Abstract
The metabolism of xenobiotics has mainly been investigated in higher plant species. We studied them in various marine macroalgae of the phyla Chlorophyta, Chromophyta, and Rhodophyta. Microsomes contained high oxidative activities for known cytochrome (Cyt) P450 substrates (fatty acids, cinnamic acid, 3- and 4-chlorobiphenyl, 2,3-dichlorobiphenyl, and isoproturon; up to 54 pkat/mg protein). The presence of Cyt P450 (approximately 50 pmol/mg protein) in microsomes of the three algal families was demonstrated by CO-difference absorption spectra. Intact algal tissue converted 3-chlorobiphenyl to the same monohydroxy-metabolite formed in vitro. This conversion was 5-fold stimulated upon addition of phenobarbital, and was abolished by the known P450 inhibitor, 1-aminobenzotriazole. It is concluded that marine macroalgae contain active species of Cyt P450 and could act as a metabolic sink for marine pollutants.
Plants and animals generally use similar enzyme systems and gene families to metabolize a wide range of xenobiotics, as summarized in the “green liver” concept (Sandermann, 1992, 1994). The evidence has been mainly based on higher plant species, in particular crop plant species. One of the major classes of oxidative enzymes is constituted by the Cyt P450 monooxygenases, which have been detected universally in animal and higher plant species. They oxidize endogenous substrates in various biosynthetic pathways as well as xenobiotic substrates, in particular herbicides. The Cyt P450 enzyme superfamily has been particularly well characterized at the protein and gene levels (for review, see Frear et al., 1972; Bolwell et al., 1994; Durst and O'Keefe, 1995). The present study concentrates on Cyt P450-type reactions in lower plant species. Emphasis was placed on marine algae because they displayed particularly high enzyme activities in a screening program with xenobiotics (Pflugmacher, 1996). Furthermore, marine macroalgae are of ecological interest because they occur in off-shore sites that are often polluted by xenobiotics, such as polychlorinated biphenyls or agrochemicals. Cyt P450 systems have been detected in Euglena gracilis (Briand et al., 1993) and in unicellular green algae (Thies et al., 1996).
First attempts to detect a mixed-function oxidase activity in marine red and green algae were unsuccessful (Payne, 1977). Microsomes from the marine seagrass Posidonia oceanica were more recently found to possess very low lauric acid hydroxylase activity (2.5−9×10−3 pkat/mg protein; Hamoutène et al., 1995). Much higher conversion rates (1–54 pkat/mg protein) have now been determined for several standard Cyt P450 substrates (fatty acids, cinnamic acid, chlorobiphenyls, and isoproturon) utilizing microsomes from several macroalgal species. 3-Chlorobiphenyl was also hydroxylated in vivo, and the presence of Cyt P450 was demonstrated spectroscopically. These results extend the green liver concept to an ecologically important segment of lower plant species.
MATERIALS AND METHODS
Chemicals
Labeled [1-14C]lauric acid, [1-14C]palmitic acid, and [7,10-14C]benzo(α)pyrene were purchased from Amersham Buchler (Braunschweig, Germany). [1-14C]Stearic acid, [ring-U-14C]-cinnamic acid, PVP, and ABT were from Sigma. [Ring-U-14C]3-chlorobiphenyl, [ring-U-14C]4-chlorobiphenyl, [ring-U-14C]2,3-dichlo-robiphenyl, [ring-U-14C]2,2′-dichlorobiphenyl, and [ring-U-14C]isoproturon were purchased from International Isotope GmbH (München, Germany). Radiochemical purities higher than 98% were in all cases determined by radio TLC (Pflugmacher, 1996). Amberlite XAD-4 was purchased from Serva Chemicals (Heidelberg, Germany) and Sephadex G-25 (PD-10) was purchased from Pharmacia. All other chemicals used were research-grade commercial materials. Glc-6-P dehydrogenase was purchased from Boehringer Mannheim. 1-Hydroxy- and monodesmethyl-isoproturon were kindly donated by Dr. W. Glässgen (Institute für Biochemische Pflanzenpathologie, Oberschleissheim, Germany).
General Methods
TLC was carried out on silica-gel 60 plates (F-254, Merck, Darmstadt, Germany) using the following solvent systems: system A, diethylether:petrol ether:formic acid (70:30:1; v/v), and system B, n-hexane:chloroform:acetone:ethanol (8:8:4:1; v/v). Radioactivity was determined using a TLC scanner (Raytest, Straubeuhardt, Germany). GC-MS was carried out on a MAT SSQ 7 000 instrument (Finnigan, Bremen, Germany) using the GC conditions of Borlakoglu and John (1989).
Plant Materials
Marine macroalgae were collected on the eastern mud flats of the North Sea island of Helgoland (Germany) and along the North Sea coasts of Neuharlingersiel, Norddeich, and the island of Spiekeroog during the summer seasons of 1993 and 1994. The freshly collected specimens were immediately frozen and stored in liquid N2. Algae were specified according to the methods of Grams (1974), Kornmann and Sahling (1993), and van den Hoek et al. (1993). Antarctic algae were provided by Dr. C. Wiencke (Alfred Wegener Institute for Polar and Marine Research, Bremerhaven, Germany). They had been collected near King George Island, grown in the laboratory under optimal conditions (Pflugmacher, 1996), and then frozen and shipped in liquid N2. Two species (Caulerpa mexicana Harv. and Halimeda opuntia [L.]) were purchased in a local aquarium store.
Enzyme Preparation
Three existing methods (Diesperger and Sandermann, 1979; Mougin et al., 1992; Schröder et al., 1992) were combined to prepare microsomal and soluble enzymes in the same preparation. Enzyme extraction was performed at 4°C. Plant material, usually 25 to 30 g fresh weight, was ground to a fine powder under liquid N2 in a mortar. This powder was transferred to another precooled mortar and extracted for 10 min with 50 mL of 0.1 m sodium phosphate, pH 6.5, containing 20% (w/v) glycerol, 14 mm DTE, 20 mm ascorbic acid, 1% insoluble PVP (preswollen in water), 1 mm EDTA, and 1 mm PMSF. After passing the extract through a 50-μm nylon net, cell debris were removed by centrifugation at 10,000g for 20 min. To remove phenolic compounds, 10% (w/w) Amberlite XAD-4 was added to the supernatant while stirring. After a second filtration step, the extract was adjusted to pH 7.0 and centrifuged at 100,000g for 60 min. The supernatant was defined as the soluble fraction, the pellet representing the microsomal fraction. The pellet was washed twice with 20 mm sodium phosphate, pH 7.0, containing 20% glycerol and 1.4 mm DTE, followed by resuspension in the same buffer to give 1 to 2 mg protein/mL. Soluble enzymes were precipitated from the initial supernatant by the addition of solid (NH4)2SO4. The pellet of the 35 to 80% (NH4)2SO4 fraction was suspended in 1.5 mL of 20 mm KH2PO4, pH 7.0, containing 5 mm DTE, followed by desalting on Sephadex G-25 (PD-10). The microsomal and soluble fractions were immediately assayed for enzyme activities. The same preparation method was used for tissue samples of fresh dog liver and dog lung (Pflugmacher, 1996). Glutathione S-transferases and different glucosyl-transferases were routinely assayed in the soluble fraction (Pflugmacher, 1996). Protein was determined according to the method of Bradford (1976) using BSA as a standard.
Measurement of Monooxygenase Activities
Measurement of P450 monooxygenase enzymes for [1-14C]lauric acid; [1-14C]stearic acid; [1-14C]palmitic acid; [ring-U-14C]3-chlorobiphenyl, -4-chlorobiphenyl, and -2,3-dichlorobiphenyl; [ring-U-14C]isoproturon; and [ring U-14C]cinnamic acid was adopted from Salaün et al. (1981, 1989), von der Trenck and Sandermann (1989), Borlakoglen and John (1989), and Mougin et al. (1992), respectively. The reaction mixtures had a final volume of 230 μL. First, 100 μL of 50 mm sodium phosphate, pH 7.0, 10 μL of 1 to 2 mm [14C] substrate in ethanol, and 100 μL of microsomal suspension were mixed and incubated for 2 min at room temperature. The reaction was then started by the addition of 20 μL of a regenerating system containing 6.7 mm Glc-6-P, 0.4 unit of Glc-6-P dehydrogenase, and 2 mm NADPH in 50 mm sodium phosphate, pH 7.0. Incubation was for 60 min at 25°C in a shaking water bath using open glass tubes. The reactions were terminated by the addition of 10 μL of 20 volume % TCA, followed by extraction with 200 μL chloroform/n-hexane (1:1; v/v) for 20 min. Aliquots of the organic phase were analyzed on TLC plates in solvent systems A or B. Enzyme activity was calculated in picokatals per milligram of protein from percent substrate conversion.
Spectroscopy
Cyt P450 content was measured by the method of Omura and Sato (1964) in a dual-beam spectrophotometer with cuvettes of a 1-cm optical path. Microsome suspensions (3–5 mg protein/mL) in 0.1 m sodium phosphate, pH 7.0, were placed into both sample and reference cuvettes. About 50 mg of solid Na2S2O4 was added to the cuvettes, and measurement of P450 was done after saturation of the sample solution with CO (Merck no. 823271) for 60 s. Spectra were measured in the range of 400 to 500 nm at 20°C using an extinction coefficient of 185 mm−1 cm−1 (A425–410) for Cyt b5 and 91 mm−1 cm−1 (A450–490) for Cyt P450 (Omura and Sato, 1964).
In Vivo Metabolism
Whole Polysiphonia urceolata tissue (10 g fresh weight) was incubated for 16 h in filtered seawater (300 mL; collected in Hochseefeld 1, North Sea) spiked with 10 μm [14C]-3-chlorobiphenyl (0.3 μCi). Illumination was by daylight lamps (20 μE/cm, L18W/11, Osram, Munich, Germany). Flasks were shaken at 40 rpm during the incubation (20°C). Phenobarbital (0.6 mm) or ABT (0.6 mm) was added to the flask in parallel experiments to modify the in vivo metabolism. After terminating the incubations by filtration, the algae were frozen in liquid N2 and ground to a fine powder. This powder was extracted twice with dichloromethane:methanol:water (1:2:0.8; v/v) overnight in a refrigerator. The homogenates were filtered, and aliquots of the organic solution were concentrated and analyzed by TLC using solvent system A.
RESULTS AND DISCUSSION
Enzyme Distributions
Enzyme activities related to Cyt P450 were examined in the microsomal fraction from various plant species. The substrates used initially were [1-14C]lauric acid, [1-14C]palmitic acid, and [1-14C]stearic acid as the endogenous substrates, and [ring-U-14C]3-chlorobiphenyl as the xenobiotic substrate. Only a few reference substances were available and product identification was based on TLC RF values and mass fragments of certain metabolites upon GC-MS. Lauric acid hydroxylase activity led in all cases to a major metabolite peak near RF 0.45 (solvent system A). Such a product has previously been characterized as an in-chain hydroxylated fatty acid (Salaün et al., 1989), but other products such as omega- or omega-1-hydroxylated fatty acids may have been formed with some of the plant species tested here (compare with Bolwell et al., 1994; Durst and O'Keefe, 1995). In the reference system of cell cultures of soybean (Glycine max L.), lauric acid hydroxylase activity was 60.0 pkat/mg protein (Pflugmacher, 1996). Microsomes from Cycas revoluta were active at 29 pkat/mg protein. Lauric acid hydroxylase acitivity could be demonstrated in marine macroalgae of the Chlorophyta, Chromophyta, and Rhodophyta families (Fig. 1). Activity of this monooxygenase reaction was particularly high in the antarctic red alga Iridaea cordata and in the brown alga Fucus spiralis. The use of palmitic and stearic acids as possible Cyt P450 substrates also led to a major product peak near RF 0.45 in solvent system A. Reference microsomes from soybean cell-suspension cultures had 4.2 (palmitic acid) and 8.2 pkat/mg protein (stearic acid). Both substrates were also utilized by microsomes from marine macroalgae from the phyla Chromophyta and Rhodophyta (Fig. 1). Palmitic acid was particularly well oxidized with 34 (Chara corallina), 8 (Enteromorpha bulbosa), and 7 (Fucus vesiculosus) pkat/mg protein. Stearic acid was particularly well oxidized with 54 (C. corallina), 52 (Laminaria hyperborea), and 36 (Cladophora rupestris) pkat/mg protein.
Figure 1.
Distribution of microsomal hydroxylase activities for lauric acid, palmitic acid, stearic acid, and 3-chlorobiphenyl among marine macroalgae. The standard procedures in Methods were used. Mean values ± sds were derived from three replicates. The numbers on the abscissa refer to the following algal species and their origins (N, North Sea; P, purchased; A, Antarctic): Chlorophyta: 1, C. mexicana Harv. (P); 2, U. lactuca (L.) (N); 3, Enteromorpha compressa (L.) Grev. (N); 4, C. rupestris (L.) Kütz (N); 5, H. opuntia (L.) Lamour. (P); Chromophyta: 6, Ascophyllum nodosum (L.) LeJol (N); 7, Cystoseira baccata (Gmel.) Silva. (N); 8, L. digitata (Huds.) Lamour. (N); 9, L. hyperborea (Gunn.) Fosl. (N); 10, L. saccharina (L.) Lamour. (A); 11, Halydris siliquosa (L.) Lyngb. (N); 12, F. vesiculosus (L.) (N); 13, F. spiralis (L.) (N); 14, Fucus serratus (L.) (N); Rhodophyta: 15, Delesseria sanguinea (Huds.) Lamour. (N); 16, Chondrus crispus Stackh. (N); 17, Plocamium cartilagineum (L.) Dixon (N); 18, Porphyra umbilicalis (L.) J.Ag. (N); 19, Cystoclonium purpureum (Huds.) Batt. (N); 20, I. cordata Kütz. (A); and 21, P. urceolata (Lightf. ex Dillw.) (A).
Variation of lauric acid between 3 and 25 μm and use of Lineweaver-Burk diagrams led to apparent Km values of 26, 34, and 22 μm for Ulva lactuca, Fucus vesiculosus, and P. urceolata, respectively. These values are close to the Km value of 20 μm obtained by Salaün et al. (1978) and Benveniste et al. (1982) for Helianthus tuberosus. There was linearity in the assay up to 150 μg of microsomal protein and up to 40 min of incubation time. The reaction had a sharp temperature optimum for incubation at 20 to 30°C. The known Cyt P450 inhibitor ABT led to strong inhibition of lauric acid hydroxylation with 50% inhibition at 1.2 ± 0.3 mm using microsomes from the above three algal species.
3-Chlorobiphenyl as the xenobiotic substrate led to a product peak at a RF of 0.27 in solvent system A. Oxygenase activity occurred at a low level among the various macroalgal species tested (Fig. 1). Particularly high activities of up to 18 pkat/mg protein occurred in microsomes from three Laminaria species: L. digitata, L. hyperborea, and L. saccharina. Some measured reaction rates are also given in Table I. An apparent Km value of 8.6 μm was determined with microsomes of U. lactuca. Upon GC-MS, the product gave a prominent mass fragment at m/z 205 and a chlorine side-peak at m/z 207, as is typical for monohydroxy-monochlorobiphenyls (Wilken et al., 1995).
Table I.
Use of xenobiotic substrates by microsomes prepared from U. lactuca (Chlorophyta), F. vesiculosus (Chromophyta), and P. urceolata (Rhodophyta) and comparison with dog liver or lung microsomes
| Substrate | Enzyme Activity
|
|||
|---|---|---|---|---|
| Dog | U. lactuca | F. vesiculosus | P. urceolata | |
| pkat/mg protein | ||||
| 3-Chlorobiphenyl | 6.3 ± 0.5 (Liver) | 0.7 ± 0.1 | 1.4 ± 0.2 | 1.1 ± 0.1 |
| 4-Chlorobiphenyl | 6.5 ± 0.6 (Liver) | 0.6 ± 0.1 | 2.8 ± 0.2 | 1.2 ± 0.1 |
| 2,3-Dichlorobiphenyl | 28 ± 0.8 (Liver) | n.d.a | 4.2 ± 0.8 | 2.1 ± 0.1 |
| Isoproturon | 23 ± 1.9 (Liver) | 3.1 ± 0.2 | 16 ± 0.4 | 8.9 ± 0.4 |
| Cinnamic acid | 19 ± 2.8 (Lung) | 2.1 ± 0.1 | 8.4 ± 0.8 | 0 |
Mean values ± sd are shown (n = 3). No products were formed with heat-inactivated microsomes (10 min, 100°C).
n.d., Not determined.
A comparison of several known Cyt P450 substrates is summarized in Table I. For comparison, dog liver or lung microsomes were prepared and incubated in parallel. 4-Chlorobiphenyl was utilized by microsomes from the three algal species and from dog liver to give a defined product at a RF of 0.27 (solvent system A). GC-MS again led to a prominent fragment at m/z 205 (parent ion of monohydroxybiphenyl, see above). Values of Km = 8.6 μm and Vmax= 3.4 pmol/mg protein were determined for the microsomal fraction of U. lactuca.
With 2,3-dichlorobiphenyl as a substrate, a monohydroxylated derivative with a prominent mass fragment at m/z 205 was also formed. Apparently, monohydroxylation with replacement of one chlorine substituent had occurred. The hydroxylation and demethylation of isoproturon, a phenylurea herbicide, has previously been demonstrated with intact, cultured plant cells and the derived microsomes (Cabanne et al., 1987). Isoproturon was also hydroxylated and demethylated by microsomes from U. lactuca, F. vesiculosus, P. urceolata, and dog liver (Table I). Metabolite analysis by TLC (solvent system B) led to two well-resolved radioactive metabolites at a RF of 0.22 (1-hydroxy-isoproturon) and at a RF of 0.46 (monodesmethyl-isoproturon). The parent substance had a RF of 0.65. Metabolite identification was by TLC comparison with the authentic standards. [Ring-U-14C]cinnamic acid was converted to a product only characterized by comigration with p-coumaric acid upon TLC in solvent system A. The rate values are given in Table I.
Spectral Studies
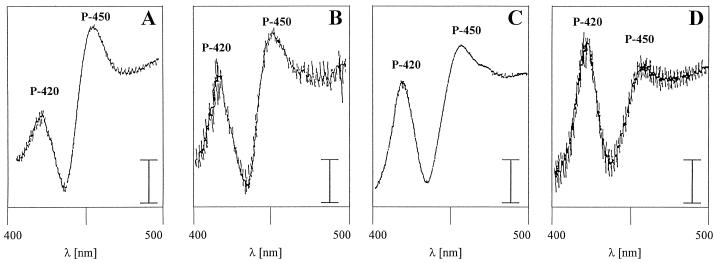
To examine for the presence of Cyt P450 in marine macroalgae, CO-difference spectra were measured by standard methods (Omura and Sato, 1964). In plants it is difficult to determine reduced CO-difference spectra, because P450 contents usually are low. The difference spectra for microsomal fractions from the marine macroalgae U. lactuca, F. vesiculosus, and P. urceolata are shown in Figure 2. All spectra contained peaks near 450 and 420 nm, corresponding to Cyt P450 and its inactivated form, respectively (Omura and Sato, 1964). Cyt b5 content was determined at 425 nm from oxidized minus reduced microsomes. High concentrations of Cyt b5 and Cyt P450 were measured in the reference microsomal fraction of soybean. All values obtained are summarized in Table II. The three algal species had P450 contents that were much lower than the P450 concentrations of about 500 pmol/mg protein given by Frear et al. (1972) for cotton and by Gabriac et al. (1991) for H. tuberosus, but similar to the value of 28 pmol/mg protein reported for Salvia officinalis (Funk and Croteau, 1993).
Figure 2.
A, CO-difference spectra of microsomes from soybean (reference); B, U. lactuca (Chlorophyta); C, F. vesiculosus (Chromophyta); and D, P. urceolata (Rhodophyta). Microsomes were divided equally between sample and reference cuvette, and reduced with solid sodium dithionite. The spectra were obtained by saturating the sample microsomes with CO. Protein concentrations were between 3 and 5 mg/mL. The bars represent 0.005 absorbance unit.
Table II.
Concentrations of Cyt b5 and Cyt P450 in microsomes from a soybean cell culture (model system) and from three species of marine macroalgae of the phyla Chlorophyta (U. lactuca), Chromophyta (F. vesiculosus), and Rhodophyta (P. urceolata)
| Plant Species | Cyt b5 | Cyt P450 |
|---|---|---|
| pmol/mg protein | ||
| Soybean (reference) | 130 | 74 |
| U. lactuca | 78 | 29 |
| F. vesiculosus | 85 | 35 |
| P. urceolata | 70 | 33 |
Amounts of the Cyts were determined as described in Methods.
In Vivo Metabolism
In vivo metabolism studies were carried out by the procedures described originally for plant cell cultures (Sandermann et al., 1984). The red alga P. urceolata was incubated with [14C]3-chlorobiphenyl and extracted with methylene chloride/methanol/water. Aliquots of the organic phase were analyzed by TLC. After 24 h of incubation one main metabolite of 3-chlorobiphenyl could be extracted from the algae. This in vivo metabolite had a RF of 0.27 in solvent system A. Upon GC-MS, a prominent mass fragment at m/z 205 was detected. These values agreed with those of the in vitro metabolite (see above). To test for an involvement of the P450 monooxygenase system, phenobarbital, a known inducer of the P450 system (Reichhart et al., 1979), and ABT, a known inhibitor of this system (Reichhart et al., 1982; Cabanne et al., 1987; Moreland et al., 1996), were used. Upon inclusion of phenobarbital (0.6 mm), the original RF 0.27 metabolite, one new, more-polar metabolite, and three new, less-polar, unidentified metabolites were detected by TLC. Total metabolism was increased 5-fold. Addition of ABT (0.6 mm) led to complete inhibition of the in vivo metabolism of 3-chlorobiphenyl. Total recovery of 14C in these in vivo experiments was 95 to 96%.
CONCLUSIONS
After an initial negative study (Payne, 1977), very low lauric acid hydroxylase activity was detected in microsomes from the seagrass Posidonia oceanica (Hamoutène et al., 1995). This study is the first one, to our knowledge, to provide reasonable evidence for Cyt P450-type oxygenases in marine algae. The evidence can be summarized as follows: (a) conversion rates of up to 54 pkat/mg protein for substrates known to be utilized by well-characterized animal and plant Cyt P450 species (lauric, palmitic, and stearic acids; 3-, 4-mono-, and 2, 3-dichlorobiphenyls; isoproturon; and cinnamic acid); (b) identity of the in vitro and in vivo monohydroxymetabolites formed from 3-chloro-biphenyl; (c) inducibility of in vivo hydroxylation by phenobarbital and inhibition by ABT; and (d) spectral detection of around 50 pmol/mg protein Cyt P450 in microsomes from three algal species.
The spectral amounts of Cyt P450 were rather low. Furthermore, only low hydroxylase activities were detected in many of the 21 algal species tested (Fig. 1). The uniform work-up and test conditions used here may have been unsuitable for some of the plant species tested. Furthermore, P450 activities are known to be highly sensitive to many environmental parameters and may therefore vary widely. Nevertheless, marine algae could act as a metabolic sink of pollutants, since 3-chlorobiphenyl was readily transformed in the in vivo experiments with P. urceolata, and high activities of glutathione S-transferases and O-, N-, and S-glucosyl transferases were also detected in the marine algae studied (Pflugmacher, 1996). For example, macroalgae contained high glucosyltransferase activities for chlorinated phenols and anilines and high glutathione S-transferase activities for the herbicides atrazine and fluorodifen. In summary, the results extend the green liver concept to an important group of lower plant species.
The induction experiment with phenobarbital indicates that at least one species of algal Cyt P450 is inducible and could serve as a biomarker for pollution in analogy to the liver Cyt P450 content of marine animal species (Bucheli and Fent, 1995). Much more work seems necessary to clarify the possible role of algal Cyt P450 systems in the removal and bioindication of marine pollutants.
ACKNOWLEDGMENTS
The authors wish to thank Dr. C. Wiencke and C. Langreder from the Alfred Wegener Institute for Polar and Marine Research (Bremerhaven) for their help with obtaining the Antarctic algal species; Prof. Buchholz, Dr. C. Buchholz, and H. Tadday from the Biologische Anstalt Helgoland, Germany, for their support during the Helgoland campaign; and Dr. H. Sahling for his help in determining the macroalgal species. We would also like to thank Dr. K. Maier (Institute of Inhalation Biology, GSF, Munich) for dog liver and lung samples.
Abbreviations:
- ABT
1-aminobenzotriazole
- DTE
dithioerythritol
Footnotes
This work was supported in part by Limagrain (Chappes, France) and by Fonds der Chemischen Industrie (Frankfurt, Germany).
LITERATURE CITED
- Benveniste I, Salaün JP, Simon A, Reichhart D, Durst F. Cytochrome P450-dependent hydroxylation of lauric acid by microsomes from pea seedlings. Plant Physiol. 1982;70:122–126. doi: 10.1104/pp.70.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Bozak K, Zimmerlin A. Plant cytochrome P450. Phytochemistry. 1994;37:1491–1506. doi: 10.1016/s0031-9422(00)89567-9. [DOI] [PubMed] [Google Scholar]
- Borlakoglu JT, John P. Cytochrome P450 dependent metabolism of xenobiotics: a comparative study of rat hepatic and plant microsomal metabolism. Comp Biochem Physiol C. 1989;94:613–617. doi: 10.1016/0742-8413(89)90121-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Briand J, Julistiono H, Beaune P, Flinois JP, Dewaziers I, Leroux JP. Presence of proteins recognized by mammalian cytochrome P-450 antibodies in Euglena gracilis. Biochim Biophys Acta. 1993;1203:199–204. doi: 10.1016/0167-4838(93)90083-4. [DOI] [PubMed] [Google Scholar]
- Bucheli TD, Fent K. Induction of cytochrome P450 as a biomarker for environmental contamination in aquatic ecosystems. Crit Rev Environ Sci Technol. 1995;25:201–268. [Google Scholar]
- Cabanne F, Huby P, Gaillardon P, Scalla R, Durst F. Effect of the cytochrome P450 inactivator 1-aminobenzotriazole on the metabolism of chlortoluron and isoproturon in wheat. Pestic Biochem Physiol. 1987;28:371–380. [Google Scholar]
- Diesperger H, Sandermann H. Soluble and microsomal glutathione S-transferase activities in pea seedlings (Pisum sativum L.) Planta. 1979;146:643–648. doi: 10.1007/BF00388845. [DOI] [PubMed] [Google Scholar]
- Durst F, O'Keefe DP, eds (1995) Plant cytochromes-P450. In Drug Metabolism and Drug Interactions, Special Issue. Freund Publishing House, London, pp 171–389 [DOI] [PubMed]
- Frear DS, Swanson HR, Tanaka FS (1972) Herbicide metabolism in plants. In VC Runeckles, TC Tso, eds, Structural and Functional Aspects of Phytochemistry. Academic Press, New York, pp 225–246
- Funk C, Croteau R. Induction and characterization of a cytochrome P-450-dependent camphor hydroxylase in tissue cultures of common sage (Salvia officinalis) Plant Physiol. 1993;101:1231–1237. doi: 10.1104/pp.101.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriac B, Werck-Reichhart D, Teutsch H, Durst F. Purification and immunocharacterization of a plant cytochrome P450: the cinnamic acid 4-hydroxylase. Arch Biochem Biophys. 1991;288:302–309. doi: 10.1016/0003-9861(91)90199-s. [DOI] [PubMed] [Google Scholar]
- Grams H (1974) Makroskopische Meeresalgen. Band Ib. Gustav-Fischer Verlag, Stuttgart, Germany
- Hamoutène D, Mathieu A, Hofmann P, Salaün J-P, Lafaurie M. Preparation and characterization of subcellular fractions suitable for studies of xenobiotic metabolism from leaf sheaths of a marine seagrass: Posidonia oceanica (Linnaeus) delile. Mar Environ Res. 1995;39:249–253. [Google Scholar]
- Kornmann P, Sahling PH. Meeresalgen von Helgoland. Benthische Grün-, Braun- und Rotalgen. Helgol. wissenschaft. Meeresunters. 1993;29:1–289. [Google Scholar]
- Moreland DE, Fleischmann TJ, Corbin FT, McFarland JE. Differential metabolism of the sulfonylurea herbicide prosulfuron (CGA-152005) by plant microsomes. Z Naturforsch C. 1996;51:698–710. [Google Scholar]
- Mougin C, Cabanne F, Scalla R. Additional observations on the chlorotoluron hydroxylase and N-demethylase activities in wheat microsomes. Plant Physiol Biochem. 1992;30:769–778. [Google Scholar]
- Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- Payne JF. Mixed function oxidase in marine organisms in relation to petroleum hydrocarbon metabolism and detection. Mar Pollution Bull. 1977;8:112–116. [Google Scholar]
- Pflugmacher S (1996) Chemo-taxonomische Untersuchungen von Enzym-systemen für die Umsetzung von Xenobiotika in niederen Pflanzen und marinen Makroalgen: Ein Beitrag zum Konzept der “grünen Leber.” PhD thesis, Ludwig-Maximilians-Universität, München, Germany
- Reichhart D, Salaün JP, Benveniste I, Durst F. Induction by manganese, ethanol, phenobarbital and herbicides of microsomal cytochrome P450 in higher plant tissues. Arch Biochem Biophys. 1979;196:301–303. doi: 10.1016/0003-9861(79)90580-0. [DOI] [PubMed] [Google Scholar]
- Reichhart D, Simon A, Durst F, Mathews JM, Ortiz de Montellano PR. Autocatalytic inactivation of plant cytochrome P-450 enzymes. I. Selective inactivation of cinnamic acid 4-hydroxylase from Helianthus tuberosus by 1-aminobenzotriazole. Arch Biochem Biophys. 1982;226:522–529. doi: 10.1016/0003-9861(82)90241-7. [DOI] [PubMed] [Google Scholar]
- Salaün JP, Benveniste I, Reichhart D, Durst F. A microsomal (cytochrome P450)-linked lauric acid monooxygenase from aged jerusalem-artichoke tuber tissues. Eur J Biochem. 1978;90:155–159. doi: 10.1111/j.1432-1033.1978.tb12586.x. [DOI] [PubMed] [Google Scholar]
- Salaün JP, Benveniste I, Reichhart D, Durst F. Induction and specificity of cytochrome P-450-dependent laurate in-chain-hydroxylase from higher plant microsomes. Eur J Biochem. 1981;119:651–655. doi: 10.1111/j.1432-1033.1981.tb05657.x. [DOI] [PubMed] [Google Scholar]
- Salaün JP, Weissbart D, Durst F, Pflieger P, Mioskowski C (1989) Epoxidation of cis and trans Δ-unsaturated lauric acid by a cytochrome P450-dependent system from higher plant microsomes. FEBS Lett 1–2: 120–126
- Sandermann H. Plant metabolism of xenobiotics. Trends Biochem Sci. 1992;17:82–84. doi: 10.1016/0968-0004(92)90507-6. [DOI] [PubMed] [Google Scholar]
- Sandermann H. Higher plant metabolism of xenobiotics: the “green liver” concept. Pharmacogenetics. 1994;4:225–241. doi: 10.1097/00008571-199410000-00001. [DOI] [PubMed] [Google Scholar]
- Sandermann H, Scheel D, Trenck T. Use of plant cell cultures to study the metabolism of environmental chemicals. Ecotoxicol Environ Saf. 1984;8:167–182. doi: 10.1016/0147-6513(84)90059-9. [DOI] [PubMed] [Google Scholar]
- Schröder P, Pflugmacher S, Rennenberg H. Biomarker für organische Schadstoffe in Fichten (Picea abies L.): Dynamik des Entgiftungsenzymes Glutathion S-Transferase. Angew Bot. 1992;66:174–179. [Google Scholar]
- Thies F, Backhaus T, Bossmann B, Grimme LH. Xenobiotic biotransformation in unicellular green algae. Involvement of cytochrome P450 in the activation and selectivity of the pyridazinone pro-herbicide metflurazon. Plant Physiol. 1996;112:361–370. doi: 10.1104/pp.112.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoek C, Jahns HM, Mann DG. Algen. 3. Neubearbeitete Auflage. Stuttgart, Germany: Georg Thieme Verlag; 1993. [Google Scholar]
- von der Trenck T, Sandermann H. Oxygenation of benzo(α)pyrene by plant microsomal fractions. FEBS Lett. 1980;119:227–231. doi: 10.1016/0014-5793(80)80258-4. [DOI] [PubMed] [Google Scholar]
- Wilken A, Bock C, Bokern M, Harms H. Metabolism of different PCB congeners in plant cell cultures. Environ Tox Chem. 1995;14(12):2017–2022. [Google Scholar]