Abstract
The molecular nature of the X chromosome inactivation process has been investigated by utilizing the techniques of DNA-mediated cell transformation of the X-linked hypoxanthine phosphoribosyltransferase (HPRT) locus. The findings indicate that purified DNA from the inactive X chromosome of a near-euploid mouse cell line is not functional in transformation for HPRT, but the DNA from its "homologous" active X readily elicits transformation for HPRT in the same hamster cell recipient. These findings suggest that there is a difference between the DNA, per se, of the active and inactive X at (or near) the HPRT locus and that this difference could account, at least in part, for its inactivation.
Full text
PDF

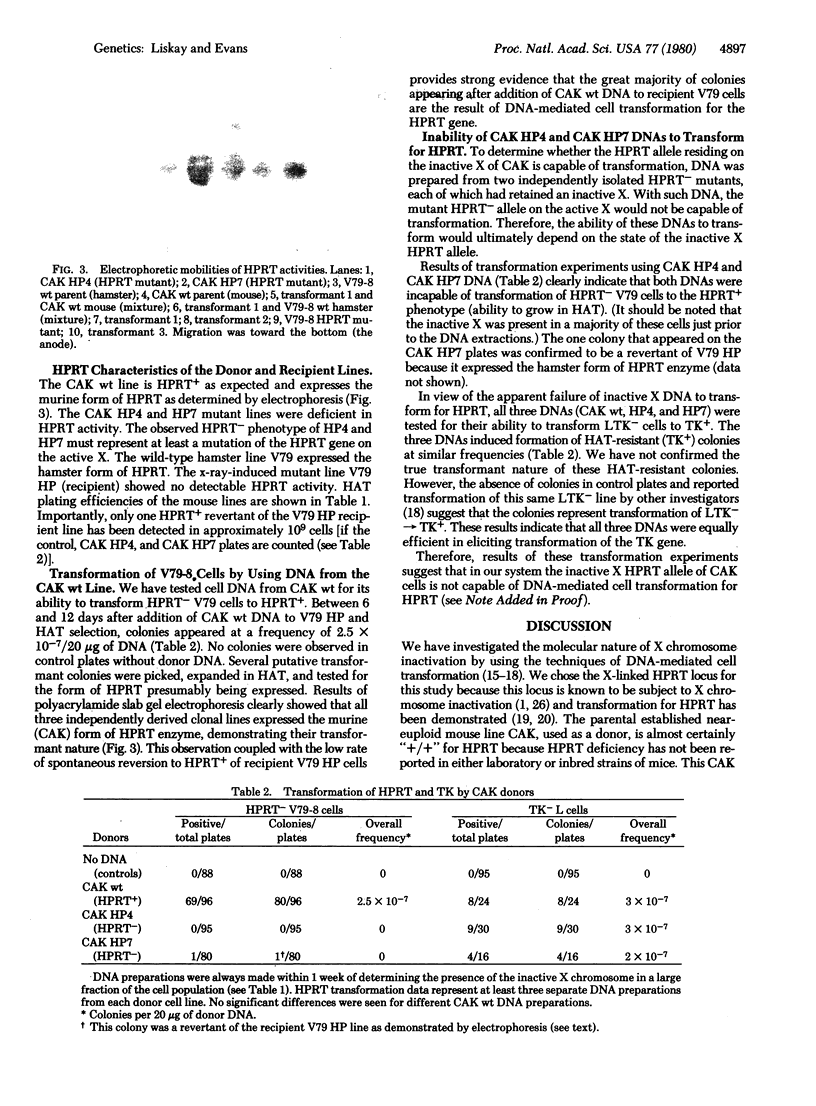

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchetti S., Graham F. L. Transfer of the gene for thymidine kinase to thymidine kinase-deficient human cells by purified herpes simplex viral DNA. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1590–1594. doi: 10.1073/pnas.74.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman V. M., Shows T. B. Somatic cell genetic evidence for X-chromosome linkage of three enzymes in the mouse. Nature. 1976 Feb 26;259(5545):665–667. doi: 10.1038/259665a0. [DOI] [PubMed] [Google Scholar]
- Cook P. R. Hypothesis on differentiation and the inheritance of gene superstructure. Nature. 1973 Sep 7;245(5419):23–25. doi: 10.1038/245023a0. [DOI] [PubMed] [Google Scholar]
- Farber R. A., Liskay R. M. Karyotypic analysis of a near-diploid established mouse cell line. Cytogenet Cell Genet. 1974;13(4):384–396. doi: 10.1159/000130288. [DOI] [PubMed] [Google Scholar]
- Francke U., Oliver N. Quantitative analysis of high-resolution trypsin-giemsa bands on human prometaphase chromosomes. Hum Genet. 1978 Dec 18;45(2):137–165. doi: 10.1007/BF00286957. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Andina R. J. Mammalian X-chromosome inactivation. Adv Hum Genet. 1976;7:99–140. doi: 10.1007/978-1-4757-0659-8_3. [DOI] [PubMed] [Google Scholar]
- Graf L. H., Jr, Urlaub G., Chasin L. A. Transformation of the gene for hypoxanthine phosphoribosyltransferase. Somatic Cell Genet. 1979 Nov;5(6):1031–1044. doi: 10.1007/BF01542658. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hellkuhl B., Grzeschik K. H. Partial reactivation of a human inactive X chromosome in human-mouse somatic cell hybrids. Cytogenet Cell Genet. 1978;22(1-6):527–530. doi: 10.1159/000131016. [DOI] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Kahan B., DeMars R. Localized Derepression on the Human Inactive X Chromosone in Mouse-Human Cell Hybrids. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1510–1514. doi: 10.1073/pnas.72.4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskay R. M., Prescott D. M. Genetic analysis of the G1 period: isolation of mutants (or variants) with a G1 perior from a Chinese hamster cell line lacking G1. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2873–2877. doi: 10.1073/pnas.75.6.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M. F. Possible mechanisms of X chromosome inactivation. Nat New Biol. 1971 Aug 25;232(34):229–232. doi: 10.1038/newbio232229a0. [DOI] [PubMed] [Google Scholar]
- Maitland N. J., McDougall J. K. Biochemical transformation of mouse cells by fragments of herpes simplex virus DNA. Cell. 1977 May;11(1):233–241. doi: 10.1016/0092-8674(77)90334-8. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Sprenkle J. A., Do T. T. Studies of human-mouse cell hybrids with respect to X-chromosome inactivation. Basic Life Sci. 1978;12:329–337. doi: 10.1007/978-1-4684-3390-6_23. [DOI] [PubMed] [Google Scholar]
- Migeon B. R. Stability of X chromosomal inactivation in human somatic cells. Nature. 1972 Sep 8;239(5367):87–89. doi: 10.1038/239087a0. [DOI] [PubMed] [Google Scholar]
- Nesbitt M. N., Gartler S. M. Replication of the mouse sex chromosomes early in the S period. Cytogenetics. 1970;9(3):212–221. doi: 10.1159/000130091. [DOI] [PubMed] [Google Scholar]
- Raskind W. H., Gartler S. M. X chromosome inactivaton and SV40 transformation of mammalian cells. Somatic Cell Genet. 1979 Nov;5(6):945–955. doi: 10.1007/BF01542653. [DOI] [PubMed] [Google Scholar]
- Riggs A. D. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- Romeo G., Migeon B. R. Stability of X chromosomal inactivation in human somatic cells transformed by SV-40. Humangenetik. 1975 Sep 10;29(2):165–170. doi: 10.1007/BF00430356. [DOI] [PubMed] [Google Scholar]
- Sager R., Kitchin R. Selective silencing of eukaryotic DNA. Science. 1975 Aug 8;189(4201):426–433. [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Willecke K., Klomfass M., Mierau R., Döhmer J. Intraspecies transfer via total cellular DNA of the gene for hypoxanthine phosphoribosyltransferase into cultured mouse cells. Mol Gen Genet. 1979 Feb 26;170(2):179–185. doi: 10.1007/BF00337794. [DOI] [PubMed] [Google Scholar]






