease progression. Thus, when assessing and comparing the PSH incidence rate with long-term follow-up, we should consider whether the primary diseases are benign or malignant.
The PSH has been treated conservatively in most patients. Surgical repair is indicated in 15 to 20% of symptomatic PSH patients with a stoma that is difficult to manipulate, obstruction, bowel incarceration and perforation. As for PSH repair techniques, local tissue repair, stoma relocation, and mesh repair have been used. However, the results of PSH repair have been disappointing, with reported recurrence rates of 30 to 76% after local aponeurotic repair, stoma relocation, and laparoscopic repair [8-15]. Because of the high frequency of PSHs and the high recurrence rate of PSHs after repair, attention has been focused on preventing theirs appearance from the very beginning, i.e., at the time of stoma creation.
A recent issue concerning PSH prevention during the primary surgical procedure has been prosthetic mesh reinforcement around the stoma. One randomized controlled trial was aborted early because it was considered unethical to continue; half of the patients in the control arm had developed a PSH compared to only 1 of 21 in the study group [21]. Several other studies reported similar results of a decreased occurrence rate of PSHs [16, 17]. However, reported rates of complications related to mesh insertion were extremely low [27]. Despite those promising results, many surgeons are probably hesitant to use prosthetic mesh because of the theoretical risk of infection from mesh placement in a contaminated environment. Also, mesh insertion is a time-consuming procedure. Therefore, a necessity exists to select patients by assessing the risk factors related to the development of the PSH and by identifying those patients that would benefit most from this technique.
The risk of occurrence of PSHs is related, in part, to the clinical characteristics of the patient and to the surgical technique employed in stoma formation. In regards to the patient-related risk factors, most authors consider them to be identical to those for any incisional hernia, namely, obesity, malnutrition, increased abdominal pressure, corticosteroid use, and advanced age. Some studies have shown that the incidence of other abdominal wall hernias is increased in patients with a PSH and that a previous history of incisional hernia is related to the development of a PSH [20]. This suggests that a possible overall weakening of the anterior abdominal wall musculoaponeurotic configuration contributes to the PSH. In this study, we investigated the clinical risk factors of age more than 60 years, sex, obesity with a BMI of more than 25 kg/m2, hypertension, cardiovascular disease, chronic lung disease, chronic liver disease, history of corticosteroids use as premedication for chemotherapy, and diabetes mellitus. Among them, age more than 60 years, female gender, a BMI of more than 25 kg/m2, and hypertension were significantly associated with a high incidence rate of PSHs in the multivariate Cox analysis. However, the scientific evidence supporting this is limited, and much of the evidence relies on the studies carried out on other forms of hernia development or on anecdotal evidence of clinicians involved in the care of the patients undergoing stoma formation. As patients get older, the muscles in the abdomen weaken, and if the abdominal muscles do not strengthen, the abdomen may be unable to provide adequate support for a stoma. A retrospective review of 782 patients with a follow-up period of more than 10 years suggested that PSHs are more likely to occur in the elderly [20], and another study reported that age was a significant factor in the development of a hernia, especially in the group of age over 60 [4]. This may be due to the fact that with age, the thickness of the rectus abdominis muscle reduces; thus, the layer of subcutaneous fat increases [28]. In addition to old age, female gender was also an independent risk factor for the occurrence of a PSH in this study. Considering the fact that women have thinner muscles and thicker fat than men regardless of age [28], this physiological difference may contribute to women's vulnerability to PSHs.
Obesity has been a traditional risk factor for incisional hernias and PSHs. Some authors argued that obesity as defined by the waist circumference was significantly associated with a PSH because of increased radial force, i.e., intraabdominal pressure [23, 29]. Also, obesity causes susceptibility to wound infection, which contributes to the incisional hernia or PSH [30]. In this regard, safety might be a major concern for prophylactic mesh insertion in obese patients due to its relation to mesh infection. In this study, obesity was defined by using a BMI of more than 25 kg/m2, and that was a significant risk factor for the occurrence of a PSH, as in other studies.
Until now, according to postoperative morbidity reports after mesh insertion for the prevention of PSHs, there were nearly no complications, such as peristomal infection, that affected mesh intolerance or chronic infection [16, 17]. However, the number of patients enrolled in those studies was so small that caution should be used when drawing any concrete conclusions on the safety of prophylactic mesh insertion in these obese patients. Thus, a large-scaled trial is strongly needed. Finding or assuming a scientific relation between hypertension, a significant risk factor for a PSH in this study, and the development a PSH is very difficult. That relation might be associated with age and obesity, considering the fact that as patients get older and are more obese, the incidence of hypertension increases.
In conclusion, in our study, the incidence of PSH increased annually and reached 37.8% at 5 years. The female gender, age more than 60 years, and BMI of more than 25 kg/m2 were found to have independent significant associations with the development of PSH. If the safety of the prophylactic mesh insertion can be guaranteed through future, large, randomized trial, patients with these risk factors may benefit from this procedure.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bass EM, Del Pino A, Tan A, Pearl RK, Orsay CP, Abcarian H. Does preoperative stoma marking and education by the enterostomal therapist affect outcome? Dis Colon Rectum. 1997;40:440–442. doi: 10.1007/BF02258389. [DOI] [PubMed] [Google Scholar]
- 2.Arumugam PJ, Bevan L, Macdonald L, Watkins AJ, Morgan AR, Beynon J, et al. A prospective audit of stomas: analysis of risk factors and complications and their management. Colorectal Dis. 2003;5:49–52. doi: 10.1046/j.1463-1318.2003.00403.x. [DOI] [PubMed] [Google Scholar]
- 3.Park JJ, Del Pino A, Orsay CP, Nelson RL, Pearl RK, Cintron JR, et al. Stoma complications: the Cook County Hospital experience. Dis Colon Rectum. 1999;42:1575–1580. doi: 10.1007/BF02236210. [DOI] [PubMed] [Google Scholar]
- 4.Mylonakis E, Scarpa M, Barollo M, Yarnoz C, Keighley MR. Life table analysis of hernia following end colostomy construction. Colorectal Dis. 2001;3:334–337. doi: 10.1046/j.1463-1318.2001.00256.x. [DOI] [PubMed] [Google Scholar]
- 5.Pearl RK. Parastomal hernias. World J Surg. 1989;13:569–572. doi: 10.1007/BF01658872. [DOI] [PubMed] [Google Scholar]
- 6.Carne PW, Robertson GM, Frizelle FA. Parastomal hernia. Br J Surg. 2003;90:784–793. doi: 10.1002/bjs.4220. [DOI] [PubMed] [Google Scholar]
- 7.Goligher JC, Lloyd-Davies OV, Robertson CT. Small-gut obstructions following combined excision of the rectum with special reference to strangulation round the colostomy. Br J Surg. 1951;38:467–473. doi: 10.1002/bjs.18003815208. [DOI] [PubMed] [Google Scholar]
- 8.Hansson BM, Bleichrodt RP, de Hingh IH. Laparoscopic parastomal hernia repair using a keyhole technique results in a high recurrence rate. Surg Endosc. 2009;23:1456–1459. doi: 10.1007/s00464-008-0253-x. [DOI] [PubMed] [Google Scholar]
- 9.Amin SN, Armitage NC, Abercrombie JF, Scholefield JH. Lateral repair of parastomal hernia. Ann R Coll Surg Engl. 2001;83:206–208. [PMC free article] [PubMed] [Google Scholar]
- 10.Burns FJ. Complications of colostomy. Dis Colon Rectum. 1970;13:448–450. doi: 10.1007/BF02616791. [DOI] [PubMed] [Google Scholar]
- 11.Kronborg O, Kramhoft J, Backer O, Sprechler M. Late complications following operations for cancer of the rectum and anus. Dis Colon Rectum. 1974;17:750–753. doi: 10.1007/BF02587634. [DOI] [PubMed] [Google Scholar]
- 12.Martin L, Foster G. Parastomal hernia. Ann R Coll Surg Engl. 1996;78:81–84. [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin MS, Schoetz DJ, Jr, Matthews JB. Parastomal hernia. Is stoma relocation superior to fascial repair? Arch Surg. 1994;129:413–418. doi: 10.1001/archsurg.1994.01420280091011. [DOI] [PubMed] [Google Scholar]
- 14.Sjodahl R, Anderberg B, Bolin T. Parastomal hernia in relation to site of the abdominal stoma. Br J Surg. 1988;75:339–341. doi: 10.1002/bjs.1800750414. [DOI] [PubMed] [Google Scholar]
- 15.Williams JG, Etherington R, Hayward MW, Hughes LE. Paraileostomy hernia: a clinical and radiological study. Br J Surg. 1990;77:1355–1357. doi: 10.1002/bjs.1800771212. [DOI] [PubMed] [Google Scholar]
- 16.Hammond TM, Huang A, Prosser K, Frye JN, Williams NS. Parastomal hernia prevention using a novel collagen implant: a randomised controlled phase 1 study. Hernia. 2008;12:475–481. doi: 10.1007/s10029-008-0383-z. [DOI] [PubMed] [Google Scholar]
- 17.Serra-Aracil X, Bombardo-Junca J, Moreno-Matias J, Darnell A, Mora-Lopez L, Alcantara-Moral M, et al. Randomized, controlled, prospective trial of the use of a mesh to prevent parastomal hernia. Ann Surg. 2009;249:583–587. doi: 10.1097/SLA.0b013e31819ec809. [DOI] [PubMed] [Google Scholar]
- 18.Morris-Stiff G, Hughes LE. The continuing challenge of parastomal hernia: failure of a novel polypropylene mesh repair. Ann R Coll Surg Engl. 1998;80:184–187. [PMC free article] [PubMed] [Google Scholar]
- 19.Ripoche J, Basurko C, Fabbro-Perray P, Prudhomme M. Parastomal hernia. A study of the French federation of ostomy patients. J Visc Surg. 2011;148:e435–e441. doi: 10.1016/j.jviscsurg.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Janes A, Cengiz Y, Israelsson LA. Preventing parastomal hernia with a prosthetic mesh. Arch Surg. 2004;139:1356–1358. doi: 10.1001/archsurg.139.12.1356. [DOI] [PubMed] [Google Scholar]
- 21.Cingi A, Cakir T, Sever A, Aktan AO. Enterostomy site hernias: a clinical and computerized tomographic evaluation. Dis Colon Rectum. 2006;49:1559–1563. doi: 10.1007/s10350-006-0681-4. [DOI] [PubMed] [Google Scholar]
- 22.De Raet J, Delvaux G, Haentjens P, Van Nieuwenhove Y. Waist circumference is an independent risk factor for the development of parastomal hernia after permanent colostomy. Dis Colon Rectum. 2008;51:1806–1809. doi: 10.1007/s10350-008-9366-5. [DOI] [PubMed] [Google Scholar]
- 23.Moreno-Matias J, Serra-Aracil X, Darnell-Martin A, Bombardo-Junca J, Mora-Lopez L, Alcantara-Moral M, et al. The prevalence of parastomal hernia after formation of an end colostomy: a new clinico-radiological classification. Colorectal Dis. 2009;11:173–177. doi: 10.1111/j.1463-1318.2008.01564.x. [DOI] [PubMed] [Google Scholar]
- 24.Gurmu A, Matthiessen P, Nilsson S, Pahlman L, Rutegard J, Gunnarsson U. The inter-observer reliability is very low at clinical examination of parastomal hernia. Int J Colorectal Dis. 2011;26:89–95. doi: 10.1007/s00384-010-1050-2. [DOI] [PubMed] [Google Scholar]
- 25.Hoer J, Lawong G, Klinge U, Schumpelick V. Factors influencing the development of incisional hernia: a retrospective study of 2,983 laparotomy patients over a period of 10 years. Chirurg. 2002;73:474–480. doi: 10.1007/s00104-002-0425-5. [DOI] [PubMed] [Google Scholar]
- 26.Israelsson LA. Parastomal hernias. Surg Clin North Am. 2008;88:113–125. ix. doi: 10.1016/j.suc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Wijeyekoon SP, Gurusamy K, El-Gendy K, Chan CL. Prevention of parastomal herniation with biologic/composite prosthetic mesh: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Surg. 2010;211:637–645. doi: 10.1016/j.jamcollsurg.2010.06.111. [DOI] [PubMed] [Google Scholar]
- 28.Kanehisa H, Miyatani M, Azuma K, Kuno S, Fukunaga T. Influences of age and sex on abdominal muscle and subcutaneous fat thickness. Eur J Appl Physiol. 2004;91:534–537. doi: 10.1007/s00421-003-1034-9. [DOI] [PubMed] [Google Scholar]
- 29.de Ruiter P, Bijnen AB. Successful local repair of paracolostomy hernia with a newly developed prosthetic device. Int J Colorectal Dis. 1992;7:132–134. doi: 10.1007/BF00360352. [DOI] [PubMed] [Google Scholar]
- 30.Israelsson LA, Jonsson T. Overweight and healing of midline incisions: the importance of suture technique. Eur J Surg. 1997;163:175–180. [PubMed] [Google Scholar]