Abstract
Kruppel-like factor 4 (KLF4) is a key transcriptional regulator of cell differentiation and proliferation and an altered expression of KLF4 has been reported in a number of human malignancies. In the present study, we investigated KLF4 expression and its role in cell proliferation in advanced epithelial ovarian cancer (EOC). We compared KLF4, Bcl-2 and Bax transcript levels in ovaries isolated from advanced EOC and normal control ovaries. In addition, the KLF4 gene was transduced into ovarian cancer cells and transcript levels of Bcl-2 and Bax and cell proliferation were analyzed by real-time RT-PCR and MTT assays, respectively. Ovarian KLF4 expression and Bcl-2/Bax ratios were downregulated in most cases of advanced EOC. In addition, KLF4 overexpression in ovarian cancer cells increased the Bcl-2/Bax ratio. However, MTT analysis indicated that the overexpression of KLF4 had no effect on the proliferation of ovarian cancer cells. The inactivation of KLF4 is frequently observed in ovarian cancers and a reduced expression of KLF4 in the ovarian cancers may lead to a reduction in the Bcl-2/Bax ratio. The latter has a role in predicting cancer grade, although its exact role in ovarian carcinogenesis requires clarification.
Keywords: KLF4, Bcl-2, Bax, epithelial ovarian cancer
Introduction
Epithelial ovarian cancer (EOC) is the most lethal of all gynecologic cancers and most women are diagnosed at an advanced stage (1). Overall mortality rates have remained relatively constant over the past several decades and the cause of EOC is largely unknown.
The Kruppel-like factor 4 (KLF4) gene encodes an epithelial cell-enriched, zinc finger-containing transcription factor that has been shown to play important roles in cell proliferation and differentiation (2,3). In total, 70% of breast carcinomas have elevated KLF4 mRNA levels, which are associated with a more aggressive phenotype (4,5). By contrast, KLF4 expression is frequently lost in various human cancers, including gastrointestinal (6), bladder (7) and advanced prostate cancer, and KLF4 has been found to exert tumor-suppressive effects (8). The dual and opposing roles of KLF4 in tumorigenesis suggest that KLF4 is one of the molecular elements that define the tissue-specific epithelial carcinogenesis pathway.
Conversely, the ultimate vulnerability of cells to apoptosis is determined by the relative ratio of various pro-apoptotic and anti-apoptotic members of the Bcl-2 family (9). The expression of Bcl-2 and Bax has been reported to be a prognostic factor in ovarian cancer (10,11). In addition, KLF4 overexpression in a leukemia cell line affected the transcriptional regulation of Bcl-2 and Bax, with effects on apoptosis and cell growth (12).
The expression of KLF4 and its role in the development of ovarian cancer has not previously been studied. Therefore, to investigate the possible role of KLF4 in ovarian carcinogenesis, we examined its expression in human ovarian cancer tissues and measured Bcl-2 and Bax mRNA levels in the same specimens. We also transduced the KLF4 gene into ovarian cancer cells to investigate the changes in Bcl-2 and Bax gene expression and the consequences in terms of cell proliferation.
Materials and methods
Patients
This study was approved by the institutional review board of Hanyang University Hospital (HYUHIRB-2009-R-50) and written informed consent was obtained from each patient. The patients included were women with surgically determined primary advanced stage (III–IV) EOC who received debulking surgery at our institution. Normal control samples obtained at the time of salpingo-oophorectomy for benign indications were used for comparative purposes. All tumor samples were snap frozen at the time of surgery and stored at −70°C until use.
Real-time RT-PCR analysis
RNA from EOC, normal tissues and ovarian cancer cells was isolated using an RNeasy extraction kit (Qiagen Inc., Valencia, CA, USA). Following quantification of RNA and verification of its integrity, 1 μg samples were reverse transcribed with an Advantage RT for PCR kit (BD Biosciences, Clontech, Palo Alto, CA, USA). Primers were designed with the Primers Express program (PE Applied Biosystems, Carlsbad, CA, USA): KLF4 forward, 5′-ATCAGATGCAGCCGCAAGTCCC-3′ and reverse, 5′-TCT TCATGTGTAAGGCGAGGTGGTCC-3′ (GenBank accession no. NM_004235.4); Bcl-2 forward, 5′-ATGTGTGTG GAGAGCGTCAA-3′ and reverse, 5′-ACAGTTCCACAA AGGCATCC-3′ (GenBank accession no. NM_000633.2); and Bax forward, 5′-GGGGACGAACTGGACAGTAA-3′ and reverse, 5′-CAGTTGAAGTTGCCGTCAGA-3′ (GenBank accession no. NM_004324.3). Amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward, 5′-CAGCCTCAAGATCATCAGCA-3′ and reverse, 5′-TGT GGTCATGAGTCCTTCCA-3′; GenBank accession no. NM_002046.3) was used to normalize each reaction (amplification product sizes 369, 136, 122 and 106 bps for KLF4, Bcl-2, Bax and GAPDH, respectively). Real-time PCR reactions were carried out in total volumes of 25 μl using SYBR-Green Supermix (Bio-Rad, Hercules, CA, USA) with an iCycler™ Thermal Cycler (Bio-Rad). PCR conditions were 10 min at 95°C, 35 cycles of 95°C for 15 sec, 60°C for 45 sec and 72°C for 1 min. Samples were run in triplicate in 96-well optical plates (Bio-Rad) and the mean values were compared with normal controls to obtain relative transcript levels.
Cell line transfection
Human ovarian cancer cell lines (SKOV3 and SNU251) were purchased from the Korean Cell Line Bank (Seoul, Korea) and transiently transfected with pCMV3xFLAG-KLF4 made by subcloning the PCR-amplified coding region of KLF4. The cells were plated in 6-well plates and incubated with 2 μg aliquots of plasmid or empty vector in the presence of Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). Following incubation for 24–48 h, the cells were harvested for the analysis of RNA, protein and cell numbers.
Cell proliferation assay
For assays examining FLAG-KLF4 abundance and turnover, cell pellets were lysed in Laemmli buffer containing β-mercaptoethanol (Bio-Rad). Samples were resolved by 4–12% NuPAGE gel electrophoresis (Invitrogen), transferred to Hybond-P membranes (Amersham Pharmacia Biotech, Arlington Heights, IL, USA) and immunoblotted with anti-FLAG M2 antibody (1:1000) (F3165, Sigma, St. Louis, MO, USA). Promega horseradish peroxidase-conjugated anti-mouse immunoglobin G (W402B) antibody was used as a secondary antibody. To ensure that lysates were loaded equally, the blots were stripped and incubated with an anti-β-actin antibody (1:1000; Sigma).
For the cell proliferation assays, cells were transferred to 96-well microplates 24 or 48 h after transfection and seeded at a density of approximately 1x105 cells per well before the assay. Cell viability was subsequently determined using an MTT cell proliferation assay kit (Cayman Chemical Company, Ann Arbor, MI, USA). Absorbance was measured at 570 nm with a microplate reader. The experiment was repeated 3 times and the data were expressed as fold changes relative to empty vector-transfected cells cultured for 24 h.
Statistical analysis
All data were analyzed using the Student’s t-test, with P<0.05 considered to indicate a statistically significant result. Data are expressed as the means ± standard deviation (SD) of triplicate measurements.
Results
Using real-time RT-PCR, we analyzed mRNA expression in pathology specimens from 3 normal ovaries and 12 cases of advanced EOC, including 3 cases each of serous, clear cell and endometrioid carcinoma and 3 mucinous cystadenocarcinomas.
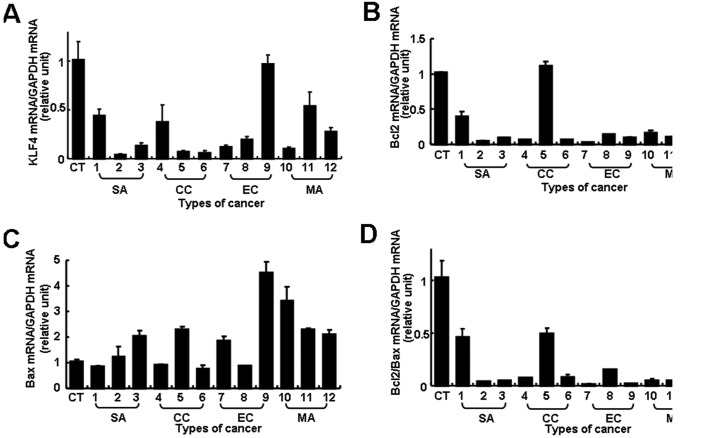
KLF4 transcript levels were substantially lower in most of the EOC samples (11 of 12 specimens) than in the normal controls, although the extent of the difference varied between tumors (Fig. 1A). The level of expression in most samples was less than half the normal level and it was particularly low (less than 0.3-fold of normal level) in 7 EOC samples. Bcl-2 or Bax mRNA expression also varied between tumors and there was no correlation with histological type (Fig. 1B and C). However, an analysis of the Bcl-2/Bax ratio showed a significantly reduced ratio, more than 2-fold lower than normal in all 12 cases and even lower in 10 cases (more than 5-fold less than normal) (Fig. 1D). Thus, both reduced KLF4 transcript levels and lower Bcl-2/Bax ratios were found in most of the EOC samples, but the extents of the changes were not always correlated.
Figure 1.
Real-time RT-PCR analysis of KLF4, Bcl-2 and Bax expression in ovarian cancer tissues. Transcript levels of (A) KLF4, (B) Bcl-2, (C) Bax and (D) Bcl-2/Bax ratios were determined in normal ovarian tissues (n=3) and advanced primary epithelial ovarian cancer tissues (n=12). CT, normal ovarian tissues; SA (1–3), serous cystadenocarcinoma; CC (4–6), clear cell carcinoma; EC (7–9), endometrioid carcinoma; MA (10–12), mucinous adenocarcinoma. GAPDH mRNA was used as an internal control. Mean values were compared with the normal control value to calculate relative amounts of transcripts. Data are represented as the means ± SD of the triplicate assays.
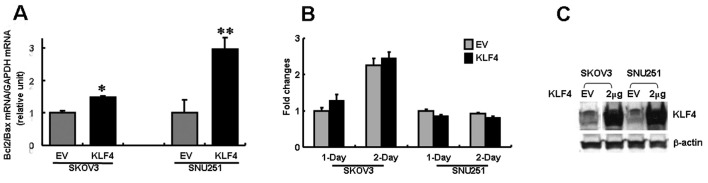
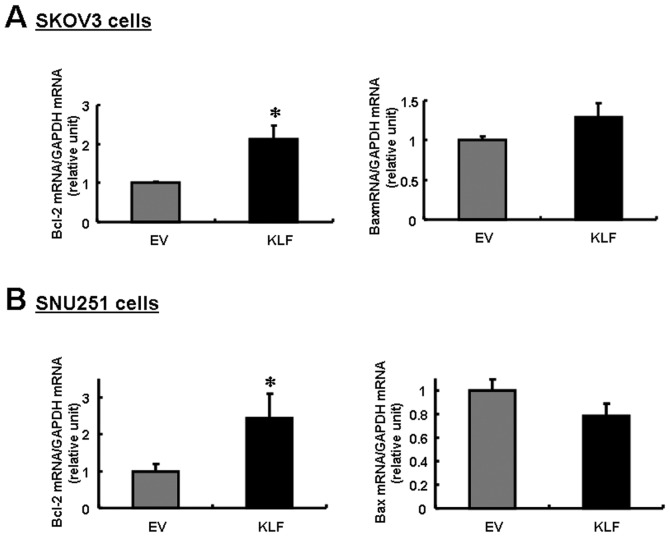
In order to examine the biological effects of KLF4 expression in ovarian cancer cells, SKOV3 and SNU251 cells were transfected with FLAG-KLF4. An immunoblot analysis confirmed marked expression of KLF4 in the transfected cells (Fig. 2C). Notably, Bcl-2 expression was upregulated in both cell lines, whereas Bax was downregulated in SNU251 cells and slightly upregulated in SKOV3 cells (Fig. 3). The differential regulation of Bcl-2 and Bax expression resulted in a decreased Bcl-2/Bax ratio in both cell lines, but the decrease was particularly significant in the SNU251 cells (P<0.03; Fig. 2A). This result suggests that the decreased expression of KLF4 in EOC is correlated with the reduced Bcl-2/Bax ratio, which is a known prognostic factor in EOC.
Figure 2.
Effect of KLF4 on Bcl-2/Bax expression and cell proliferation. (A) Real-time RT-PCR analysis of Bcl-2/Bax expression in KLF4-expressing and control vector-only SKOV3 and SNU251 cells. GAPDH mRNA was used as an internal control. Mean values were compared with the value of empty vector-transfected cells to calculate relative amounts of transcripts; (B) MTT cell proliferation assays of KLF4-expressing and vector-only SKOV3 and SNU251 cells from 1 and 2-day cultures. Each bar represents the fold change compared to the value for empty vector-transfected cells cultured for 24 h; (C) Immunoblot analysis of KLF4 protein in transfected cells. β-actin was used as an internal control. EV, empty vector; KLF4, pCMV3xFLAG-KLF4 plasmid (2 μg). Data are the means ± SD of 3 separate experiments. P-values were compared to EVs using the Student’s t-test (*P=0.024; **P=0.014).
Figure 3.
Real-time RT-PCR analysis of Bcl-2 and Bax mRNA expression in (A) SKOV3 and (B) SNU251 cells overexpressing KLF4. GAPDH mRNA was used as an internal control. Mean values were compared with the normal control value (empty vector-transfected cells) to calculate relative amounts of transcripts. Data are represented as the means ± SD of the triplicate assays. P-values were compared to empty vectors using the Student’s t-test (*P<0.05).
To evaluate the effect of KLF4 on cell proliferation, we used the MTT assay for quantitative analysis. In contrast to the known inhibition of cell growth and induction of apoptosis by KLF4 in a number of non-gonadal cancer cells, the in vitro assays indicated that cell proliferation following KLF4 gene transfection was unaffected at both 24 or 48 h of culture (P>0.05; Fig. 2B).
Discussion
This study is the first to suggest that KLF4 may play an important role in the development and progression of ovarian cancer. We found that both the expression of KLF4 and the Bcl-2/Bax ratio were downregulated in many advanced EOC cases, and that KLF4 overexpression in ovarian cancer cells resulted in an increased Bcl-2/Bax ratio, which is known to indicate a favorable prognosis in ovarian cancer.
Several lines of evidence indicate that KLF4 has variable effects on cell cycle arrest and inhibition of apoptosis depending on the cellular context (7,8,12,13). Although the expression of KLF4 was found to be downregulated in ovarian cancers in this study, which is consistent with the results for many human cancers, we observed no change in cell proliferation due to KLF4 overexpression (Fig. 2B). Given that the multiplication of human Sertoli cells is not affected by a lack of KLF4 (14), it is likely that the different effects of KLF4 on cell proliferation depend on unidentified cellular factors.
In this study we found that Bax and Bcl-2 expression levels varied among tumors, as demonstrated in other studies (10,11). Thus, it has been suggested that the ratio of Bcl-2 to Bax, rather than the absolute concentration of either, is predictive of cell fate (9), and their relative expression has been reported to be a better predictor of outcome for both progression-free survival and overall survival (10,11). The transcription of Bcl-2 and Bax in leukemia cells is affected by KLF4 overexpression (12). We also demonstrated in this study that the transgenic expression of KLF4 in ovarian cancer cells modulates Bcl-2/Bax gene expression (Fig. 2A). Previously, a microarray study showed that decreased KLF4 expression was associated with chemotherapy resistance in ovarian cancers (15). In principle, chemotherapeutics act on rapidly proliferating cells and promote cell cycle arrest or apoptosis. Thus, lower expression of KLF4 linked to a lower Bcl-2/Bax ratio may possibly contribute to resistance or relapse in advanced EOC. This increases the possibility that a crucial function of KLF4 is to increase the Bcl-2/Bax ratio, at least in ovarian cells, and that this is essential for a favorable prognosis in ovarian cancer.
Although further research should be devoted to understanding why KLF4 regulates cell growth and apoptosis in many other cancer cells (6–8) but not in ovarian cancer cells, our data indicate that the downregulation of KLF4 may be a frequent step in ovarian carcinogenesis. The decreased expression of KLF4 in ovarian cancer may modulate Bcl-2/Bax expression, a known prognostic factor for cancer grade, although its exact role in ovarian carcinogenesis needs to be clarified.
Acknowledgments
We thank Professor Hyun Kook (Medical Research Center for Gene Regulation, Chonnam National University Medical School, Gwangju, Korea) for the gift of the pCMV3-FLAG-KLF4 plasmid construct. This study was supported by a Korea Research Foundation Grant funded by the Korean Government (KRF 2009-0074679 and 2009-0065769).
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Garrett-Sinha LA, Eberspaecher H, Seldin MF, de Crombrugghe B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J Biol Chem. 1996;271:31384–31390. doi: 10.1074/jbc.271.49.31384. [DOI] [PubMed] [Google Scholar]
- 3.Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster KW, Frost AR, McKie-Bell P, Lin CY, Engler JA, Grizzle WE, Ruppert JM. Increase of GKLF messenger RNA and protein expression during progression of breast cancer. Cancer Res. 2000;60:6488–6495. [PubMed] [Google Scholar]
- 5.Pandya AY, Talley LI, Frost AR, Fitzgerald TJ, Trivedi V, Chakravarthy M, Chhieng DC, Grizzle WE, Engler JA, Krontiras H, et al. Nuclear localization of KLF4 is associated with an aggressive phenotype in early-stage breast cancer. Clin Cancer Res. 2004;10:2709–2719. doi: 10.1158/1078-0432.ccr-03-0484. [DOI] [PubMed] [Google Scholar]
- 6.Ghaleb AM, Katz JP, Kaestner KH, Du JX, Yang VW. Kruppel-like factor 4 exhibits antiapoptotic activity following gamma-radiation-induced DNA damage. Oncogene. 2007;26:2365–2373. doi: 10.1038/sj.onc.1210022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohnishi S, Ohnami S, Laub F, Aoki K, Suzuki K, Kanai Y, Haga K, Asaka M, Ramirez F, Yoshida T. Downregulation and growth inhibitory effect of epithelial-type Kruppel-like transcription factor KLF4, but not KLF5, in bladder cancer. Biochem Biophys Res Commun. 2003;308:251–256. doi: 10.1016/s0006-291x(03)01356-1. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Place RF, Huang V, Wang X, Noonan EJ, Magyar CE, Huang J, Li LC. Prognostic value and function of KLF4 in prostate cancer: RNAa and vector-mediated overexpression identify KLF4 as an inhibitor of tumor cell growth and migration. Cancer Res. 2010;70:10182–10191. doi: 10.1158/0008-5472.CAN-10-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 10.de la Torre FJ, Garcia A, Gil-Moreno A, Planaguma J, Reventos J, Ramon y Cajal S, Xercavins J. Apoptosis in epithelial ovarian tumours Prognostic significance of clinical and histopathologic factors and its association with the immunohistochemical expression of apoptotic regulatory proteins (p53, bcl-2 and bax) Eur J Obstet Gynecol Reprod Biol. 2007;130:121–128. doi: 10.1016/j.ejogrb.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 11.Schuyer M, van der Burg ME, Henzen-Logmans SC, Fieret JH, Klijn JG, Look MP, Foekens JA, Stoter G, Berns EM. Reduced expression of BAX is associated with poor prognosis in patients with epithelial ovarian cancer: a multifactorial analysis of TP53, p21, BAX and BCL-2. Br J Cancer. 2001;85:1359–1367. doi: 10.1054/bjoc.2001.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Zhao J, Li Q, Yang W, Song Q, Li W, Liu J. KLF4 promotes hydrogen-peroxide-induced apoptosis of chronic myeloid leukemia cells involving the bcl-2/bax pathway. Cell Stress Chaperones. 2010;15:905–912. doi: 10.1007/s12192-010-0199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]
- 14.Godmann M, Kosan C, Behr R. Kruppel-like factor 4 is widely expressed in the mouse male and female reproductive tract and responds as an immediate early gene to activation of the protein kinase A in TM4 Sertoli cells. Reproduction. 2010;139:771–782. doi: 10.1530/REP-09-0531. [DOI] [PubMed] [Google Scholar]
- 15.Jazaeri AA, Awtrey CS, Chandramouli GV, Chuang YE, Khan J, Sotiriou C, Aprelikova O, Yee CJ, Zorn KK, Birrer MJ, Barrett JC, Boyd J. Gene expression profiles associated with response to chemotherapy in epithelial ovarian cancers. Clin Cancer Res. 2005;11:6300–6310. doi: 10.1158/1078-0432.CCR-04-2682. [DOI] [PubMed] [Google Scholar]