Abstract
In the present study, we investigated the antitumor activity of HMQ1611, a novel synthetic taspine derivative, in vivo and evaluated associated potential antiangiogenesis mechanisms. The proliferation of A549 cells was examined by WST-1 assay in vitro. Tube formation and lung tissue vessel models were used to observe the antiangiogenic activity of HMQ1611. In addition, vascular enodthelial growth factor (VEGF) secretion and KDR kinase activities were measured by ELISA and the HTRF®KinEASE™-TK assay. In vivo, the antitumor activity was assessed by implantation of A549 cells in athymic mice. The results showed that HMQ1611 inhibited A549 cell proliferation and VEGF secretion, while it significantly inhibited tube formation and tissue vascularization. Furthermore, HMQ1611 inhibited A549 xenograft tumor growth. In conclusion, the results of our study suggest that HMQ1611 has latent properties for the inhibition of angiogenesis which are involved in its antitumor activity.
Keywords: HMQ1611, A549 cell, angiogenesis, vascular endothelial growth factor
Introduction
Angiogenesis is the process of new blood vessel formation from pre-existing vasculature, and is essential for new organ development, differentiation and growth (1,2). Angiogenesis plays an important role in the growth and metastasis of solid tumors (1,3). It is a complex process which must be accurately regulated by pro- and antiangiogenic factors (4). Among the large number of proangiogenic factors, vascular endothelial growth factor (VEGF) has been confirmed to be key in angiogenesis in a number of preclinical and clinical studies (5,6). VEGF is often overexpressed in most tumors (7) and its expression is linked to tumor growth, metastasis and angiogenesis. The overexpression of VEGF has been associated with a poor prognosis in non-small cell lung cancer (NSCLC) (8). Lung cancer has become one of the most common malignancies and is the leading cause of cancer-related mortality in males and females throughout the world (9). NSCLC accounts for 85% of all cases of lung cancer. However, chemotherapy combinations appear to have reached a therapeutic plateau of activity in the treatment of advanced NSCLC (10).
In recent years, a number of antiangiogenic agents have been developed, including monoclonal antibodies (mAbs), targeting specifically the proangiogenic factors (11,12), and synthetic tyrosine kinase inhibitors, targeting multiple proangiogenic factors (13,14). A number of clinical studies have now verified the antiangiogenic agents used in various types of cancer and many patients treated with angiogenesis inhibitors survive for a longer period (15). Therefore, it is important to study antitumor activity which occurs via the inhibition of tumor angiogenesis (16).
Taspine, isolated from Radix et Rhizoma Leonticsi, has been shown to have antitumor angiogenesis activity (17,18). HMQ1611, a taspine derivative, was prepared in our laboratory to obtain a better water-solubility and a weaker toxicity. In this study, we aimed to investigate the in vivo effect of HMQ1611 on the growth of human lung cancer by establishing an A549 animal-transplanted lung cancer tumor model. We also evaluated the inhibition of microvessel formation in the lung tissue and used ECV304 cells to study the in vitro antiangiogenic effect of HMQ1611 on tube formation. We also analyzed the antiangiogenic effect in vitro using a tissue model. To further understand the molecular mechanism, we investigated the HMQ1611 inhibitory effect on VEGF secretion and KDR, which play key roles in tumor angiogenesis.
Materials and methods
Reagents
HMQ1611 was provided by the Natural Drug Research and Engineering Center of Xi’an Jiaotong University (Shaanxi, China). Stock concentration of HMQ1611 (20 mM) was prepared with dimethyl sulfoxide (DMSO) and stored at 4°C. The stock solution was further diluted with the serum-free RPMI-1640 medium immediately before use. WST-1 reagent was purchased from Roche Diagnostics (Indianapolis, IN, USA). DMSO, RPMI-1640, DMEM and trypsin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Matrigel was obtained from BD Biosciences (Franklin Lakes, NJ, USA). Thrombin was obtained from Guoao Pharmaceutical (Changchun, China). Enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, MN, USA). KDR kinase was obtained from Carna Biosciences (Kobe, Japan). HTRF® package insert was purchased from Cisbio Bioassays (Bedford, MA, USA).
Cells
The A549 human NSCLC cell line and ECV304 cells were purchased from the Shanghai Institute of Cell Biology in the Chinese Academy of Sciences (Shanghai, China). A549 and ECV304 cells were cultured in RPMI-1640 supplemented with 10% FBS and incubated at 37°C in a 5% CO2 atmosphere.
Mice
BALB/c mice (4–6 weeks old) were purchased from the Shanghai Institute of Experimental Animals in the Chinese Academy of Sciences. Kunming mice (15–18 g) were purchased from Animal Research center of Xi’an Jiaotong University. The mice were maintained under laminar air flow conditions with a 12-h light (6:00–18:00)/12-h dark (18:00-6:00) cycle. Laboratory food and water were freely available. Animal care was in accordance with the National Institute of Health guidelines and the Animal Research Committee of Xi’an Jiaotong University.
Antitumor effect of HMQ1611 on A549 cell line xenografted in athymic mice
BALB/c immunodeficient mice were purchased from the Shanghai Institute of Experimental Animals in the Chinese Academy of Sciences. A549 cells (2×107 cells/ml) were implanted into the right axilla of athymic mice (0.2 ml/mouse) to form solid tumors. Athymic mice with solid tumors were randomly divided into groups which received HMQ1611 (100 mg/kg and 200 mg/kg in 0.5% CMC-Na; n=8) or vehicle alone (normal saline; n=8). Drugs were administered once a day for two weeks beginning when the tumor volumes became noticeable. The tumors were measured with calipers three times and tumor volume was calculated as: tumor volume = (length x width2)/2. The weight of the mice and the tumor volumes were recorded when the mice were sacrificed.
Cell proliferation assay
A549 (1×105 cells/well) cells were seeded in a 96-well plate (in RPMI-1640 medium with 10% FBS) and cultivated for 24 h. Then a series of different concentrations of HMQ1611 in serum-free RPMI-1640 medium was added to the 96-well plate for 48 h. After 48 h, 10 μl/well WST-1 was added to the 96-well plate, which was then incubated for variable time periods (0.5 to 4 h) in a humidified atmosphere (at 37°C under 5% CO2). The plate was agitated thoroughly for 1 min on a shaker prior to every measurement. The absorbance was measured at 450 nm in a microplate reader (Bio-Rad, Hercules, CA, USA). The results are expressed as a percentage of the proliferation ratio: percentage of proliferation ratio = [(ODtreatment group - ODblank group) / (ODcontrol group - ODblank group)] × 100%.
Tube formation assay
The tube formation assay was performed to determine the effect of HMQ1611 on angiogenesis in vitro. Briefly, matrigel was diluted in serum-free DMEM to 3 mg/ml of the final concentration. A 48-well plate was coated with 200 μl/well matrigel and 5 μl/well thrombin (50 U/ml) and incubated at 37°C for 30 min to form a gel layer. Following gel formation, 1×105 ECV304 cells were added to each well in 500 μl 10% FBS-containing medium and various concentrations of HMQ1611 were applied to each well. The plates were incubated at 37°C for 18 h. Images of the formation of capillary tubes were then captured randomly under a microscope. The area of the tubes was measured with using Graphpad Prism 5 software, with three images from separate experiments for each data point. The inhibition rate of tube formation was calculated as: [1 - (tube areatreated / tube areacontrol)] × 100%.
Inhibition of angiogenesis of lung tissue
An assay of mouse lung tissue was used as the in vitro angiogenesis model. For preparation of fibrin matrices, 3 mg/ml solution of fibrinogen containing 300 μg/ml ε-aminocaproic acid (in DMEM) was incubated on ice for 10 min. An aliquot of this solution (250 μl) was mixed with 1 unit of thrombin and quickly pipetted into the wells of a 24-well plate (Costar, Corning, NY, USA). The mixture was incubated at 37°C for 30 min, during which time a male mouse was anesthetized with diethyl ether and sacrificed. The lung tissues were cut into 0.5–1.0 mm3 sections and placed on top of the previously prepared fibrin matrix. Lung tissues were then covered with a second layer of fibrin matrix that was covered with DMEM containing 20% fetal bovine serum. The lung tissues were incubated with the growth media, which consisted of DMEM with or without HMQ1611 (0, 5, 10 and 45 μM). The plates were stored in an incubator at 37°C and 5% CO2. The number of sprouting vessels was analyzed with the image analysis program Image Pro Plus (IPP, version 5.1, Media Cybernetics, Bethesda, MD, USA).
ELISA
A549 cells were seeded at a density of 1×105 cells/well in a 6-well plate and grown to confluence. The various concentrations of HMQ1611 were added to the well for 48 h and then the supernatant was removed and immediately assayed. The quantitation of VEGF was determined by ELISA, which was performed according to the manufacturer’s instructions.
Kinase assay
The ability of HMQ1611 to inhibit the phosphorylation of a peptide substrate by KDR kinase was evaluated in a microtiter plate format using homogeneous time-resolved fluorescence (HTRF). Initially, 2 μl kinase (Km=0.003767 ng/μl) and 2 μl substrate (Km=121.4 nM) were separately added to a 384-well plate, and variable concentrations of HMQ1611 (diluted in kinase buffer) were then added to the assay plate. ATP (2 μl, Km=1.332 μM) was added and the reaction was allowed to proceed at 37°C for 30 min. The TK-Antibody labeled with Eu3+-cryptate and streptavidin-XL665 were then added with EDTA to detect the phosphorylated product at room temperature for 1 h. Then the fluorescence was measured at 615 nm (cryptate) and 665 nm (XL665) using the Perkin-Elmer victor 2030 multilabel plate reader. Finally, the results were calculated as follows: ratio = (OD665 nm / OD615 nm) × 104.
Statistical analysis
All data are expressed as mean ± SEM (standard error of the mean). The statistical software SPSS 18.0 was used to analyze statistical data and ANOVA was used to analyze differences between groups of data. P<0.05 was considered to indicate a statistically significant result.
Results
Effect of HMQ1611 on the growth of A549 lung cancer cells in athymic mice
The antitumor properties of HMQ1611 were evaluated using human tumor models xenografted in athymic mice. HMQ1611 significantly inhibited tumor growth in A549-xenografted athymic mice. Compared with the control group, the group treated with HMQ1611 significantly inhibited the tumor growth at a rate of 28.28% and 54.76%, respectively. Furthermore, there was no substantial change in the body weight of the athymic mice during the experiment (the mean body weight at the start was 20.79±2.15, 20.21±1.56 and 20.43±1.89 g in the control, 100 mg/kg and 200 mg/kg groups; at the end the mean body weight was 23.89±3.49, 24.48±4.02 and 23.93±4.29 g, respectively), which could be considered as the antitumor activity of HMQ1611 overcoming the toxicity in athymic mice.
Effect of HMQ1611 on proliferation of A549 cells
The WST-1 assay demonstrated that the HMQ1611 significantly inhibited the growth of A549 cells in a dose-dependent manner. HMQ1611 had hardly any effect on A549 proliferation at 2 μM. The inhibition increased with increasing concentrations, with an inhibitory percentage of 98.79% at the 50 μM dose. The IC50 was 26.70 μM.
Effect of HMQ1611 on tube formation of ECV304 cells
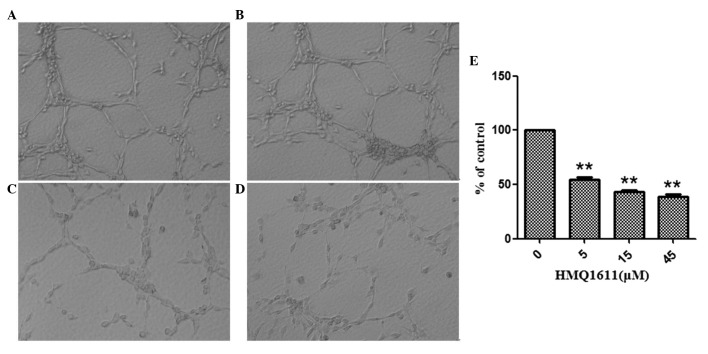
Tube formation is an important process in the differentiation of resting endothelial cells into new vessels. Fig. 1B, C and D showed that treatment with HMQ1611, ranging from 5 to 45 μM, markedly inhibited the tube formation in a dose-dependent manner. The inhibitory percentages were 45.55, 56.96 and 61.39% at 5, 15 and 45 μM, respectively.
Figure 1.
Effect of HMQ1611 on the tube formation of ECV304 cells. Cells were treated with (A) 0 μM, (B) 5 μM, (C) 15 μM and (D) 45 μM HMQ1611. (E) Quantitation data of inhibition by HMQ1611 on the tube formation. Values are expressed as mean ± SEM (n=3). **P<0.01 vs. control. All samples were run in triplicate. VEGF, vascular endothelial growth factor.
Effect of HMQ1611 on angiogenesis in the lung tissue model
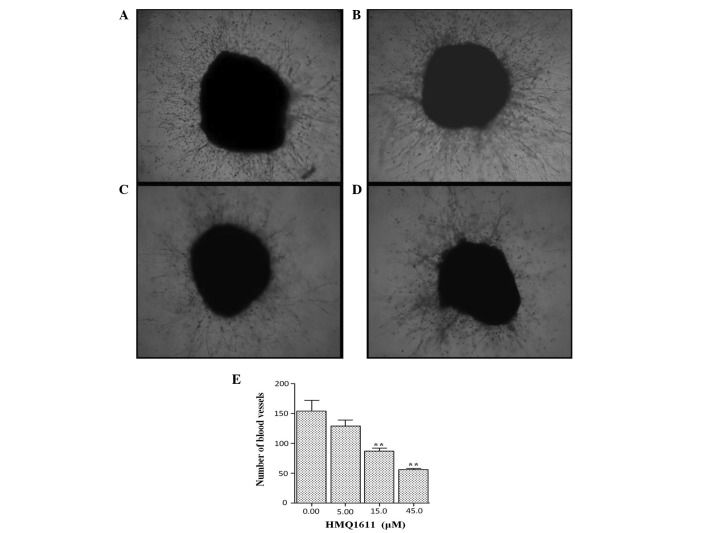
The lung tissue model was established to imitate angiogenesis in vivo. As shown in Fig. 2, HMQ1611 markedly inhibited the formation of new blood vessels compared with the control group. The quantitative data of the number of blood vessel indicated that HMQ1611 significantly reduced vascularization of the lung tissue at the concentrations of 5, 15 and 45 μM in a dose-dependent manner.
Figure 2.
Effects of HMQ1611 on microvessel outgrowth from the cultured lung tissue. (A) Microvessel outgrowth from mice lung tissue in the control group after 6 days; (B–D) Microvessel outgrowth from rat lung tissue in the HMQ1611 group after 6 days. Animals were treated with (B) 5 μM, (C) 15 μM and (D) 45 μM HMQ1611; (x100 magnification). (E) Analysis of angiogenesis in the cultured lung tissue. Data represent the mean ± SEM (n=5). **P<0.01 vs. the untreated control.
Effect of HMQ1611 on VEGF secretion
A sandwich ELISA was used to determine the level of VEGF released from A549 cells. In A549 cells that were cultured with or without HMQ1611, Fig. 3 showed that HMQ1611 inhibited VEGF production in a dose-dependent manner from 5 to 45 μM compared with the negative control. The VEGF amount was significantly decreased at all used concentrations.
Figure 3.
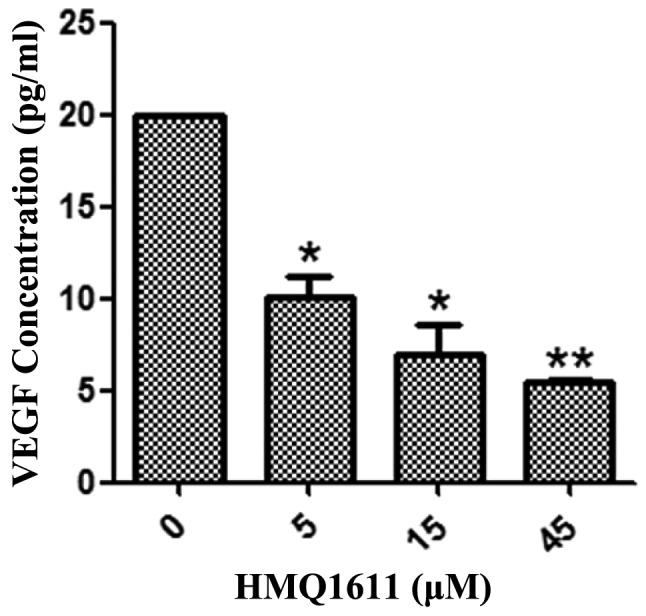
Effect of HMQ1611 on VEGF secretion by A549 cells. A549 cells were treated with HMQ1611 for 48 h, then the supernatant was collected and used to quantitate the VEGF secretion. VEGF expression was inhibited in a dose-dependent manner compared with the untreated group. Values are expressed as mean ± SEM (n=3). *P<0.05, **P<0.01 vs. control. All samples were run in triplicate. VEGF, vascular endothelial growth factor.
Kinase assay
The LANCE™ assay was used to assess the effects of HMQ1611 on KDR kinase activity. The IC50 of HMQ1611 on KDR kinase activity was >5,000 nM, suggesting that HMQ1611 did not markedly alter KDR kinase activity.
Discussion
Previous studies in our laboratory have revealed that taspine had antitumor activity and was able to markedly inhibit the proliferation of ECV304 cells and angiogenesis (17,19). In the current study, we investigated HMQ1611, a taspine derivative, its action on NSCLC and its potential antiangiogenic mechanisms.
We initially evaluated the effect of HMQ1611 on tumor growth in vivo by xenografts of A549 cells in athymic mice. It was shown that the tumor development of BALB/c mice with the implantation of A549 cells was inhibited by the administration of HMQ1611. The tumor formation of BALB/c mice was significantly suspended in the HMQ1611 group. The average tumor volume and weight were decreased in the administration group compared with the control group; however, the final body weight of the mice in the two groups was essentially identical. Meanwhile, xenografts in athymic mice treated with HMQ1611 at different concentrations exhibited different inhibition rates. These results demonstrated the antitumor effect of HMQ1611 and that it had no marked toxicity.
We then used the WST-1 assay to observe the effect of HMQ1611 on A549 cell proliferation in vitro. The results showed that HMQ1611 exhibited significant inhibition of A549 cell proliferation in a dose-dependent manner. This assay also provided an effective non-toxic concentration for use in further study. Angiogenesis has been recognized as an important driver in the initiation and progression of several types of human cancer (20), while endothelial cell tube formation is the final stage of angiogenesis (21,22). Therefore, we used the tube formation of endothelial cell on matrigel to validate the in vitro antiangiogenic properties of HMQ1611. The results showed that the tubes exhibited different numbers at different concentrations of HMQ1611. Compared with the control group, the formation of tubes in animals treated with HMQ1611 was postponed, indicating that the compound inhibited the tube formation. The tissue vessel model established in our laboratory was used to verify the role of HMQ1611 in angiogenesis at the tissue level. Following treatment with HMQ1611, new vessels on the periphery of the lung tissues grew slower than in the control group; moreover, the number of vessels reduced markedly. These facts indicate that HMQ1611 effectively disturbed the vessel formation that is critical for the supply of nutrients and oxygen to the tumor. Therefore, we hypothesized that the antitumor activity of HMQ1611 most likely occurs via the inhibition of tumor angiogenesis. VEGF, recognized as one of the key proangiogenic factors in angiogenesis, is an endothelial cell-specific mitogenic and chemotactic agent (23) and the inhibition of VEGF in endothelial cells should block the process of angiogenesis. Subsequently, we explored the potential mechanism of HMQ1611 inhibition of angiogenesis by detecting VEGF secretion and KDR kinase activity in vitro. The data showed that HMQ1611 inhibited VEGF secretion and did not markedly inhibit KDR kinase. These results indicate that HMQ1611 most likely only reduced VEGF secretion and did not block the receptor of KDR.
In conclusion, the present study demonstrated that HMQ1611 inhibits tumor growth in xenografted A549 cells in nude mice by inhibiting the growth of neovessels. In other words, the results show that HMQ1611 is an inhibitor of angiogenesis which functions by downregulating VEGF. Our study suggests that HMQ1611 is a promising candidate for a treatment strategy in angiogenesis-related disease.
Acknowledgments
This study was supported by National Natural Science Foundation of China (Grant No. 81001447) and Shaanxi young star of science and technology Program (Grant No. 2012KJXX-06).
References
- 1.Folkman J. Anti-angiogenesis: a new concept for therapy of solid tumors. Ann Surg. 1972;175:409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9:2–10. doi: 10.1634/theoncologist.9-suppl_1-2. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 4.Hirte H. Novel developments in angiogenesis cancer therapy. Curr Oncol. 2009;16:50–54. doi: 10.3747/co.v16i3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 6.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 8.Imoto H, Osaki T, Taga S, Ohgami A, Ichiyoshi Y, Yasumoto K. Vascular endothelial growth factor expression in non-small-cell lung cancer: prognostic significance in squamous cell carcinoma. J Thorac Cardiovasc Surg. 1998;115:1007–1014. doi: 10.1016/S0022-5223(98)70398-8. [DOI] [PubMed] [Google Scholar]
- 9.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 10.Carney DN. Lung cancer - time to move on from chemotherapy. N Engl J Med. 2002;346:126–128. doi: 10.1056/NEJM200201103460211. [DOI] [PubMed] [Google Scholar]
- 11.Halatsch ME, Schmidt U, Behnke-Mursch J, Unterberg A, Wirtz CR. Epidermal growth factor receptor inhibition for the treatment of glioblastoma multiforme and other malignant brain tumors. Cancer Treat Rev. 2006;32:74–89. doi: 10.1016/j.ctrv.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Harding J, Burtness B. Cetuximab: an epidermal growth factor receptor chimeric human-murine monoclonal antibody. Drugs Today (Barc) 2005;41:107–127. doi: 10.1358/dot.2005.41.2.882662. [DOI] [PubMed] [Google Scholar]
- 13.Glade Bender J, Cooney EM, Kandel JJ, Yamashiro DJ. Vascular remodeling and clinical resistance to antiangiogenic cancer therapy. Drug Resist Updat. 2004;7:289–300. doi: 10.1016/j.drup.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 15.Ribatti D. Novel angiogenesis inhibitors: addressing the issue of redundancy in the angiogenic signaling pathway. Cancer Treatment Rev. 2011;37:344–352. doi: 10.1016/j.ctrv.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Jekunen AP, Kairemo KJA. Inhibition of malignant angiogenesis. Cancer Treat Rev. 1997;23:263–266. doi: 10.1016/s0305-7372(97)90014-1. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, He L, Meng L, Luo W. Taspine isolated from Radix et Rhizoma Leonticis inhibits proliferation and migration of endothelial cells as well as chicken chorioallantoic membrane neovascularisation. Vascul Pharmacol. 2008;148:129–137. doi: 10.1016/j.vph.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YM, He LC, Wang HY. Inhibitory effect of taspine on mouse S180 sarcoma and its mechanism. Zhongguo Zhong Yao Za Zhi. 2007;32:953–956. (In Chinese) [PubMed] [Google Scholar]
- 19.Zhang Y, He L, Zhou Y. Taspine isolated from Radix et Rhizoma Leonticis inhibits growth of human umbilical vein endothelial cell (HUVEC) by inducing its apoptosis. Phytomedicine. 2008;15:112–119. doi: 10.1016/j.phymed.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Grepin R, Pages G. Molecular mechanisms of resistance to tumor antiangiogenic strategies. J Oncol. 2010;2010:835680. doi: 10.1155/2010/835680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis LM. Epidermal growth factor receptor in tumor angiogenesis. Hematol Oncol Clin North Am. 2004;18:1007–1021. doi: 10.1016/j.hoc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 23.Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]