Abstract
The light-saturated rate of photosynthetic O2 evolution in Chlamydomonas reinhardtii declined by approximately 75% on a per-cell basis after 4 d of P starvation or 1 d of S starvation. Quantitation of the partial reactions of photosynthetic electron transport demonstrated that the light-saturated rate of photosystem (PS) I activity was unaffected by P or S limitation, whereas light-saturated PSII activity was reduced by more than 50%. This decline in PSII activity correlated with a decline in both the maximal quantum efficiency of PSII and the accumulation of the secondary quinone electron acceptor of PSII nonreducing centers (PSII centers capable of performing a charge separation but unable to reduce the plastoquinone pool). In addition to a decline in the light-saturated rate of O2 evolution, there was reduced efficiency of excitation energy transfer to the reaction centers of PSII (because of dissipation of absorbed light energy as heat and because of a transition to state 2). These findings establish a common suite of alterations in photosynthetic electron transport that results in decreased linear electron flow when C. reinhardtii is limited for either P or S. It was interesting that the decline in the maximum quantum efficiency of PSII and the accumulation of the secondary quinone electron acceptor of PSII nonreducing centers were regulated specifically during S-limited growth by the SacI gene product, which was previously shown to be critical for the acclimation of C. reinhardtii to S limitation (J.P. Davies, F.H. Yildiz, and A.R. Grossman [1996] EMBO J 15: 2150–2159).
Photosynthesis and plant growth can be limited by macronutrient (N, P, S, and C) availability. Growth in terrestrial and freshwater ecosystems is often limited for P (Wetzel, 1983). Even in environments in which P levels are high, much of it is in the form of insoluble phosphate salts of Ca, Al, or Fe, which are not easily accessible to plants (Raich et al., 1996). S availability may also limit plant productivity, and the fertilization of soils with sulfate can result in improved crop yields (Mahler and Maples, 1987; Samosir et al., 1993; Warman and Sampson, 1994). Free sulfate, the source of S preferred by plants, is often a minor component of the soil S; most is in the form of sulfate esters and sulfonates, which may be difficult for plants to exploit (Stanko-Golden and Fitzgerald, 1991; Houle and Cargnan, 1992).
We are interested in how photosynthetic organisms respond to limitations in P and S and, in particular, how the photosynthetic apparatus is controlled as nutrient levels decline. Photosynthesis can be divided into the light reactions, in which light energy is stored as ATP and NADPH, and the dark reactions, in which the products of the light reactions are used to reduce inorganic C. There must be a coordination of the light and dark reactions for the efficient utilization of excitation energy. A lack of coordination could result in the generation of toxic, reduced O2 species either as a consequence of the generation of triplet excited chlorophyll molecules or by direct reduction of O2 by PSI (Asada, 1994). Therefore, the flux of electrons through the electron-transport chain is modulated by the rate of ATP and NADPH consumption, which is affected by the physiological state of the cells. Numerous groups have demonstrated that P deprivation causes a decline in the in vivo, light-saturated rate of photosynthesis (Brooks, 1986; Dietz and Heilos, 1990; Jacob and Lawlor, 1993; Plesnicar et al., 1994). This decline has been attributed to a limitation in the rate of C reduction because of a depletion in the pool of phosphorylated intermediates of the reductive pentose-phosphate cycle (Brooks, 1986; Jacob and Lawlor, 1993). Furthermore, S limitation results in selective degradation of Rubisco in Lemna minor (Ferreira and Teixeira, 1992).
Studies have demonstrated that the light reactions and photosynthetic electron transport may also be altered during nutrient limitation. Spinach leaves have fewer functional PSII centers when the plants are starved of Mg and S (Godde and Hefer, 1994). Similarly, when the cyanobacterium Synechococcus sp. strain PCC 7942 is deprived of S, a decline in O2 evolution probably results from the degradation of PSII reaction centers (Collier et al., 1994). The efficiency with which absorbed light energy is used is also modulated during nutrient limitation. Cells starved for nutrients may convert excess absorbed excitation energy to heat within the LHC (qE) and/or undergo a state transition in which an increased proportion of the absorbed light energy is directed away from PSII (qT; Peltier and Schmidt, 1991; Jacob and Lawlor, 1993; Levy et al., 1993; Collier et al., 1994; Plesnicar et al., 1994; Braun et al., 1996).
Furthermore, reaction centers that are damaged by excess radiation may also quench absorbed light energy. Nutrient-deprived cells have been shown to accumulate the xanthophylls antheraxanthin and zeaxanthin (Lers et al., 1991; Levy et al., 1993), which correlates with increased qE (Demmig-Adams and Adams, 1992; Björkman and Demmig-Adams, 1994; Horton et al., 1996). These xanthophylls, plus lutein, appear to serve a photoprotective role by facilitating the conversion of excess absorbed light energy to heat (Demmig-Adams and Adams, 1992; Niyogi et al., 1997). Also contributing to photoprotection during nutrient starvation is a state transition in which the photosynthetic apparatus is transformed from state 1 (much of the light energy absorbed by LHCII is directed into the PSII reaction centers) to state 2 (less of the light energy absorbed is directed into the PSII reaction centers; Peltier and Schmidt, 1991; Allen, 1992). There appear to be a number of different ways in which photosynthetic activity is modulated during nutrient-limited growth.
The responses of the unicellular green alga Chlamydomonas reinhardtii to macronutrient limitation (de Hostos et al., 1989; Ball et al., 1990; Davies et al., 1994, 1996; Yildiz et al., 1994, 1996; Quisel et al., 1996) include the secretion of hydrolytic enzymes (sulfatases and phosphatases), kinetic changes in the uptake of the limiting nutrient, the cessation of growth, and changes in metabolic processes (e.g. increased respiration and accumulation of starch, which include a decrease in the rate of photosynthetic electron transport). The purpose of this study was to determine the effects of S and P limitation on photosynthetic electron-transport activity.
MATERIALS AND METHODS
Strains and Growth Conditions
Chlamydomonas reinhardtii strain CC125 (mt+; wild type), ars 5–4 (mt+ arg7 nit1 sac1::ARG7; the sac1 mutant), and ars 5–4 C11 (mt+ arg7 nit1 sac1::ARG7 NIT1 SAC1; the complemented sac1 mutant) were grown to the mid-logarithmic phase (0.5 to 3 × 106 cells mL−1) in TAP medium (Gorman and Levine, 1966). To induce P or S starvation, the cells were harvested by centrifugation at 5000g for 1 min, washed twice with TAP-P medium (Quisel et al., 1996) or TAP-S medium (Davies et al., 1996), and resuspended to a final density of 1 × 106 cells mL−1. After 2 d, P-deprived cells were diluted to 1 × 106 cells mL−1 with fresh TAP-P medium (cells divided three to four times following the imposition of P starvation). All cultures were grown in Erlenmeyer flasks with constant shaking and illumination at 80 μmol photons m−2 s−1 at 27°C.
Chlorophyll Determination and Cell Viability
Cells were counted using a hemacytometer and the viability of the cells was assessed by viability staining (Davies et al., 1996). For chlorophyll determinations cells were suspended in 0.01% Tween 20 (United States Biochemical) and pelleted by centrifugation for 30 s at 16,000g. The cell pellet was extracted in 90% acetone, cellular debris were removed by centrifugation (30 s at 16,000g), and the chlorophyll a and b levels were determined spectrophotometrically (Jeffrey and Humphrey, 1975).
In Vivo Measurements of O2 Evolution
O2 evolution in the light and dark respiration was measured with a Clark-type O2 electrode (Hansatech, King's Lynn, UK). Measurements of whole-cell O2 evolution were conducted at 27°C on vigorously stirred samples containing 10 μg chlorophyll a mL−1 and 5 mm bicarbonate, pH 8.1, in either TAP-P or TAP-S medium. The photosynthetic rates were calculated by adding the dark respiration rate to the rate of O2 evolution at various light intensities. Maximal photosynthetic rates were determined at 800 μmol photons m−2 s−1 and were not significantly different from values measured at 400 μmol photons m−2 s−1.
In Vitro Measurements of Electron Flow
Five milliliters of cells at 10 μg chlorophyll a mL−1 was sonicated (at a setting of 3 out of 10) for 6 s in a 10-mL glass beaker using the microtip of the Sonic Dismembrator 550 (Fisher Scientific). These sonication parameters yielded optimal rates of ferricyanide-stimulated O2 evolution during both starved and nonstarved conditions. Electron flow from water to MV, DCPIPH2 to MV, and DHQ to MV were measured as O2 uptake (Curtis et al., 1975; White et al., 1978). All reactions involving MV-stimulated O2 uptake included 2 mm cyanide to inhibit peroxide reduction and methylamine to uncouple electron transport from ATP synthesis.
The light-driven reduction of DCPIP by DPC was assayed as a decline in A600; the DCPIP reduction was linear for approximately 5 min. DPC was prepared in methanol and added to heat-treated (5 min, 60°C), sonicated cells to a final concentration of 2.5 mm (Vernon and Shaw, 1969). All of the partial reactions were shown to be light dependent and inhibited by the photosynthetic electron-transport inhibitors DCMU or DBMIB (except electron flow from DCPIPH2 to MV, which is not blocked by the electron-transport inhibitors used). Furthermore, the assays for DCPIPH2 to MV and DHQ to MV contained DCMU, and that for DPC to DCPIP contained DBMIB to prevent light-dependent alternative electron transport. Because the DHQ-to-MV partial reaction is not completely inhibited by DBMIB (inhibition is approximately 60%), we set the portion of electron transport sensitive to DBMIB as 100%.
PAM Fluorometry and PSII Characteristics during Nutrient Limitation
Fluorescence characteristics (Schreiber et al., 1986; van Kooten and Snel, 1990) were determined with a PAM fluorometer (model OS-100, PP Systems, Haverhill, MA) connected to a personal computer containing the OS-Log program (version 1.5, PP Systems). Samples were continuously stirred and maintained at 27°C in a water-jacketed chamber. The intensity of the measuring beam was <0.1 μmol photons m−2 s−1 (setting 70), which gave a significant signal with cultures containing 10 μg chlorophyll a mL−1. The actinic light source was an incandescent light of 659 μmol photons m−2 s−1, and the saturating pulse exceeded 3000 μmol photons m−2 s−1. The F0, Fv/Fmax, and Fmax of all samples were determined after a 1-min dark adaptation; the ΔF/Fmax' was determined after the cells were exposed to actinic light for 10 min. When included in the assay, nigericin was used at a final concentration of 20 μm (equilibrated for 2 min in the dark).
PSII reaction centers were quantitated by measuring the light-induced A320 change, which is attributed to the reduction of QA (Guenther et al., 1990; Neale and Melis, 1990). This measurement estimates the number of PSII centers capable of a stable charge separation. The A320 change was measured with a split-beam spectrophotometer as previously described (Guenther et al., 1990). The number of PSII centers was also estimated by western-blot analysis using a monospecific antibody raised against spinach D1 that was previously shown to recognize D1 of C. reinhardtii (Guenther et al., 1988; Kim et al., 1993). Polypeptides that cross-reacted with the antibody were detected with peroxidase-linked anti-rabbit IgG using a chemiluminescence kit (Boehringer Mannheim), and the levels of cross-reacting polypeptides were quantified by densitometry.
Xanthophyll Pigment Analysis
Cells in 1 mL of culture were chilled in liquid N2 and pelleted by centrifugation at 16,000g for 30 s, and the pellet was immediately frozen in liquid N2 and kept at −80°C until analysis. The levels of zeaxanthin, antheraxanthin, violaxanthin, and lutein were determined as previously described (Niyogi et al., 1997).
Measurements of Fluorescence Induction
Thylakoid membranes were isolated (Neale and Melis, 1990), diluted to <20 μg chlorophyll mL−1, and placed in a 3-mL quartz cuvette. Ferricyanide was added to a concentration of 500 μm to prevent reduction of the PQ pool and to obtain the Fpl. The suspension was exposed to 1 s of green light at 50 μmol photons m−2 s−1, and the output of the photomultiplier tube was recorded by a digital voltmeter (model 3437A, Hewlett-Packard) in 2-ms intervals. F0 and Fpl were obtained from these data. To determine the Fmax value, DCMU was added to a final concentration of 20 μm and the measurement was repeated.
77 K Fluorescence Emission Spectra
Prior to measuring fluorescence emission spectra, cells were maintained in TAP, TAP-S, or TAP-P medium at 80 μmol photons m−2 s−1 from fluorescent tubes or were exposed to far-red light (λ > 714 nm, approximately 3 μmol photons m−2 s−1) for 20 min. Fluorescence emission spectra were determined with a single-beam fluorometer (PTI, New Brunswick, NJ), as previously described (Collier et al., 1994). Aliquots of cells containing 5 μg chlorophyll a mL−1 were loaded into a cuvette that was submerged in liquid N2 (77 K). The samples were excited at 435 nm (2.74 μmol photons m−2 s−1), and the fluorescence emission was measured between 650 and 750 nm; the fluorescence of samples containing no cells (just the appropriate growth medium) was subtracted.
RESULTS
Photosynthetic Activity Decreases during Nutrient Starvation
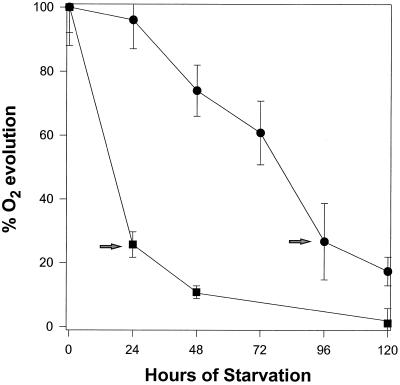
When C. reinhardtii was starved of P or S, there was a dramatic reduction in the level of photosynthesis. After 4 d of P starvation, the light-saturated rate of photosynthesis as measured by O2 evolution declined by approximately 75% compared with the rate measured in cells maintained in complete medium (Fig. 1; Table I). P-starved cells remained 100% viable for up to 7 d after P was eliminated from the medium (data not shown). C. reinhardtii cultures that were transferred to medium lacking S also showed a decline in the photosynthetic rate, but the decline was much more rapid. Twenty-four hours after the transfer of cells to medium lacking S, photosynthetic O2 evolution decreased by 76%, whereas after 48 h the photosynthetic rate had declined by nearly 90% (Fig. 1). There was no decrease in cell viability for at least 4 d following the initiation of S starvation (Davies et al., 1996). Furthermore, cells deprived of P for 4 d or S for 1 d exhibited no change in their chlorophyll a/b ratio and a 14 to 20% decrease in the level of chlorophyll per cell (Table I). We have chosen 4 d of P starvation and 1 d of S starvation for further characterizations because at these times the cells did not lose viability and showed a similar reduction in the rate of O2 evolution.
Figure 1.
The rate of O2 evolution in saturating light for P- (•) and S-deprived (▪) cells expressed as a percentage of nutrient-replete cells. The percentage of O2 evolution was calculated by dividing the rate of O2 evolution per cell in starved cultures by the rate in replete cultures. Each point represents the mean of at least five separately grown cultures and the bars indicate the ses. The arrows indicate times at which the cells were used for the remaining characterizations presented in this manuscript.
Table I.
Photosynthesis and chlorophyll levels in cells starved of S and P
| Condition | O2 Evolution | Chl Level | Chl a/Chl b | |
|---|---|---|---|---|
| μmol mg−1 Chl h−1 | μmol 109 cells−1 h−1 | pg cell−1 | ||
| TAP | 182.2 ± 15.8 | 1410 ± 120 | 7.71 ± 0.79 | 2.60 ± 0.08 |
| TAP-P | 53.9 ± 9.6 (29.6%) | 359 ± 64 (25.5%) | 6.66 ± 0.21 | 2.54 ± 0.11 |
| TAP-S | 56.4 ± 8.0 (31.0%) | 344 ± 49 (24.4%) | 6.10 ± 0.34 | 2.60 ± 0.23 |
All values were calculated from at least five separately grown cultures and ses are given. Values in parentheses are relative to unstarved cells. Chl, Chlorophyll.
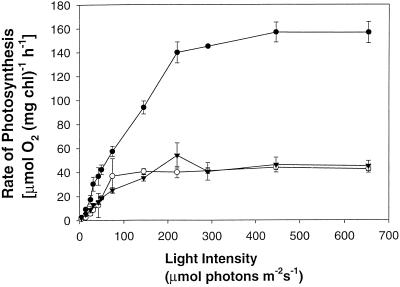
To determine the quantum yield of photosynthesis at subsaturating light intensities for cells deprived of P and S, we measured O2 evolution as a function of light intensity. Figure 2 shows the light-response curves of O2 evolution of cells either grown in complete medium or starved for P and S. Not only was the light-saturated rate of photosynthesis less, but the rate of increase in O2 evolution as a function of light intensity was also less in P- and S-starved cells compared with that of cells grown in nutrient-replete medium. Therefore, nutrient-deprived cells exhibited a reduced efficiency of light-energy utilization.
Figure 2.
Light-response curves of O2 evolution after S (▴) and P (○) starvation and in nutrient-replete (•) cells. Each point represents the mean of three measurements and the bars indicate the ses. This experiment was performed twice and the trends were identical.
PSII Is the Site of Inhibition in Photosynthetic Electron Transport during Nutrient Starvation
The decline in the light-saturated rate of in vivo O2 evolution that accompanies nutrient limitation could be an indirect effect caused by a decline in the activity of the reductive pentose-phosphate pathway, or it could be a direct effect on the rate of noncyclic electron flow. To distinguish between these possibilities, we determined the light-saturated rate of photosynthetic electron transport in crude cell membrane preparations in which photosynthetic electron transport was uncoupled from CO2 fixation and photophosphorylation (Table II; Curtis et al., 1975). Electron transport from water to MV for P- and S-starved cells was 30 to 40% of that of unstarved cells. This decline in electron-transport activity was similar to the decline in whole-cell O2 evolution (Table I) and the ΔF/Fmax' (the fluorescence measurement that reflects the rate of electron transport; Genty et al., 1989; Table III) observed in nutrient-deprived cells.
Table II.
Summary of partial reactions
| Partial Reaction | Electron Donor | Electron Acceptor | Ratea
|
|
|---|---|---|---|---|
| TAP-P | TAP-S | |||
| % | ||||
| In vivo O2 evolution | 30 ± 6 | 31 ± 4 | ||
| PSII and PSI | H2O | MV | 39 ± 3 | 37 ± 4 |
| PSII | H2O | Ferricyanide | 45 ± 13 | 56 ± 6 |
| PSI | DCPIPH2 | MV | 85 ± 12 | 135 ± 16 |
| PQ, Cyt b6-f, and PSI | DHQ | MV | 82 ± 17 | 88 ± 13 |
| PSII (after O2-evolving complex) | DPC | DCPIP | 55 ± 14 | 51 ± 3 |
Absolute values for partial reactions in unstarved cells (μmol O2 mg−1 Chl h−1) range from: −658 to −593 (H2O to MV), +304 to +377 (H2O to ferricyanide), −869 to −716 (DCPIPH2 to MV), and −1122 to −979 (DHQ to MV). DPC to DCPIP was measured photometrically. Values are the result of three independent experiments.
Percentage ± se of the absolute values of unstarved cells.
Table III.
PAM fluorescence characteristics
| Condition | Fluorescence Parametera
|
||||||
|---|---|---|---|---|---|---|---|
| Fmax | Fmax (+ N) | Fmax (+ FR) | Fmax (+ FR + N) | F0 (+ FR) | Fv/Fmax (+ FR) | ΔF/Fmax′b | |
| TAP | 206 | 211 | 210 | 212 | 55 | 0.738 | 0.459 |
| TAP-P | 172 | 183 | 187 | 198 | 75 | 0.599 (81%) | 0.151 (33%) |
| TAP-S | 156 | 184 | 201 | 209 | 88 | 0.562 (76%) | 0.107 (23%) |
| Sacl-S | 170 | 188 | 207 | 215 | 64 | 0.624 (84%) | 0.398 (87%) |
Measurements are the means of two independently grown cultures on equal amounts of chlorophyll. The difference between the mean of the measurements is less than 5%, except for the F0 and the ΔF/Fmax of replete-grown cells, which is 6%. Values in parentheses are relative to unstarved wild-type cells.
N, Nigericin; FR, far-red light.
During exposure to 659 μmol photons m−2 s−1.
Partial reactions of photosynthetic electron transport were performed to locate the site at which electron transport was inhibited. PSI activity, assayed as electron flow from DCPIPH2 (which donates electrons to plastocyanin) to MV, was approximately the same for unstarved and P- and S-starved cells (Table II). Furthermore, the rate of electron transfer from DHQ (which donates electrons to the PQ pool) to MV showed little change during nutrient limitation. These results suggested that nutrient limitation resulted in a decrease in linear electron transport prior to the PQ pool.
To measure PSII activity we used ferricyanide and p-benzoquinone. The electron flow from water to ferricyanide (Table II) or to p-benzoquinone (data not shown) was reduced by approximately 50% in starved cells. Electron transfer from DPC (which donates electrons to the PSII reaction center) to DCPIP (which accepts electrons from the PQ pool) was also inhibited by approximately 50%. These results demonstrated that S and P starvation caused a reduction in the rate of electron transfer between the site of DPC electron donation (TyrZ) and the PQ pool and could reflect a decline in the rate of electron transfer from QA to the PQ pool and/or a reduction in the total number of active PSII reaction centers.
Loss of Functional PSII Reaction Centers during Nutrient Starvation
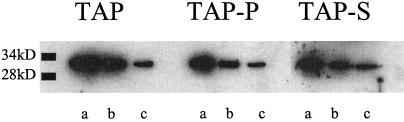
To examine changes in the maximum quantum yield of PSII that occurred during P and S starvation, we measured Fv/Fmax (Krause and Weis, 1991; Lavergne and Briantais, 1996; Table III). Limitation of either P or S resulted in a 20 to 25% decrease in the Fv/Fmax, suggesting that some of the PSII centers were damaged. However, the Fv/Fmax is a relative measure of the maximal PSII quantum yield and does not provide information about the absolute number of functional PSII reaction centers present in nutrient-limited cells. To determine the absolute number of functional PSII reaction centers, we spectrophotometrically measured the amount of QA that could be reduced (Table IV). Cells starved of either P or S exhibited a 30% decline in the amount of functional QA relative to unstarved cells. In addition, we quantitated the D1 protein content in thylakoid membrane preparations from starved and unstarved cells (Fig. 3). Quantitation from three separate preparations (one representative experiment is shown in Fig. 3) indicated that there was also a reduction in the absolute level of the D1 polypeptide by approximately 25% during nutrient limitation. Together, these results suggested that less than half of the decline in O2 evolution that occurred during S and P limitation reflected a loss of functional PSII reaction centers (centers devoid of the D1 protein).
Table IV.
Measurement of PSII centers using change of A320
| Condition | Chlorophyll | QA | |
|---|---|---|---|
| mol chlorophyll mol−1 QA | 105 cells−1 | % | |
| TAP | 417 ± 51 | 126 ± 14 | 100 |
| TAP-P | 513 ± 74 | 88.5 ± 15 | 70 |
| TAP-S | 471 ± 34 | 88.3 ± 6.9 | 70 |
Values are the means ± ses of three thylakoid membrane preparations.
Figure 3.
Western-blot analysis using D1 antibody against thylakoid membrane preparations from nutrient-replete (TAP), P-starved (TAP-P), and S-starved (TAP-S) cells. Three different concentrations of thylakoids (in micrograms of chlorophyll) were electrophoresed in 15% polyacrylamide-urea gels: a, 5.12; b, 2.56; and c, 1.28.
Accumulation of PSII Centers Unable to Reduce PQ during Nutrient Starvation
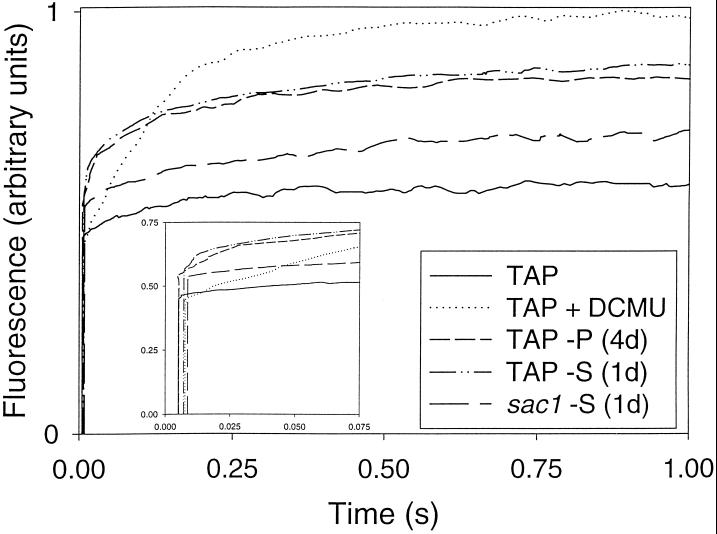
In addition to damage to PSII, reduced electron flow through PSII may occur as a consequence of the retardation of the transfer of electrons from the reaction center to the PQ pool. This can be assessed by the analysis of fluorescence-induction curves that provide information about the reduction state of QA, the primary quinone electron acceptor from P680 (Melis, 1991). When QA is reduced (i.e. in the presence of DCMU, which blocks the transfer of electrons from QA to QB), the fluorescence rapidly reaches a maximum. When QA is oxidized (i.e. in low light in the presence of electron acceptors), the fluorescence approaches F0. Under our illumination conditions, in the presence of DCMU, the variable fluorescence yield increases with characteristic biphasic kinetics (Melis, 1991; Fig. 4). In the absence of DCMU and in the presence of ferricyanide, the variable fluorescence yield gradually attained the intermediate Fpl level.
Figure 4.
Fluorescence induction upon illumination with 50 μmol photons m−2 s−1 of green light. Ferricyanide was added to the membrane preparation to oxidize all of the functional PSII reaction centers. The Fmax was determined in the presence of DCMU. These curves were normalized to Fmax = 100 units. The inset shows the F0 of each curve. The F0 values are 0.46, 0.44, 0.55, 0.56, 0.53 for TAP, TAP plus DCMU, TAP-P, TAP-S, and sac1-TAP-S, respectively. The data presented are from one experiment, but numerous replicates gave nearly identical results.
The increase from F0 to Fpl in the presence of ferricyanide is attributed to the reduction of QA present in a small pool of PSII reaction centers (Govindjee, 1990; Melis, 1991). Such centers, termed PSII QB-nonreducing centers (Chylla and Whitmarsh, 1989), oxidize QA− at a rate that is approximately 1000 times slower than that of QB-reducing centers (Ort and Whitmarsh, 1990) and are essentially nonproductive in O2 evolution (Melis, 1991). In both S- and P-starved cells, the Fpl was significantly higher than in unstarved cells, indicating an increase in the level of QB-nonreducing centers under both conditions. Because the thylakoid membranes used in these experiments were washed and the assays were performed in the presence of ferricyanide, the apparent increase in QB-nonreducing centers in nutrient-deprived cells was not a consequence of partial PQ reduction by cellular metabolites.
Calculations of (Fpl − F0)/(Fmax − F0) allowed for an estimate of the percentage of PSII reaction centers that are QB nonreducing. As shown in Table V, during nutrient-replete growth these centers made up approximately 29% of all PSII reaction centers. In thylakoid preparations from P- and S-starved cells the (Fpl − F0)/(Fmax − F0) was greater by 83 and 65%, respectively, than for thylakoid preparations from unstarved cells.
Table V.
Accumulation of PSII QB-nonreducing centers
| Condition | (Fpl − F0)/(Fmax − F0) (n)a | Increase in Fpl |
|---|---|---|
| %b | ||
| CC125 (wild type) | ||
| TAP | 0.298 ± 0.013 (4) | |
| TAP-P | 0.546 ± 0.059 (4) | 83 ± 26 |
| TAP-S | 0.492 ± 0.033 (3) | 65 ± 19 |
| sacl mutant | ||
| TAP | 0.285 ± 0.027 (3) | |
| TAP-S | 0.335 ± 0.017 (3) | 18 ± 17 |
| TAP-P | 0.574 ± 0.066 (2) | 101 ± 47 |
| Complemented sacl mutant | ||
| TAP | 0.253 ± 0.015 (3) | |
| TAP-S | 0.496 ± 0.015 (3) | 96 ± 19 |
Values are means ± se.
Number of independent thylakoid preperations measured.
Relative to unstarved cells.
Light Energy Is Directed Away from PSII during Nutrient Starvation
The decrease in quantum yield of O2 evolution at subsaturating light levels suggested that less of the absorbed light energy was being directed to functional PSII centers. Previously, the decrease in quantum yield of O2 evolution at subsaturating light levels was correlated with a decline in Fv/Fmax (Krause and Weis, 1991); however, a decline in the slope of the light-saturation curves during nutrient starvation (Fig. 2) can result from any process that causes the dissipation of excitation energy before it reaches the PSII reaction center.
Utilization of absorbed light energy was examined in more detail via measurements of chlorophyll fluorescence. Room-temperature chlorophyll fluorescence, primarily a measure of fluorescence from the chlorophyll a of LHCII (Krause and Weis, 1991), was analyzed by PAM fluorometry (Schreiber et al., 1986). Fluorescence measurements on light-grown cells containing equal amounts of chlorophyll demonstrated that the Fmax of P- and S-starved cells was 83 and 76%, respectively, of cells grown in complete medium (Table III). This reduction in the Fmax suggested that more of the light energy absorbed by LHCII of nutrient-deprived cells relative to unstarved cells was (a) dissipated as heat within the LHCII (quenching of fluorescence of singlet excited chlorophyll, qE); (b) dissipated as a consequence of the formation of damaged PSII reaction centers (qI); and/or (c) directed away from PSII because of a state transition (transition from state 1 to state 2, qT).
Experiments were performed to determine the role of qE and qT in the depression of Fmax in nutrient-deprived cultures (maintained at 80 μmol photons m−2 s−1). Nigericin, which dissipates the ΔpH necessary for maintaining qE, had no effect on the Fmax of cells maintained in complete medium. In contrast, the addition of nigericin to P- and S-starved cells caused a 6 and 13% increase in the Fmax, respectively (Table III). Both P- and S-starved cells treated with nigericin had an Fmax that was 89% of the value determined for unstarved cells. Therefore, at least part of the reduction in Fmax (between 30 and 50%) observed for nutrient-deprived cells appeared to result from qE.
Previous work correlated the accumulation of de-epoxidated xanthophylls with the development of qE in nutrient-deprived photosynthetic organisms (Plesnicar et al., 1994; Braun et al., 1996; Niyogi et al., 1997). Using HPLC analysis (Table VI), we found that both P- and S-starved cells accumulated zeaxanthin (Z), antheraxanthin (A), and violaxanthin (V), the pigments of the xanthophyll cycle (Demmig-Adams and Adams, 1992; Björkman and Demmig-Adams, 1994), even during growth in relatively low light. Furthermore, the de-epoxidation state of the xanthophylls ([Z + 0.5A]/[Z + A + V]) also increased following exposure of the cells to P or S limitation, suggesting that the xanthophyll cycle and the dissipation of excess absorbed light energy as heat contributes to the Fmax depression observed in starved cells. In addition to the xanthophyll cycle pigments, nutrient-deprived cells accumulated lutein, which may also function in the dissipation of excess absorbed light energy (Niyogi et al., 1997).
Table VI.
HPLC analysis
| Condition | Violaxanthin | Antheraxanthin | Zeaxanthin | De-Epoxidation State | Lutein | Chlorophyll a | Chlorophyll b |
|---|---|---|---|---|---|---|---|
| TAP | 104 | 2.0 | 1.0 | 0.019 | 66 | 1000 | 370 |
| TAP-P | 142 | 37 | 42 | 0.27 | 236 | 1000 | 407 |
| TAP-S | 96 | 17 | 18 | 0.20 | 122 | 1000 | 374 |
| sacl TAP-S | 55 | 19 | 19 | 0.31 | 134 | 1000 | 296 |
All values are normalized to moles of chlorophyll a and are the means of two independent measurements. The deviation from the mean for all measurements was 10% or less.
Because the addition of nigericin to nutrient-deprived cells did not completely restore the Fmax to that of nutrient-replete cells (Table III), qE did not entirely explain the lower Fmax observed in P- and S-starved cells. Exposing P- and S-starved cells to 20 min of far-red light (far-red light preferentially excites PSI, oxidizes the PQ pool, and promotes a transition to state 1) also resulted in a substantial increase in the Fmax, indicating that the cells were originally in state 2 and that qT was also important during the acclimation of C. reinhardtii to nutrient limitation (Table III). Furthermore, the simultaneous addition of nigericin and exposure to far-red light brought the Fmax of S-starved cells to the level observed for nutrient-replete cells, suggesting that qE and qT were essentially the only processes contributing to the reduction in the Fmax.
In contrast, nigericin and far-red light treatments were not able to fully restore the Fmax in P-deprived cells, suggesting a contribution of qI. To further test whether nutrient-deprived cells were in states 1 or 2, we measured fluorescence emission from whole cells at 77 K, both before and after exposure of the cells to 20 min of far-red light. The relative ratio of PSII-associated fluorescence emission (684 nm) to PSI-associated fluorescence emission (714 nm) was less for P- and S-starved cells than for unstarved cells (Table VII). However, when the starved cells were exposed to 20 min of far-red light prior to the measurement, the relative fluorescence emission from PSII increased dramatically; far-red light treatment had only a small effect on fluorescence emission from unstarved cells. Furthermore, dark adaptation for 20 min or treatment with nigericin led to relatively small increases in the 684/714 ratio (10–30% change relative to that observed following far-red light treatment). Therefore, much of the change in the 684/714 ratio during nutrient starvation appears to be a consequence of a state transition, which is consistent with the increase in Fmax observed in P- and S-starved cultures after a 20-min exposure to far-red light (Table III). Together the results presented above indicate that cells starved of P or S are in state 2, while unstarved cells are primarily in state 1.
Table VII.
PSII fluorescence/PSI fluorescence emission measured at 77 K
| Condition | Fluorescence Emissiona
|
|
|---|---|---|
| Continuous lightb | 20-min far-red light | |
| TAP | 1.74 ± 0.12 | 2.04 ± 0.08 |
| TAP-P | 1.18 ± 0.04 | 1.69 ± 0.04 |
| TAP-S | 1.23 ± 0.03 | 2.01 ± 0.07 |
| Sacl TAP-S | 1.25 ± 0.06 | 1.77 ± 0.09 |
Values are means ± sd of at least three measurements.
684/714-nm emission.
80 μmol photons m−2 s−1.
Photosynthetic Parameters of the sac1 Mutant during S Limitation
Using the sac1 mutant of C. reinhardtii, we were able to determine which responses to S starvation were actively regulated by the SacI polypeptide. The rate of O2 evolution in the sac1 mutant does not decline significantly (based on the number of viable cells) after 24 h of S starvation (Davies et al., 1996), whereas photosynthesis in wild-type cells declines by more than 70%. Furthermore, the sac1 mutant showed little reduction in the ΔF/Fmax' (Table III) and in the light-driven transfer of electrons from water to MV (data not shown) following 24 h of S deprivation. Chlorophyll levels declined by 10% (data not shown), whereas the Fv/Fmax declined by 16% (Table III) in the sac1 strain during S starvation. However, more than 10% of the cells in the S-starved culture of sac1 were dead within 24 h of starvation, suggesting that the decline in the Fv/Fmax (and perhaps in chlorophyll levels) was not part of the same mechanism controlling the reduction of PSII activity in wild-type cells during S starvation.
Furthermore, unlike wild-type cells, the sac1 mutant did not accumulate PSII QB-nonreducing centers during S starvation (Table V; Fig. 4). This effect was specific for S deprivation (as expected), since an increase in (Fpl − F0)/(Fmax − F0) was observed when the sac1 mutant was starved for P (Table V). The complemented sac1 mutant strain (ars5–4 C11) exhibited an increase in (Fpl − F0)/(Fmax − F0) during S starvation that was similar to that of wild-type cells (Table V). Therefore, the accumulation of PSII QB-nonreducing centers is part of the S-acclimation response and is governed by the SacI gene product.
To determine whether the transition of light-grown cells to state 2 during S-limited growth is an active process also controlled by the SacI protein, we determined the state of the light-grown (80 μmol photons m−2 s−1), S-starved sac1 strain (Table VII). Like wild-type cells, the sac1 mutant was in state 2 during S starvation in the light and shifted to state 1 upon exposure to far-red light, demonstrating that the SacI protein is not required for this aspect of the S-stress response. In addition, the de-epoxidation state of the xanthophylls (Table VI) and nigericin-sensitive quenching of Fmax in the sac1 mutant during S starvation were approximately the same as in wild-type cells (Table III). The overall pool size of the xanthophyll cycle pigments were somewhat lower in the S-starved sac1 mutant than in S-starved wild-type cells.
DISCUSSION
This study demonstrates that P and S deprivation cause similar alterations to photosynthetic electron transport in C. reinhardtii, which exhibited a 75% decrease in maximal in vivo O2 evolution within 4 d of P deprivation or 1 d of S deprivation. The longer time required to observe the effects of P limitation may reflect the accumulation of much larger intracellular reserves of P than of S (Wetzel, 1983). The decline in photosynthetic O2 evolution was shown to occur at the level of photosynthetic electron transport using fluorescence measurements (ΔF/Fmax') and in vitro quantitation of the activities of specific sections of the electron-transport chain. The latter analyses demonstrated that electron flow was inhibited at PSII, whereas PSI activity was essentially unchanged in starved cells. The continued operation of PSI during nutrient-limited growth would provide energy for cell maintenance and for transporting the limiting nutrient into the cell when it becomes available.
The decreased maximal electron flow through PSII is a consequence of at least two processes. First, between 20 and 30% of the PSII reaction centers appear to be inactivated because of photodamage. Quantitation of photodamage is complicated by the inherent inaccuracies in quantitating protein levels by western analysis and the potential contribution of PSII QB-nonreducing centers to the loss of Fv (which can result from an apparent increase in the F0 value). However, analyses of fluorescence using a very low-intensity measuring beam (<0.01 μmol photons m−2 s−1) had little effect on either F0 or Fv/Fmax (<5%), and the measurements of QA levels (Table IV) demonstrated an absolute loss of functional PSII (by approximately 30%). These results indicate that nutrient limitation results in a loss of active PSII centers.
Nutrient limitation also promoted the formation of PSII QB-nonreducing centers, which cannot rapidly transfer electrons from QA− to QB (Chylla and Whitmarsh, 1989). Others have demonstrated an increase in the percentage of PSII QB-nonreducing centers during nutrient limitation or exposure to excessive light (Falk et al., 1992; Godde and Hefer, 1994). These centers may result from the reduction and subsequent loss of the quinone from the QB-binding site (Godde and Hefer, 1994), although it is possible that other alterations of PSII may also result in centers that are unable to reduce QB. It has been postulated that PSII QB-nonreducing centers serve as intermediates in the D1 repair cycle and constitute a PSII reservoir that can be rapidly activated (Guenther and Melis, 1990). Furthermore, these centers are resistant to photoinhibition and may facilitate the dissipation of excess absorbed light energy (Neale and Melis, 1990; Falk and Samuelsson, 1992). Therefore, increased photodamage and increased formation of QB-nonreducing centers in cells deprived of S or P may be directly linked.
There is still little mechanistic understanding of the modifications to the photosynthetic machinery that occur during nutrient-limited growth. In previous reports it was suggested that P deprivation caused a decline in the levels of reductive pentose-phosphate cycle intermediates, which led to decreased levels of terminal energy acceptors and consequently depressed photosynthetic electron-transport activity (Brooks, 1986; Dietz and Heilos, 1990; Jacob and Lawlor, 1993). The accumulation of specific reductive pentose-phosphate cycle metabolites may also serve to inhibit electron transport, which is consistent with the finding that the rate of electron flow from water to MV in vitro was unaffected by P starvation in spinach (Brooks, 1986).
In addition, some studies have shown a direct effect of nutrient deprivation on photosynthetic electron transport. Both C. reinhardtii and Hematococcus pluvialis exhibit a significant decrease in the level of the Cyt b6-f complex as the cells become starved for specific nutrients (Peltier and Schmidt, 1991; Bulte and Wollman, 1992; Tan et al., 1995). During N deprivation vegetative cells differentiate into gametes and the loss of the Cyt b6-f complex parallels the reduction in O2 evolution. Other studies have demonstrated that nutrient limitation can directly affect PSII activity (Herzig and Falkowski, 1989; Collier et al., 1994; Godde and Dannehl, 1994), which is consistent with the results presented in this manuscript.
In addition to a reduction in the rate of light-saturated photosynthetic electron flow, nutrient limitation caused a decline in the quantum yield of O2 evolution at subsaturating light levels. Such a decline can result from a less- efficient transfer of excitation energy to the PSII reaction centers. Mechanistically, the decrease in the quantum yield of O2 evolution at subsaturating light could be a consequence of nonphotochemical quenching, which is composed of qE, qI, and qT. A nigericin-induced increase in Fmax and increased levels of xanthophyll cycle pigments in nutrient-deprived cells suggested that qE was at least partially responsible for the decrease in the quantum yield of O2 evolution. However, the observation that nutrient-deprived cells were primarily in state 2, and cells maintained on complete medium were primarily in state 1, demonstrated a role for qT as well. Although probably less important than qE or qT, qI may become important to some extent during P deprivation and in more severely starved cells. Cultures starved of P for 4 d were unable to return to state 1 as readily as cultures starved of S for 1 d, suggesting that P limitation causes alterations in the structure of LHCII (or PSII) that are not readily reversible by far-red illumination.
The reduction in photosynthetic electron flow that develops during nutrient limitation of C. reinhardtii cells is an active process and is necessary for survival. During S deprivation the reduction in photosynthetic electron flow appears to be controlled by the SacI gene product, which may be involved in sensing the S status of the environment (Davies et al., 1996). A sac1 mutant strain becomes light sensitive during S deprivation because it cannot alter photosynthetic electron transport. S starvation of sac1, as in wild-type cells, results in the induction of both qE and qT. These processes may be triggered by metabolic changes in nutrient-starved cells that are independent of the SacI signal-transduction pathway. However, in contrast to wild-type cells, the level of damaged PSII centers in the sac1 mutant reflects cell death and the mutant strain is unable to form PSII QB-nonreducing centers. DCMU, which phenocopies the formation of QB-nonreducing centers, rescues the lethal phenotype (Davies et al., 1996). This suggests that either the formation of O2 radicals generated by photosynthetic electron transport or the hyperreduction of electron transport components downstream of the QB-binding site, or both, leads to a loss of viability in the sac1 mutant.
In summation, similar alterations in photosynthetic electron transport occur during both P and S starvation. First, the levels of chlorophyll a and b decline by approximately 15%. Second, there is a 20 to 30% decrease in functional PSII reaction centers. Third, fewer of the functional centers can rapidly reduce the PQ pool. Fourth, more of the light harvested by LHCII is dissipated as heat or directed away from PSII reaction centers, possibly toward PSI, via a transition to state 2. These alterations result in a decline in linear electron flow and a reduction in the efficiency of energy transfer to PSII, while possibly allowing PSI cyclic electron flow to continue. If nutrient-starved cells maintain PSI cyclic electron flow with little PSII activity, the cells would produce less reductant (which is no longer required at high levels) but would continue to generate a ΔpH across the thylakoid membrane, allowing for significant nonphotochemical quenching and the production of ATP for the maintenance of vital cellular processes.
ACKNOWLEDGMENTS
We thank Krishna Niyogi and Klaas van Wijk for critically reading the manuscript.
Abbreviations:
- DBMIB
2,5-dibromo-3-methyl-6-isopropyl-1,4-benzoquinone
- DCPIP
2,6-dichlorophenol-indo-phenol
- DCPIPH2
DCPIP (reduced form)
- ΔF
Fmax', steady-state fluorescence during actinic light
- DHQ
durohydroquinone
- DPC
sym-diphenylcarbazide
- ferricyanide
potassium ferricyanide
- Fmax
maximal fluorescence when QA is fully reduced
- Fmax'
Fmax during exposure to actinic light
- Fpl
fluorescence plateau in the presence of ferricyanide
- F0
initial fluorescence when QA is fully oxidized
- Fv
variable fluorescence (Fmax − F0)
- LHC
light-harvesting complex
- MV
methyl viologen
- PAM
pulse-amplitude-modulated
- PQ
plastoquinone
- qE
energy-dependent chlorophyll fluorescence quenching
- qI
chlorophyll fluorescence quenching due to photodamage
- qT
chlorophyll fluorescence quenching due to a state transition
- TAP
Tris-acetate-P
- TAP-P
TAP without P
- TAP-S
TAP without S
Footnotes
D.D.W. was supported as a predoctoral trainee by the National Institutes of Health (grant no. GM07276). This work was also supported by the Carnegie Institution of Washington, the U.S. Department of Agriculture (grant no. 9302076), and the National Science Foundation (grant no. IBN950-6254). This is Carnegie Institution of Washington Department of Plant Biology publication no. 1327.
LITERATURE CITED
- Allen JF. Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta. 1992;1098:275–335. doi: 10.1016/s0005-2728(09)91014-3. [DOI] [PubMed] [Google Scholar]
- Asada K. Production and action of active oxygen species in photosynthetic tissues. In: Foyer Mullineaux PM, editor. Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. Boca Raton, FL: CRC Press; 1994. pp. 77–104. [Google Scholar]
- Ball SG, Dirick L, Decq A, Martiat J-C, Matagne RF. Physiology of starch storage in the monocellular alga Chlamydomonas reinhardtii. Plant Sci. 1990;66:1–9. [Google Scholar]
- Björkman O, Demmig-Adams B. Regulation of photosynthetic light energy capture, conversion, and dissipation in leaves of higher plants. In: Schulze E-D, Caldwell MM, editors. Ecophysiology of Photosynthesis. Berlin: Springer-Verlag; 1994. pp. 17–47. [Google Scholar]
- Braun P, Banet G, Tal T, Malkin S, Zamir A. Possible role of Cbr, an algal early-light-induced protein, in nonphotochemical quenching of chlorophyll fluorescence. Plant Physiol. 1996;110:1405–1411. doi: 10.1104/pp.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A. Effects of phosphorus nutrition on ribulose-1,5-bisphosphate carboxylase activation, photosynthetic quantum yield and amounts of some Calvin-cycle metabolites in spinach leaves. Aust J Plant Physiol. 1986;13:221–237. [Google Scholar]
- Bulte L, Wollman FA. Evidence for a selective destabilization of an integral membrane protein, the cytochrome b6/f complex, during gametogenesis in Chlamydomonas reinhardtii. Eur J Biochem. 1992;204:327–336. doi: 10.1111/j.1432-1033.1992.tb16641.x. [DOI] [PubMed] [Google Scholar]
- Chylla RA, Whitmarsh J. Inactive photosystem II complexes in leaves. Plant Physiol. 1989;90:765–772. doi: 10.1104/pp.90.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier JL, Herbert SK, Fork DC, Grossman AR. Changes in the cyanobacterial photosynthetic apparatus in response to macronutrient deprivation. Photosynth Res. 1994;42:173–183. doi: 10.1007/BF00018260. [DOI] [PubMed] [Google Scholar]
- Curtis VA, Brand JJ, Togasaki RK. Partial reactions of photosynthesis in briefly sonicated Chlamydomonas. Plant Physiol. 1975;55:183–186. doi: 10.1104/pp.55.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JP, Yildiz F, Grossman AR. Mutants of Chlamydomonas with aberrant responses to sulfur deprivation. Plant Cell. 1994;6:53–63. doi: 10.1105/tpc.6.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JP, Yildiz FH, Grossman AR. Sac1, a putative regulator that is critical for survival of Chlamydomonas reinhardtii during sulfur deprivation. EMBO J. 1996;15:2150–2159. [PMC free article] [PubMed] [Google Scholar]
- de Hostos EL, Schilling J, Grossman AR. Structure and expression of the gene encoding the periplasmic arylsulfatase of Chlamydomonas reinhardtii. Mol Gen Genet. 1989;218:229–239. doi: 10.1007/BF00331273. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW., III Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. [Google Scholar]
- Dietz K-J, Heilos L. Carbon metabolism in spinach leaves as affected by leaf age and phosphorus and sulfur nutrition. Plant Physiol. 1990;93:1219–1225. doi: 10.1104/pp.93.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk S, Leverenz JW, Samuelsson G, Oquist G. Changes in photosystem II fluorescence in Chlamydomonas reinhardtii exposed to increasing levels of irradiance in relationship to the photosynthetic response to light. Photosynth Res. 1992;31:31–40. doi: 10.1007/BF00049534. [DOI] [PubMed] [Google Scholar]
- Falk S, Samuelsson G. Recovery of photosynthesis and photosystem II fluorescence in Chlamydomonas reinhardtii after exposure to three levels of high light. Physiol Plant. 1992;85:61–68. [Google Scholar]
- Ferreira RMB, Teixeira ARN. Sulfur starvation in Lemna leads to degradation of ribulose-bisphosphate carboxylase without plant death. J Biol Chem. 1992;267:7253–7257. [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Godde D, Dannehl H. Stress-induced chlorosis and increase in D1-protein turnover precede photoinhibition in spinach suffering under magnesium/sulphur deficiency. Planta. 1994;195:291–300. [Google Scholar]
- Godde D, Hefer M. Photoinhibition and light-dependent turnover of the D1 reaction centre polypeptide of photosystem II are enhanced by mineral-stress conditions. Planta. 1994;193:290–299. [Google Scholar]
- Gorman DS, Levine RP. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1966;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindjee Photosystem-II heterogeneity—the acceptor side. Photosynth Res. 1990;25:151–160. doi: 10.1007/BF00033157. [DOI] [PubMed] [Google Scholar]
- Guenther JE, Melis A. The physiological significance of photosystem II heterogeneity in chloroplasts. Photosynth Res. 1990;23:105–109. doi: 10.1007/BF00030070. [DOI] [PubMed] [Google Scholar]
- Guenther JE, Nemson JA, Melis A. Photosystem stoichiometry and chlorophyll antenna size in Dunaliella salina (green algae) Biochim Biophys Acta. 1988;934:108–117. [Google Scholar]
- Guenther JE, Nemson JA, Melis A (1990) Development of photosystem II in dark grown Chlamydomonas reinhardtii. A light-dependent conversion of PSIIβ, QB-nonreducing centers to the PSIIα, QB-reducing form. Photosynth Res 24: 35–46 [DOI] [PubMed]
- Herzig R, Falkowski PG. Nitrogen limitation in Isochrysis galbana (haptophyceae). I. Photosynthetic energy conversion and growth efficiencies. J Phycol. 1989;25:462–471. [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants: indication by nonphotochemical quenching of chlorophyll fluorescence. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Houle D, Cargnan R. Sulfur speciation and distribution in soils and above ground biomass of a boreal coniferous forest. Biogeochemistry. 1992;16:63–82. [Google Scholar]
- Jacob J, Lawlor DW. In vivo photosynthetic electron transport does not limit photosynthetic capacity in phosphate-deficient sunflower and maize leaves. Plant Cell Environ. 1993;16:785–795. [Google Scholar]
- Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167: 191–194
- Kim JH, Nemson JA, Melis A. Photosystem II reaction center damage and repair in Dunaliella salina (green alga) Plant Physiol. 1993;103:181–189. doi: 10.1104/pp.103.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:313–349. [Google Scholar]
- Lavergne J, Briantais JM. Photosystem II heterogeneity. In: Ort DR, Yocum CF, editors. Oxygeneic Photosynthesis: The Light Reactions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 265–287. [Google Scholar]
- Lers A, Levy H, Zamir A. Co-regulation of a gene homologous to early light-induced genes in higher plants and β-carotene biosynthesis in the alga Dunaliella bardawil. J Biol Chem. 1991;266:13698–13705. [PubMed] [Google Scholar]
- Levy H, Tal T, Shaish A, Zamir A. Cbr, an algal homolog of plant early light-induced proteins, is a putative zeaxanthin binding protein. J Biol Chem. 1993;268:20892–20896. [PubMed] [Google Scholar]
- Mahler RJ, Maples RL. Effect of sulfur additions on soil and the nutrition of wheat. Commun Soil Sci Plant Anal. 1987;18:653–673. [Google Scholar]
- Melis A. Dynamics of photosynthetic membrane composition and function. Biochim Biophys Acta. 1991;1058:87–106. [Google Scholar]
- Neale PJ, Melis A. Activation of a reserve pool of photosystem II in Chlamydomonas reinhardtii counteracts photoinhibition. Plant Physiol. 1990;92:1196–1204. doi: 10.1104/pp.92.4.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Björkman O, Grossman AR. Chlamydomonas xanthophyll cycle mutants identified by video imaging of chlorophyll fluorescence quenching. Plant Cell. 1997;9:1369–1380. doi: 10.1105/tpc.9.8.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Whitmarsh J. Inactive photosystem II centers. Photosynth Res. 1990;23:101–104. doi: 10.1007/BF00030069. [DOI] [PubMed] [Google Scholar]
- Peltier G, Schmidt GW. Chlororespiration: an adaptation to nitrogen deficiency in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1991;88:4791–4795. doi: 10.1073/pnas.88.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesnicar M, Kastori R, Petrovic N, Pankovic D. Photosynthesis and chlorophyll fluorescence in sunflower (Helianthus annuus L.) leaves as affected by phosphorus nutrition. J Exp Bot. 1994;45:919–924. [Google Scholar]
- Quisel JQ, Wykoff DD, Grossman AR. Biochemical characterization of the extracellular phosphatases produced by phosphorus-deprived Chlamydomonas reinhardtii. Plant Physiol. 1996;111:839–848. doi: 10.1104/pp.111.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich JW, Russell AE, Crews TE, Farrington H, Vitousek PM. Both nitrogen and phosphorus limit plant production on young Hawaiian lava flows. Biogeochemistry. 1996;32:1–14. [Google Scholar]
- Samosir SSR, Blair GJ, Lefroy RDB. Effects of placement of elemental S and sulfate on the growth of two rice varieties under flooded conditions. Aust J Agric Res. 1993;44:1775–1788. [Google Scholar]
- Schreiber U, Schliwa U, Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res. 1986;10:51–62. doi: 10.1007/BF00024185. [DOI] [PubMed] [Google Scholar]
- Stanko-Golden KM, Fitzgerald JW. Sulfur transformations and pool sizes in tropical forest soils. Soil Biol Biochem. 1991;23:1053–1058. [Google Scholar]
- Tan S, Cunningham FX, Youmans M, Grabowski B, Sun Z, Gantt E. Cytochrome f loss in astaxanthin-accumulating red cells of Haematococcus pluvialis (chlorophyceae): comparison of photosynthetic activity, photosynthetic enzymes, and thylakoid membrane polypeptides in red and green cells. J Phycol. 1995;31:897–905. [Google Scholar]
- van Kooten O, Snel JFH. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- Vernon LP, Shaw ER. Photoreduction of 2,6-dichlorophenolindophenol by diphenylcarbazide. A photosystem 2 reaction catalyzed by tris-washed chloroplasts and subchloroplast fragments. Plant Physiol. 1969;44:1645–1649. doi: 10.1104/pp.44.11.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warman PR, Sampson HG. Effect of sulfur additions on the yield and elemental composition of canola and spring wheat. J Plant Nutr. 1994;17:1817–1825. [Google Scholar]
- Wetzel RG (1983) Limnology. Saunders College Publishing, Fort Worth, TX
- White CC, Chain RK, Malkin R. Duroquinol as an electron donor for chloroplast electron transfer reactions. Biochim Biophys Acta. 1978;502:127–137. doi: 10.1016/0005-2728(78)90137-8. [DOI] [PubMed] [Google Scholar]
- Yildiz FH, Davies JP, Grossman AR. Characterization of sulfate transport in Chlamydomonas reinhardtii during sulfur-limited and sulfur-sufficient growth. Plant Physiol. 1994;104:981–987. doi: 10.1104/pp.104.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Davies JP, Grossman AR. Sulfur availability and the SAC1 gene control adenosine triphosphate sulfurylase gene expression in Chlamydomonas reinhardtii. Plant Physiol. 1996;112:669–675. doi: 10.1104/pp.112.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]