Abstract
Connexins (Cxs), a conserved family of trans-membrane proteins, function in the organization of cell-cell communicatin via gap junctions in multicellular organisms. However, the role of Cxs in acute myeloid leukemia (AML) is poorly understood. In this study, we investigated the relationship between cell proliferation and expression of connexin 43 (Cx43) and connexin 32 (Cx32) mRNA and proteins in acute myeloid leukemia in vitro. Proliferation was observed using a growth curve and the rate of proliferation was detected by MTT assay in the acute myeloid leukemia cell lines OCI-AML3 and OCIM2. Cell cycle and cell proliferation index were assessed by flow cytometry analysis. The mRNA expression of the gap junction genes Cx43 and Cx32 was detected by RT-PCR. The expression of Cx43 and Cx32 proteins in the cell lines was analyzed by western blot analysis and immunofluorescence. The doubling time of OCI-AML3 and OCIM2 was 48 h and 36 h, respectively. In OCIM2, the percentage of cells in the S phase fraction was 59.47±9.6%, and the proliferation rate was 78.12±8.9%; however, in OCI-AML3, the percentage of cells in the S phase was 24.95±5.8%, and the proliferation rate was 35.21±6.7%. At the mRNA level, both cell lines expressed Cx43 and Cx32, and there was no significant difference in the expression of Cx43 and Cx32 mRNA in the two cell lines. At the protein level, there was a significant difference in the expression of Cx43, but not of Cx32. The proliferation ability of OCIM2 was higher than OCI-AML3, and OCIM2 exhibited higher Cx43 western blot and fluorescence intensities compared with OCI-AML3. The results suggest that a higher expression of Cx43 in AML cells may play a significant role in the proliferation ability.
Keywords: connexins, acute myeloid leukemia, proliferation, expression
Introduction
Acute myeloid leukemia (AML) is the most common type of hematological malignancy characterized by key properties, including blocked differentiation, enhanced self-renewal and increased proliferation. Although there have been great developments in the understanding and treatment of AML, the mortality of AML is still high. Due to the difficulties in tolerance of treatment, the molecular mechanisms of uncontrolled proliferation of AML have been a major research project all over the world.
Gap junctions consist of arrays of intercellular channels composed of two hemichannels or connexons, one of which is formed by six protein subunits, termed connexins (Cxs). Cxs are a conserved family of transmembrane proteins which regulate the passage of biological molecules and allow the exchange of signaling molecules smaller than 1 kDa between the cytoplasm of two neighboring cells, e.g. Ca2+, cyclic adenosine monophosphate (cAMP) and inositol triphosphate (IP3) (1–3). To date, at least 21 different human Cxs have been identified and they have been divided into two groups based on the primary amino acid sequence homology (3). It suggests that the dysregulation of Cx expression is related to uncontrolled proliferation, embryogenesis, tissue homeostasis and carcinogenesis (4). Recently, abnormal or defective gap junction communication in various solid tumors including liver, bladder, breast and prostate cancers was found (5), and accumulating research has also shown that restoring Cx gene expression and gap junction by gene therapy in Cx-deficient tumor cells could decrease tumor cell growth. However, despite much existing evidence, the exact contribution of the Cx channel family still remains controversial, as gap junction and cxs may furnish cell survival signals. The gap junction and cxs could exert their effect on promoting cancer cell proliferation (6,7). Each Cx shows tissue cell-specific expression, and Cx43 is the major component of hematopoietic tissue (8–11). Cx32 was also found to be important in bone marrow stromal cells (12,13). Until now, there have been few studies focusing on the expression of the gap junction genes in the AML cell lines, and little is known regarding the correlation between cell proliferation and Cxs. In this study, the AML cell lines OCI-AML3 and OCIM2 were employed to investigate the expression of Cx32 and Cx43 in AML and their role in proliferation.
Materials and methods
Cell culture
The OCI-AML3 and OCIM2 were kindly provided by M.D. Minden (Ontario Cancer Institute, Toronto, ON, Canada). OCI/AML3 (FAB M4) was established from a patient with AML, and OCIM2 (FAB M6) from a patient with erythroleukemia. The cells were grown in RPMI-1640 supplemented 10% fetal bovine serum (FCS). Cultures were maintained in a humidified atmosphere with 5% CO2 at 37˚C.
Chemicals and antibodies
Thiazolyl blue tetrazolium bromide (MTT), dimethysulfoxide (DMSO), prodidum iodide (PI) and Hoechst 33258 were bought from Sigma (Sigma-Aldrich, St. Louis, MO, USA). Polyclonal rabbit antibody against Cx43 and Cx32 were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA) and PTG (Chicago, IL, USA). γ-tubulin was bought from Jackson ImmunoResearch (Jackson, WY, USA).
Cell growth curve
The OCI-AML3 and OCIM2 cells were seeded in 24-well plates (2x104 per well), cultured in a humidified atmosphere with 5% CO2 at 37˚C. The cells were harvested and counted on days 1–4, three wells each time and three times each well, to obtain an average to draw the growth curve.
Cell proliferation assay
The proliferative rate of OCI-AML3 and OCIM2 was determined by MTT assay following the method of Mosmann (14). The OCI-AML3 and OCIM2 cells (1x104 per well) were cultured for 24, 48 and 72 h in 96-well plates. Thereafter, 20 μl MTT solution was added to each well. After continued incubation for 4 h, the supernatant was discarded and 150 μl DMSO was added. Once the blue crystals were dissolved, the optical density (OD) was measured at 490 nm with background substraction of 630 nm using a plate microreader (Tecan Spectra, Wetzlar, Germany). The experiments were performed in triplicate. The proliferation rate was determined using the following formula: Cell proliferation (%) = OD of the experimental samples / OD of the control x 100% (n=3, mean ± SD).
Cell cycle analysis
The OCI-AML3 and OCIM2 (1x106 cells) were harvested. After being washed, the cells were fixed with 75% ice-cold ethanol and maintained overnight at 4˚C. The cells were collected and resuspended in PBS containing 40 μg/ml PI, 0.1 mg/ml RNase, and 5% Triton X-100, and then incubated at 37˚C for 30 min. The cells were evaluated by flow cytometry (FCM) using FACS (BD, San Diego, CA, USA). At least 10,000 counts were made for each sample. The percentage distribution in the cell cycle phases was analyzed using CellQuest. The proliferation index (PI) was determined using the following formula: PI = (S + G2/M) / (G0/G1 + S + G2/M).
Semi-quantitative reverse transcription-PCR (RT-PCR)
Total RNA was extracted from OCI-AML3 and OCIM2 cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Following quantification by spectrophotometry, the first-strand cDNA was synthesized from 2 μg of total RNA with the RevertAid First-Strand cDNA Sythesis kit. Cx43: forward 5′-TCGCCTATGTCTCCTCCTGG-3′, reverse 5′-GCTGGCTCTGCTTGAAGGTC-3′, with a PCR product of 270 bp; Cx32: forward 5′-AAATGCTACGGCTTGA GGGC-3′, reverse 5′-CGGAACACCACGCTGATGAC-3′, which can amplify a 115 bp fragment; β-actin: forward 5′-G CG G GA A ATCGTG CGTGACAT TA-3′, reverse 5′-GACTCGTCATACTCCTGCTTGCTGAT-3′, with an expected PCR product of 480 bp. The products were electrophoresesed on 2% agarose gel and the ratio between the target gene and β-actin gene band density was used for quantitative evaluation.
Western blot analysis
For preparation of total cell lysates, cells were collected and lysed in lysis buffer (50 mM HEPES, 150 mM NaCl, 1% Triton X-100, 5 mM EGTA, 50 mM glycerophosphate, 20 mM NaF, 1 mM Na3VO4, 2 mM phenylmethyl sulfonyl fluoride, 10 μg/ml leupeptin and 10 μg/ml aprotinin) by incubating on ice for 30 min. Lysates were then centrifuged at 12,000 x g for 15 min at 4˚C. The supernatant was collected and the total protein concentrations were determined using the BCA assay by spectrophotometer (Biotech Instruments, NY, USA). Samples were separated on 10% SDS-PAGE and transferred onto nitrocellulose membranes. After blocking with 5% non-fat dry milk in blocking buffer (25 mM Tris, pH 7.5, 150 mM NaCl, and 0.1% Tween-20), the membranes were incubated with primary antibodies at 4˚C overnight. The membranes were then incubated with appropriate peroxidaseconjugated secondary antibodies, and the protein expression was detected by ECL substrate solution (Thermo Scientific, Rockford, IL, USA). Densitometric analysis was performed using Quantity One software.
Fluorescent immunostaining
OCI-AML3 and OCIM2 cells were collected and washed with PBS and then fixed with 40 mg/l paraformaldehyde for 10 min before deposition on polylysine-coated coverslips. After washing, samples were blocked with 10% goat serum albumin for 30 min. The cells were reacted overnight at 4˚C with a drop of 1:100 diluted Cx43 antibody, then washed and incubated with a drop of 1:500 diluted Cy3-labeled goat anti-rabbit IgG (Sigma, USA) for 1 h. After that, cells were stained with Hoechst 33258 for 30 min at 37˚C. Finally, the slides were mounted with 50% glycerol and observed by Olympus BH-2 fluorescence microscope (Tokyo, Japan).
Statistical analysis
The statistical significance of difference between control and treatment groups was determined by the Student’s t-test. Values are shown as the mean ± SD, and P<0.05 was considered to indicate a statistically significant difference.
Results
Growth curve and proliferation rate in AML cell lines OCI-AML3 and OCIM2
The OCI-AML3 and OCIM2 cells were seeded and cultured for 1–4 days, and cells were harvested and counted each day. According to the growth curve, as shown in Fig. 1A, the doubling time of OCI-AML3 and OCIM2 was 48 and 36 h respectively, and OCIM2 grew faster than OCI-AML3. To further confirm the proliferation rate of OCI-AML3 and OCIM2, the cells were seeded and cultured for 24, 48 and 72 h and detected by MTT. As shown in Fig. 2, the proliferation rate of OCIM2 was nearly twice that of OCI-AML3.
Figure 1.
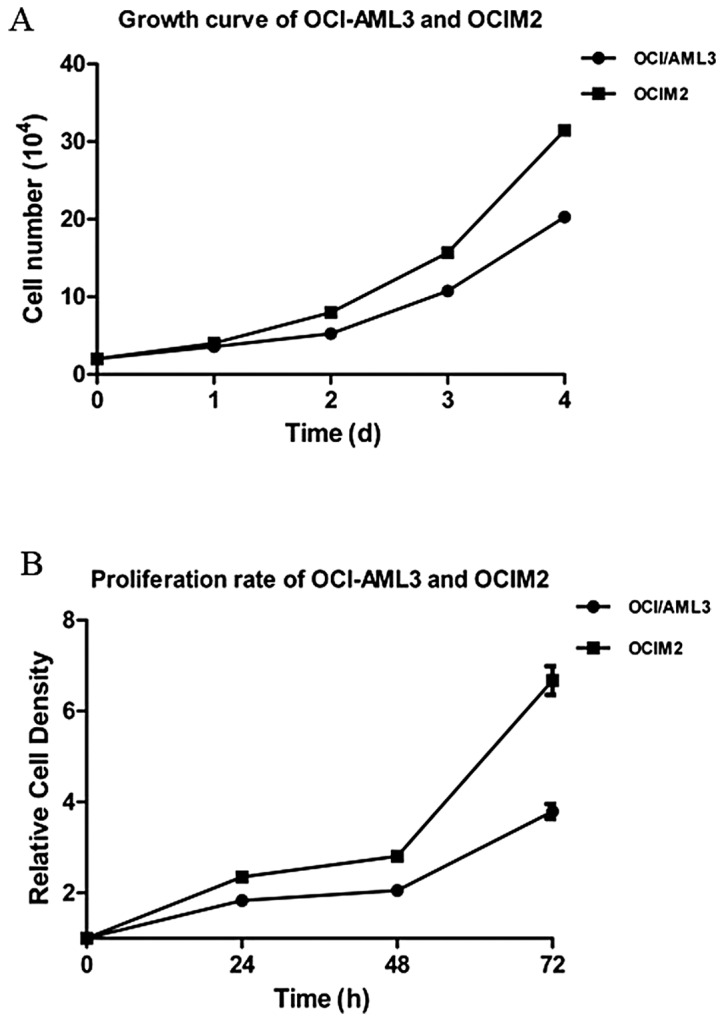
Assessment of proliferation rate of OCI-AML3 and OCIM2. (A) The OCI-AML3 and OCIM2 cells were seeded and cultured for 1, 2, 3 and 4 days. The cell number was counted each day. (B) The cells were seeded and cultured for 24 h, 48 h and 72 h. Viable cells were detected by MTT assay and the proliferation rate was calculated. The data shown is the mean ± SD from three independent experiments.
Figure 2.
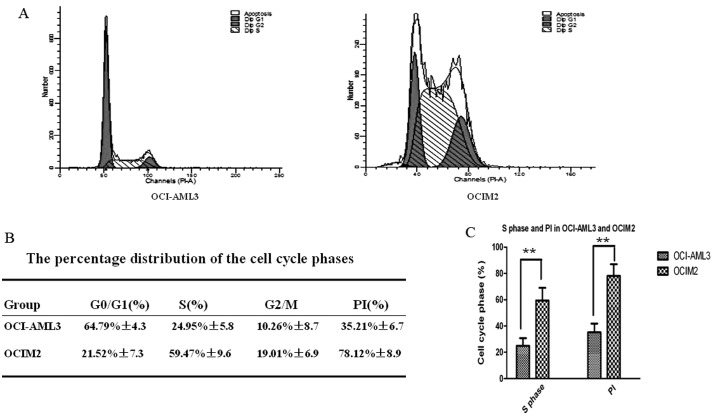
Assessment of cell cycle phase and proliferation index (PI) of OCI-AML3 and OCIM2. (A) The OCI-AML3 and OCIM2 cells were harvested and evaluated by flow cytometry using FACS. (B) The percentage distribution in the cell cycle phases was analyzed using CellQuest. The PI was determined using the following formula: PI = (S + G2/M) / (G0/G1 + S + G2/M). (C) S phase and PI are shown in histogram. Statistical significance was determined by the Student’s t-test. The data shown is the mean ± SD from three independent experiments. **P<0.01.
Cell cycle distribution and PI in AML cell lines OCI-AML3 and OCIM2
As shown in Fig. 2A, the cell cycle distribution of AML cell lines OCI-AML3 and OCIM2 was determined by FCM. As shown in Fig. 2B, the percentage of OCI-AML3 cells in the S phase fraction was 24.95±5.8%; however, the percentage of OCIM2 cells was much higher, at OCI-AML3, at 59.47±9.6%. At the same time, the PI of OCI-AML3 and OCIM2 was calculated to further confirm the role of the cell cycle in the proliferation rate of these two cell lines. As shown in Fig. 2B, that of OCIM2 was 78.12±8.9%, however, the PI of OCI-AML3 was only 35.21±6.7%. There were significant differences in S phase distribution and PI between OCI-AML3 and OCIM2, as shown in Fig. 2C (P<0.01).
Expression of Cx32, Cx43 mRNA and proteins in AML cell lines OCI-AML3 and OCIM2
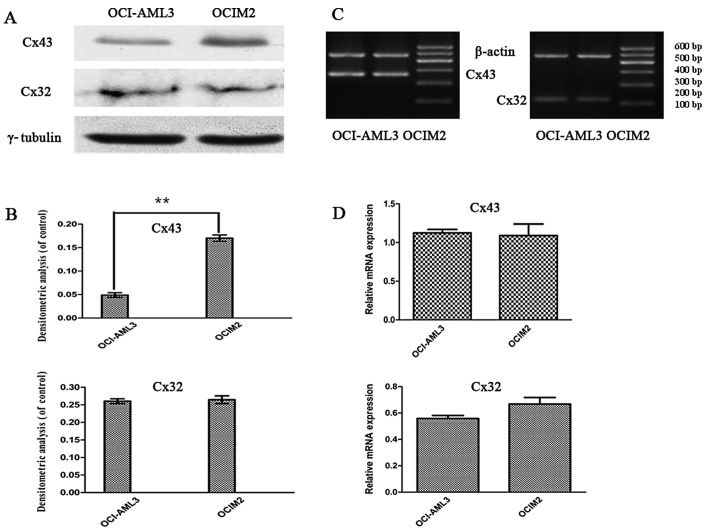
To investigate whether there was a difference in the expression of Cxs in AML cell lines OCI-AML3 and OCIM2, the expression of Cx32, and Cx43 mRNA in OCI-AML3 and OCIM2 was detected by RT-PCR. The protein levels of Cx32 and Cx43 were determined by western blot assay and immunofluorescence. As shown in Fig. 3C and D, the RT-PCR assay showed that the expression of Cx43 and Cx32 mRNA in these two cell lines was not significantly different (P>0.05). In contrast, a clear difference was noted between OCI-AML3 and in OCIM2 in the expression of Cx43 protein. As shown in Fig. 3A and B, the protein level of Cx43 in OCIM2 was notably higher than that of OCI-AML3 (P<0.01).
Figure 3.
The expression of Cx43 and Cx32 mRNA and protein in OCI-AML3 and OCIM2. (A) The OCI-AML3 and OCIM2 cells were harvested and the protein level of Cx43 and Cx32 was determined by western blot assay. (B) The values for Cx43 and Cx32 were corrected relative to γ-tubulin and are shown in the histogram. (C) The OCI-AML3 and OCIM2 cells were harvested and the mRNA levels of Cx43 and Cx32 were determined by RT-PCR. β-actin was used as an equal loading control. (D) The bands were quantified by densitometric analysis and are shown in the histogram. Statistical significance was determined by the Student’s t-test. The data is represented as the mean ± SD from three independent experiments. **P<0.01.
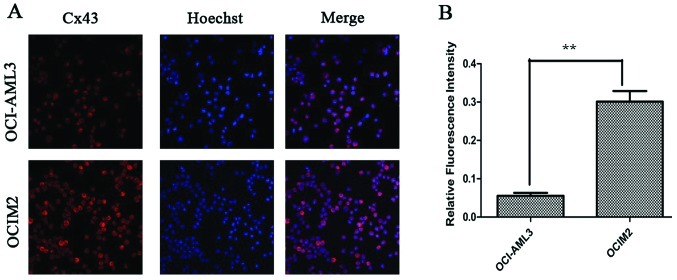
Cx43 proteins appeared as a collection of red spots intracytoplasmically and in certain parts of the plasma membrane, as shown in Fig. 4A. The immunofluorescence assay showed that the fluorescence intensity of Cx43 protein in OCIM2 was higher than that in OCI-AML3. OCIM2 cells showed both stronger western blot and fluorescence intensity. These results suggested that the expression of Cx43 in OCIM2 was much higher than OCI-AML3 at the protein level, but there was no difference at the mRNA level.
Figure 4.
(A) The expression of Cx43 in OCI-AML3 and OCIM2 detected by immunofluorescence. The representative images are shown. (B) The intensity of Cx43 was quantitated by densitometric analysis and is shown in the histogram. Statistical significance was determined by the Student’s t-test. The data is represented as the mean ± SD from three independent experiments. **P<0.01.
Discussion
Deviation of physiological cell cycling, as a consequence of homeostatic imbalance, may lead to uncontrolled cell proliferation activity (15–17). The S phase (synthesis phase) is the part of the cell cycle in which DNA is replicated, occurring between the G1 and G2 phase, which is a reliable marker to indicate how fast a tumor is growing. In clinical practice, the S phase fraction could be used as an independent prognostic factor in node-negative invasive breast carcinoma (18). The proliferative index (PI) is a measure of the number of proliferated cells in a tumor, which is also an indicator of the cell proliferation rate. Our results revealed that OCIM2 grew much faster than OCI-AML3 and that the proliferation ability of OCIM2 was much stronger than that of OCI-AML3. In addition, the results showed that the S phase percentage and PI of OCIM2 was higher than OCI-AML3. Since acute erythroleukemia is an uncommon subtype of AML associated with a very poor prognosis compared to other subtypes, such as OCI-AML3, these results support the use of the S phase fraction as a parameter to predict the proliferation rate in AML cells.
It is well known that gap junctions, a group of specialized cell-to-cell junctions composed of Cx proteins (19–21), are critical gatekeepers of cell proliferation, controlling the intercellular exchange of essential growth regulators (22). The role of Cxs in leukemogenesis and leukemic cell functions are not well defined. Previous studies have clearly shown that soluble mediators alter AML cell proliferation and apoptosis both when the leukemic cells are cultured alone and when co-cultured with fibroblasts, endothelial cells and osteoblasts (23). Furthermore, direct cell-cell interactions between AML cells also alter AML cell function. Even though the function of Cx building blocks affects the regulation of several cellular functions, including growth and apoptosis of normal and malignant cells, and also appears to be important for chemosensitivity, little is known about their role in leukemic cell functions. Certain reports demonstrate that overexpression or genetic mutation of Cx could induce tumor cell growth and inhibit G1/S cell cycle arrest (12,24,25). In contrast, several studies have demonstrated that the presence of Cxs is indispensable for the enrollment of cell proliferation (26–29). Our research demonstrated a lower expression of Cx43 in OCI-AML3 than OCIM2, but RT-PCR results revealed no decrease of Cx43 mRNA in these two cell lines. It is of note, however, that the same level of Cx32 mRNA and proteins was detected in OCIM2 and OCI-AML3 cell lines. It appears that Cx43 gene transcription was not responsible for the aberrant expression of Cx43 proteins. Although there was no difference in the expression of Cx32 both at the transcriptional and protein level, we found that there was a significant difference in the expression of Cx43 at the protein level. Therefore, the results of the present study indicate that Cx43 could be considered as a potential tumor promoter which exerts its effect by promoting the exchange of growth factors or facilitating proliferation by itself as a pro-survival signal, and may also have a correlation with malignant AML cell proliferation. Further investigation is required to understand the precise mechanism in which intercellular communication participates in neoplastic growth in the hematopoietic process.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (no. 81070429).
Reference
- 1.Oyamada M, Oyamada Y, Takamatsu T. Regulation of connexin expression. Biochim Biophys Acta. 2005;1719:6–23. doi: 10.1016/j.bbamem.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 3.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cronier L, Crespin S, Strale PO, Defamie N, Mesnil M. Gap junctions and cancer: new functions for an old story. Antioxid Redox Signal. 2009;11:323–338. doi: 10.1089/ars.2008.2153. [DOI] [PubMed] [Google Scholar]
- 5.Mesnil M, Crespin S, Avanzo JL, Zaidan-Dagli ML. Defective gap junctional intercellular communication in the carcinogenic process. Biochim Biophys Acta. 2005;1719:125–145. doi: 10.1016/j.bbamem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Giardina SF, Mikami M, Goubaeva F, Yang J. Connexin 43 confers resistance to hydrogen peroxide-mediated apoptosis. Biochem Biophys Res Commun. 2007;362:747–752. doi: 10.1016/j.bbrc.2007.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freidin M, Asche S, Bargiello TA, Bennett MV, Abrams CK. Connexin 32 increases the proliferative response of Schwann cells to neuregulin-1 (Nrg1) Proc Natl Acad Sci USA. 2009;106:3567–3572. doi: 10.1073/pnas.0813413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Xu YB, Wang Q, et al. Leukemogenic AML1-ETO fusion protein upregulates expression of connexin 43: the role in AML 1-ETO-induced growth arrest in leukemic cells. J Cell Physiol. 2006;208:594–601. doi: 10.1002/jcp.20695. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Zhang X, Li ZJ, Chen XH. Up-regulation of Cx43 expression and GJIC function in acute leukemia bone marrow stromal cells post-chemotherapy. Leuk Res. 2010;34:631–640. doi: 10.1016/j.leukres.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Zhang X, Si YJ, Gao L, Chen XH. [Connexin 43 expression and interacellular communicating function in acute leukemia bone marrow stroma cells] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15:679–682. [PubMed] [Google Scholar]
- 11.Krenacs T, Rosendaal M. Connexin43 gap junctions in normal, regenerating, and cultured mouse bone marrow and in human leukemias: their possible involvement in blood formation. Am J Pathol. 1998;152:993–1004. [PMC free article] [PubMed] [Google Scholar]
- 12.Sato H, Hagiwara H, Ohde Y, Senba H, Virgona N, Yano T. Regulation of renal cell carcinoma cell proliferation, invasion and metastasis by connexin 32 gene. J Membr Biol. 2007;216:17–21. doi: 10.1007/s00232-007-9020-5. [DOI] [PubMed] [Google Scholar]
- 13.Hirabayashi Y, Yoon BI, Tsuboi I, et al. Protective role of connexin 32 in steady-state hematopoiesis, regeneration state, and leukemogenesis. Exp Biol Med (Maywood) 2007;232:700–712. [PubMed] [Google Scholar]
- 14.Spinner DM. MTT growth assays in ovarian cancer. Methods Mol Med. 2001;39:175–177. doi: 10.1385/1-59259-071-3:175. [DOI] [PubMed] [Google Scholar]
- 15.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 16.Suryadinata R, Sadowski M, Sarcevic B. Control of cell cycle progression by phosphorylation of cyclin-dependent kinase (CDK) substrates. Biosci Rep. 2010;30:243–255. doi: 10.1042/BSR20090171. [DOI] [PubMed] [Google Scholar]
- 17.van den Heuvel S. Cell-cycle regulation. WormBook. 2005:1–16. doi: 10.1895/wormbook.1.28.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moureau-Zabotto L, Bouchet C, Cesari D, et al. Combined flow cytometry determination of S-phase fraction and DNA ploidy is an independent prognostic factor in node-negative invasive breast carcinoma: analysis of a series of 271 patients with stage I and II breast cancer. Breast Cancer Res Treat. 2005;91:61–71. doi: 10.1007/s10549-004-7047-1. [DOI] [PubMed] [Google Scholar]
- 19.Vinken M, Vanhaecke T, Papeleu P, Snykers S, Henkens T, Rogiers V. Connexins and their channels in cell growth and cell death. Cell Signal. 2006;18:592–600. doi: 10.1016/j.cellsig.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Vinken M, Henkens T, De Rop E, Fraczek J, Vanhaecke T, Rogiers V. Biology and pathobiology of gap junctional channels in hepatocytes. Hepatology. 2008;47:1077–1088. doi: 10.1002/hep.22049. [DOI] [PubMed] [Google Scholar]
- 21.Vinken M, De Rop E, Decrock E, et al. Epigenetic regulation of gap junctional intercellular communication: more than a way to keep cells quiet? Biochim Biophys Acta. 2009;1795:53–61. doi: 10.1016/j.bbcan.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Decrock E, Vinken M, De Vuyst E, et al. Connexin-related signaling in cell death: to live or let die? Cell Death Differ. 2009;16:524–536. doi: 10.1038/cdd.2008.196. [DOI] [PubMed] [Google Scholar]
- 23.Hatfield K, Ryningen A, Corbascio M, Bruserud O. Microvascular endothelial cells increase proliferation and inhibit apoptosis of native human acute myelogenous leukemia blasts. Int J Cancer. 2006;119:2313–2321. doi: 10.1002/ijc.22180. [DOI] [PubMed] [Google Scholar]
- 24.Roger C, Mograbi B, Chevallier D, et al. Disrupted traffic of connexin 43 in human testicular seminoma cells: overexpression of Cx43 induces membrane location and cell proliferation decrease. J Pathol. 2004;202:241–246. doi: 10.1002/path.1509. [DOI] [PubMed] [Google Scholar]
- 25.Sato H, Fukumoto K, Hada S, et al. Enhancing effect of connexin 32 gene on vinorelbine-induced cytotoxicity in A549 lung adenocarcinoma cells. Cancer Chemother Pharmacol. 2007;60:449–457. doi: 10.1007/s00280-006-0406-3. [DOI] [PubMed] [Google Scholar]
- 26.Chadjichristos CE, Matter CM, Roth I, et al. Reduced connexin43 expression limits neointima formation after balloon distension injury in hypercholesterolemic mice. Circulation. 2006;113:2835–2843. doi: 10.1161/CIRCULATIONAHA.106.627703. [DOI] [PubMed] [Google Scholar]
- 27.Ozawa H, Mutai H, Matsunaga T, et al. Promoted cell proliferation by connexin 30 gene transfection to head-and-neck cancer cell line. Anticancer Res. 2009;29:1981–1985. [PubMed] [Google Scholar]
- 28.Wang HH, Kung CI, Tseng YY, et al. Activation of endothelial cells to pathological status by down-regulation of connexin43. Cardiovasc Res. 2008;79:509–518. doi: 10.1093/cvr/cvn112. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Furuya T, Li D, et al. Connexin 26 expression correlates with less aggressive phenotype of intestinal type-gastric carcinomas. Int J Mol Med. 2010;25:709–716. doi: 10.3892/ijmm_00000395. [DOI] [PubMed] [Google Scholar]