Abstract
The majority of successful existing vaccines rely on neutralizing antibodies, which may not require specific anatomical localization of B cells. However, efficacious vaccines that rely on T cells for protection have been difficult to develop, as robust systemic memory T cell responses do not necessarily correlate with host protection1. In peripheral sites, tissue-resident memory T cells provide superior protection compared to circulating memory T cells2,3. Here, we describe a simple and non-inflammatory vaccine strategy that enables the establishment of a protective memory T cell pool within peripheral tissue. The female genital tract, which is a portal of entry for sexually transmitted infections (STIs), is an immunologically restrictive tissue that prevents entry of activated T cells in the absence of inflammation or infection4. To overcome this obstacle, we explored a vaccine strategy we term “prime and pull” to establish local tissue-resident memory T cells at a site of potential viral exposure. This approach relies on two steps: 1) conventional parenteral vaccination to elicit systemic T cell responses (prime), followed by 2) recruitment of activated T cells via topical chemokine application to the restrictive genital tract (pull), where such T cells establish a long-term niche and mediate protective immunity. Prime and pull protocol reduces the spread of infectious HSV-2 into the sensory neurons and prevents development of clinical disease. These results reveal a promising vaccination strategy against HSV-2, and potentially against other STIs such as HIV-1.
Viral sexually transmitted infections (STIs) such as human immunodeficiency virus-1 (HIV-1) and herpes simplex virus-2 (HSV-2) account for significant morbidity and mortality around the world. Strong preclinical evidence for the role of T cells in controlling viral STIs has led to the design of prophylactic vaccines that elicit systemic cellular immunity, and yet these vaccines have not been efficacious1,5. While systemic memory T cells can migrate freely through organs such as the spleen and liver, others such as the intestines, lung airways, central nervous system, skin and vagina, are restrictive for memory T cell entry6. In the latter tissues, inflammation or infection is often required to permit entry of circulating activated T cells to establish a tissue-resident memory T cell pool that composes a separate compartment from the circulating pool2,7,8. Given that side effects of inflammation in the reproductive tissue may preclude the use of a live prophylactic vaccine given vaginally, we investigated an alternative approach to recruit virus-specific T cells into the vaginal mucosa without inducing local inflammation or infection.
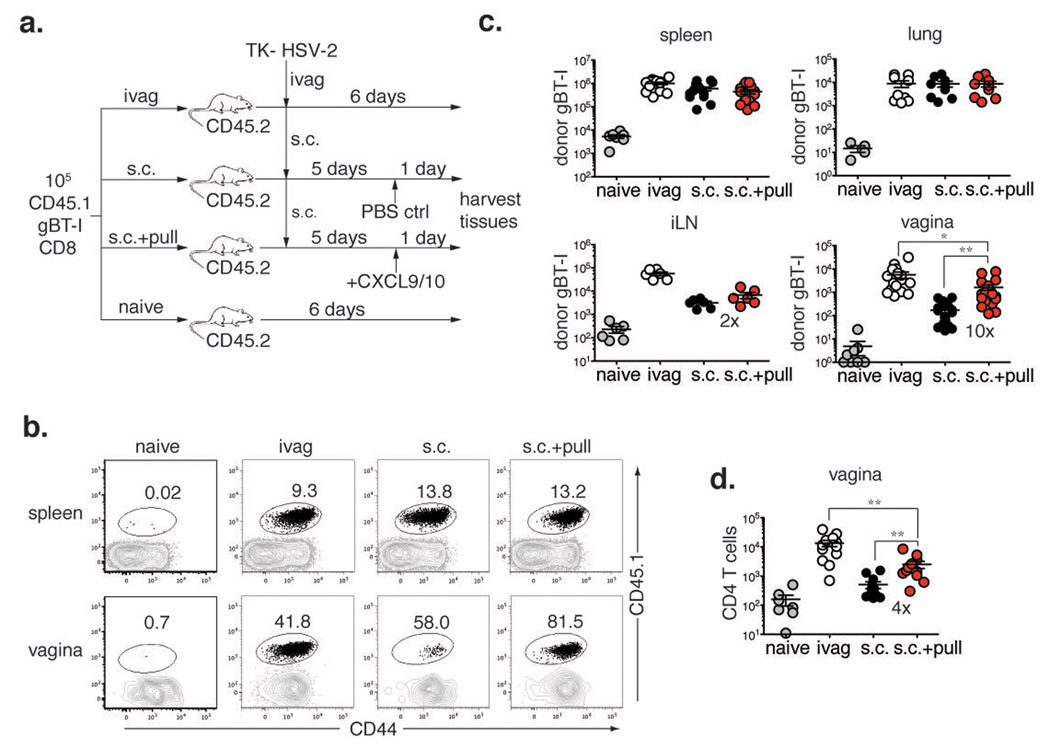
Following genital HSV-2 infection, CXCL9 and CXCL10 expression is induced by IFN-γ secreted by CD4 T cells and mediates recruitment of effector CD8 T cells to the infected tissue via CXCR3 (Ref 4). CXCR3 is expressed by both effector Th1 cells and CD8 T cells, as well as other cell types9. Thus, we hypothesized that the topical application of chemokines CXCL9 and CXCL10 might be sufficient to recruit effector T cells to the vagina in the absence of infection. To test this hypothesis, we utilized TCR transgenic CD8 T cells that recognize an epitope within glycoprotein B (gBT-I)10 to track the HSV-2-specific CD8 T cell population. Naïve female C57BL/6 mice were transplanted with 105 congenically marked gBT-I cells and immunized with an attenuated strain of HSV-2 that lacks thymidine kinase (TK- HSV-2)11 subcutaneously (s.c.) (Fig. 1a). As expected, this route of immunization resulted in minimal migration of activated CD8 T cells into the vagina (Fig. 1b & c). In order to recruit or “pull” activated HSV-specific CD8 T cells, the chemokines CXCL9 and CXCL10 were topically applied to the vaginal cavity of s.c. immunized mice (Fig. 1a). Another group of mice was immunized intravaginally (ivag) with TK- HSV-2, which served as a positive control for maximal CD8 T cell recruitment to the vagina (Fig. 1b, c). At day 6 post-infection, all three treatment groups exhibited primary CD8 T cell responses of similar magnitudes, as indicated by the numbers and percentages of systemic gBT-I CD8 T cells found in the spleen (Fig. 1b, c). However, the frequency and number of gBT-I CD8 T cells in the vagina were significantly higher in mice treated with the chemokine pull (s.c. + pull) as compared to the control s.c. immunized mice (Fig. 1b, c). Furthermore, the action of the chemokine pull was restricted to the genital mucosa, as gBT-I CD8 T cell recruitment to the vagina-draining lymph nodes was limited (Fig. 1c). Activated CD4 T cells were also strongly recruited to the vagina by the chemokine pull (Fig. 1d). Antigen in the vagina was not responsible for the recruitment, as HSV-2 genomic DNA was absent from the genital tract after s.c. immunization (Supplementary Fig. 1). To mimic a vaccination scenario more closely, we also tested whether endogenous virus-specific T cells could be recruited by prime and pull. Like gBT-I CD8 T cells, the systemic endogenous HSV-specific CD8 T cell response was similar in all immunized groups (Supplementary Fig. 2a). However, significantly greater numbers of HSV-specific CD8 T cells as well as CD4 T cells were present in the genital tracts of mice treated with the chemokine pull as compared to s.c. immunization alone (Supplementary Fig. 2). Thus, these data show that the prime and pull method is capable of recruiting a large number of parenterally primed T cells to the genital tract with a single topical application of chemokines.
Figure 1. Effector T cells are recruited to the vagina by topical chemokine treatment.
a, Experimental schematic. Donor gBT-I CD8 T cell recipients were unimmunized (naïve), or immunized either ivag or s.c. with TK- HSV-2. Five days post-infection, s.c. immunized mice was treated vaginally with either the chemokines (pull) or PBS. One day post-pull, the frequency (b) and number (c, d) of donor gBT-I CD8 T cells (b, c) and CD4 T cells (d) were determined in the indicated tissues. Plots are gated on total CD8 T cells and numbers indicate % gBT-I (CD45.1+) (b). Numbers in graphs indicate fold difference in T cell number between s.c. vs. s.c. + pull. Statistical significance was determined by two-tailed unpaired Student’s t-test. Data are pooled from 2–7 independent experiments; n = 6–21 per group. All error bars show standard error (SEM), and *; p = 0.05–0.01, **; p = 0.01 – 0.001, ***; p < 0.001 throughout the manuscript (individual p values are found in Supplementary Information).
To assess the possible inflammatory consequences of topical chemokine application to the vagina, we next examined the presence of innate inflammatory cells after the chemokine pull. Other cell types, including NK cells and plasmacytoid dendritic cells (pDC), express CXCR3 (Ref 9). However, no significant increase in the number of pDC, NK cells, granulocytes, DCs, monocytes, macrophages and monocyte-derived DCs was elicited by the chemokine treatment (s.c. + pull) compared to the s.c. immunized control (Supplementary Fig. 3). These data indicated that topical chemokines do not induce appreciable recruitment of NK or pDCs to the vagina, and selectively recruited effector T cells without inducing a general inflammatory response.
During genital HSV infection, CD4 T cells act as a pioneering population for the migration of virus-specific CD8 T cells by inducing the production of critical chemokines within the tissue4. To determine whether the recruitment of gBT-I CD8 T cells to the genital tract was similarly dependent on CD4 T cell help during prime and pull, s.c. immunized mice were injected with a CD4 depleting antibody on day 3 and day 5 p.i. in order to preserve normal CD8 T cell priming12, and then treated with the chemokine pull (Supplementary Fig. 4a). In CD4 T cell-depleted mice (Supplementary Fig. 4b), both systemic gBT-I CD8 T cell numbers and migration to the vagina were unaffected (Supplementary Fig. 4c, d), indicating that recruitment of effector CD8 T cells to the vagina after chemokine treatment bypasses the requirement for CD4 T cell help.
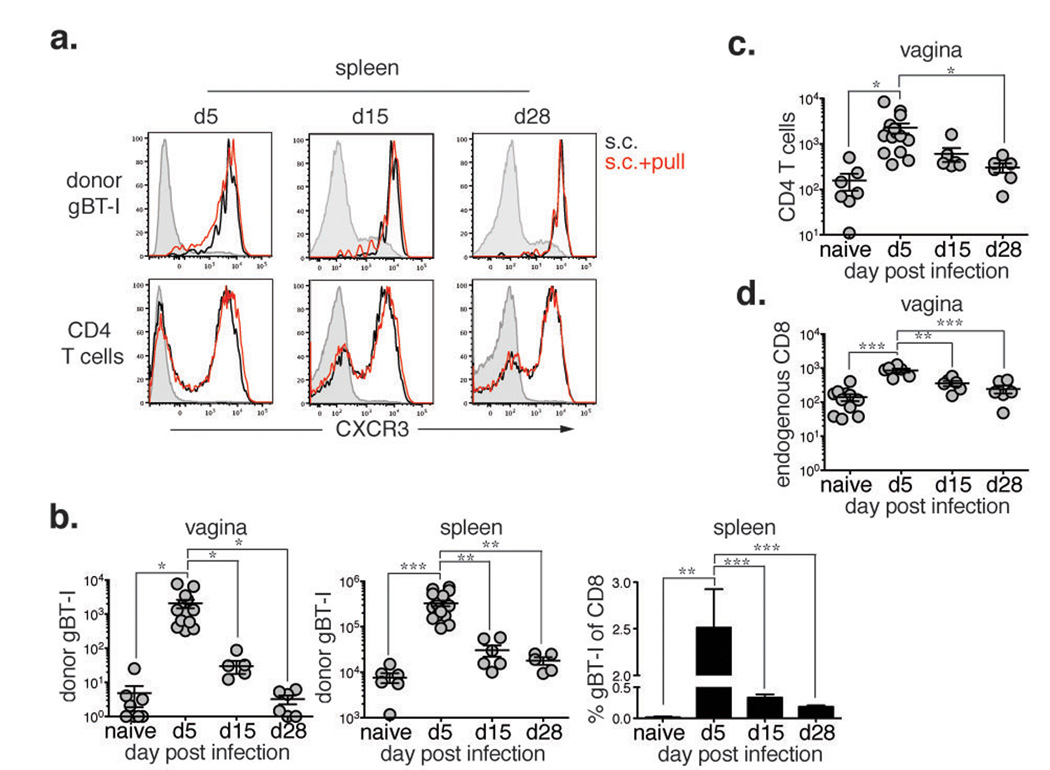
CXCR3 is upregulated on T cells upon activation and remains high through the effector and memory stages9. Having demonstrated that CXCL9 and CXCL10 could recruit CXCR3+ effector T cells to the vagina, we next examined the efficacy of the chemokine pull at different stages of T cell priming. Following s.c. TK- HSV-2 immunization, CXCR3 was upregulated on both gBT-I and CD4 T cells throughout the response (Fig. 2a), suggesting that both effector and memory T cells should be capable of responding to the chemokine pull. As previous reports have shown that early effector CD8 T cells had a heightened ability to migrate to peripheral tissues7, we next determined whether the timing of chemokine pull dictated the efficacy of T cell recruitment to the genital tract. When s.c. immunized mice were treated with the chemokine pull at the effector (d5), contraction (d15) and memory (d30) phase of the T cell response13, we found that the chemokine pull was most effective at recruiting antigen-specific CD8 T cells during the effector (d5) phase, which correlated with the heightened frequency and number of systemic gBT-I CD8 T cells (Fig. 2b). Despite similar CXCR3 expression (Fig. 2a), memory gBT-I CD8 T cells were not present in the tissue after pull (Fig. 2b). We speculate that this may be due to altered homing patterns7,14 and the reduced number and frequency of gBT-I in circulation at the memory time point. Recruitment of CD44+ CD4 T cells (Fig. 2c) and endogenous CD8 T cells (Fig. 2d) followed a similar pattern. Collectively, these data indicate that the chemokine pull is most effective at recruiting recently activated effector CD8 T cells that are circulating at high frequency, establishing a specific time frame within which the chemokine pull should be administered after priming.
Figure 2. Chemokine pull is specific for highly activated effector T cells.
Mice were s.c. immunized and given chemokines or PBS at day 5, 15 or 28 post-infection and analyzed one day post-pull. a, CXCR3 expression on donor gBT-I or CD44+ CD4 T cells from the spleen one day post pull from s.c. immunized mice (open black) and s.c. + pull. (open red). Shaded histograms are CD44lo CD8 or total CD4 T cells. b, The gBT-I cell number in the vagina (left) or spleen (middle) and frequency in the spleen (right) were examined one day post-pull. c, d, The number of CD44+ CD4 T cells (c) and endogenous CD44+ CD8 T cells (d) was determined in the vagina one day post-pull on the indicated days. Statistical significance was determined by two-tailed unpaired Student’s t-test. Data are pooled from 2–3 independent experiments; n = 6–18 per group.
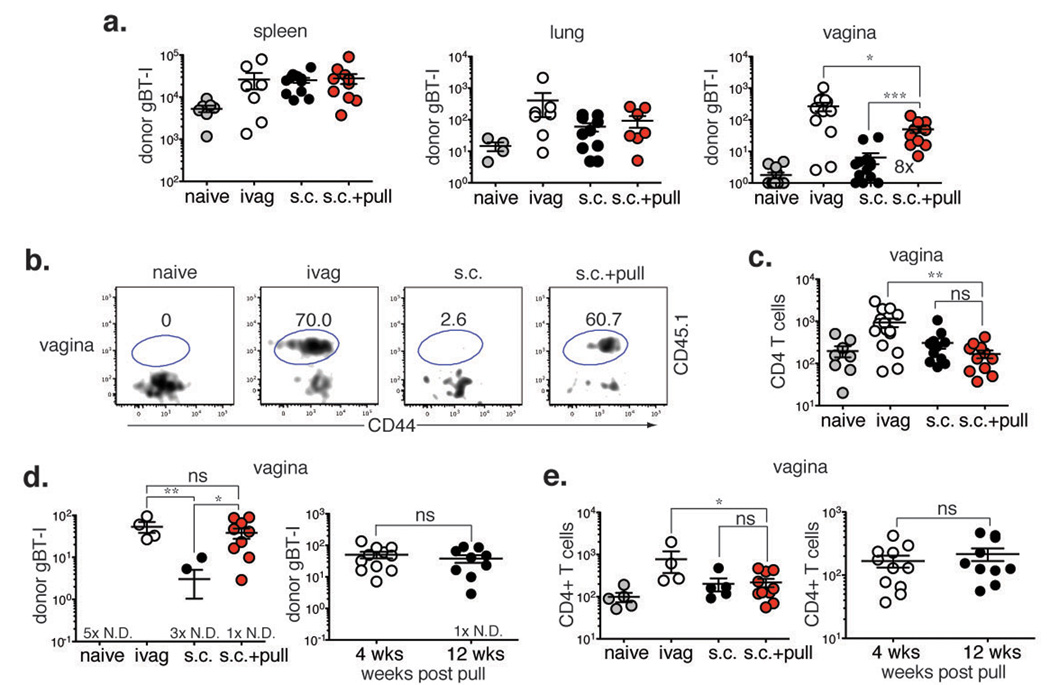
For the prime and pull approach to be an effective vaccine strategy, pathogen-specific T cells must be retained within the tissue for an extended time and establish a pool of memory cells. To determine whether the effector gBT-I CD8 T cells recruited into the vagina after prime and pull were capable of establishing a long-term population of memory CD8 T cells, we examined the presence of gBT-I CD8 T cells 4 weeks after the chemokine pull. The number of systemic memory gBT-I CD8 T cells, while decreased compared to d1 post-pull (Fig. 1c) due to contraction of the T cell response, was similar regardless of immunization route or treatment (Fig. 3a). However, a significantly greater number and frequency of memory gBT-I CD8 T cells was present in the genital tract of s.c. immunized mice treated with the chemokine (s.c. + pull) compared to untreated mice (s.c.) (Fig. 3a, b). Despite significant recruitment during the effector phase (Fig. 1d), CD4 T cells were not retained within the vagina long-term (Fig. 3c), reminiscent of CD4 T cell behavior after dermal HSV-1 infection in which the cells leave the site of infection to mediate immunosurveillance15. Thus, CD4 T cells may require additional signals, such as those generated during HSV-2 infection16,17, to be retained long term within the vagina.
Figure 3. Virus-specific T cells recruited by chemokine pull are retained in the vagina long-term.
Mice were immunized and treated as shown in Figure 1a. Four (a-c) or twelve (d, e) weeks post-pull, T cell numbers and frequency were assessed in various tissues., At 4 weeks post-pull, numbers of gBT-I cells (a) and endogenous CD4 T cells (c) were determined in the indicated tissues. Number in graph shows fold difference in gBT-I number between s.c. vs. s.c. + pull groups. b, Frequency of gBT-I cells was measured in the vagina. Plots are gated on total CD8 T cells. Numbers show % gBT-I (CD45.1+). At 12 weeks post-pull, the numbers of gBT-I cells (d) and endogenous CD4 T cell (e) were determined in the vagina (left) and compared to numbers at 4 weeks post-pull (right). # × N.D. = number of animals with no cells detected in the tissue. Statistical significance was determined by two-tailed unpaired Student’s t-test; ns = not significant. Data are pooled from 2–3 independent experiments; n = 4–15 per group.
To investigate the stability of this tissue-resident population of memory gBT-I CD8 T cells, we also examined T cell numbers at 12 weeks post-pull. Donor gBT-I CD8 T cells numbers in the vagina were significantly higher after prime and pull than after s.c. immunization alone (Fig. 3d). Furthermore, the number of memory gBT-I CD8 T cells did not decline between 4 weeks and 12 weeks (Fig. 3d), suggesting that this tissue-resident population was stable and was retained long-term. CD4 T cell numbers in the vagina remained low at week 12 after prime and pull and were comparable to numbers detected at week 4 (Fig. 3e). Thus, a single chemokine pull given to mice during the effector phase is sufficient to establish a long-term population of tissue-resident memory CD8 T cells, but not CD4 T cells, within the vagina.
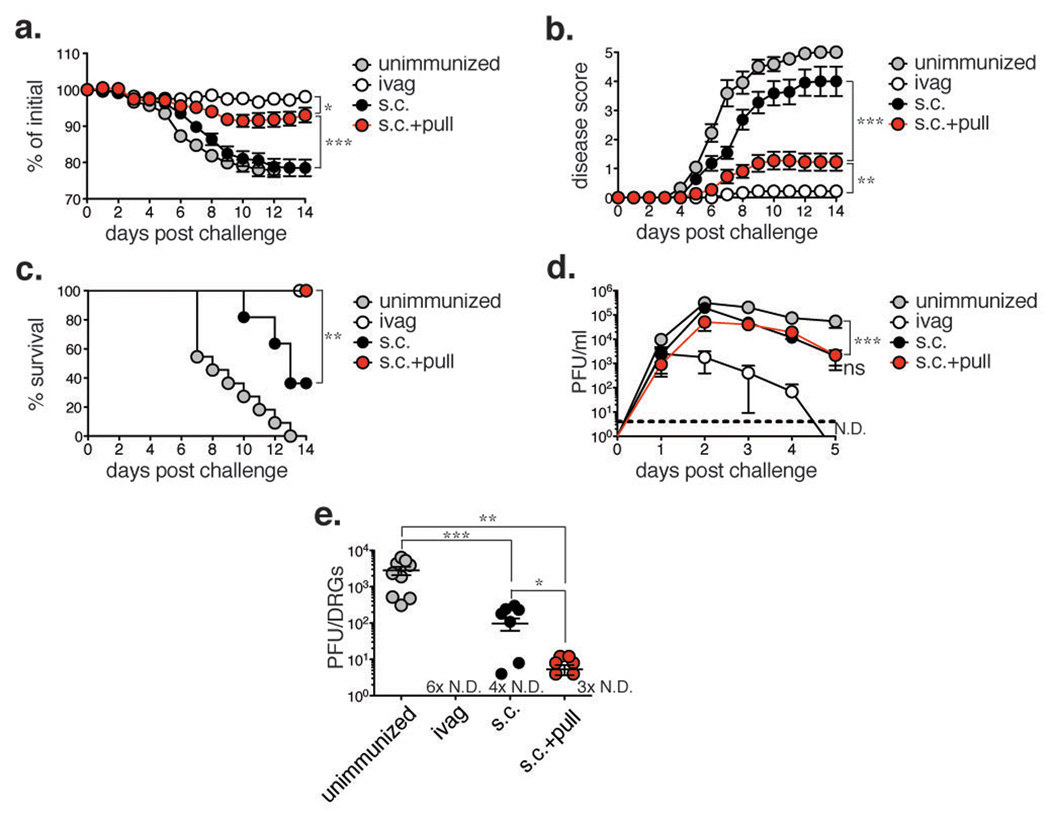
Tissue-resident memory T cells are effective in mediating immunity against local infections2,3. HSV-2 spreads from its initial replication site at the epithelium to the innervating neurons and subsequently establishes latency within the dorsal root ganglia (DRG)5. Reactivation from latency leads to viral shedding and formation of genital lesions that are commonly associated with genital herpes5. Thus, preventing the spread of virus from the mucosal epithelium to the DRG is key in preventing disease and transmission of the virus. As a single chemokine pull administered after s.c. immunization is capable of establishing a population of tissue-resident memory CD8 T cells within the vagina long term, we next examined whether the prime and pull strategy would provide enhanced immunity against genital HSV-2 infection. Mice were challenged ivag with a lethal dose of WT HSV-2 four weeks after prime and pull and monitored for disease and survival. Notably, mice treated with the chemokine pull (s.c. + pull) lost significantly less weight than either the unimmunized or s.c. immunized controls (Fig. 4a). Furthermore, prime and pull almost completely prevented the development of clinical symptoms, which were observed in both unimmunized and s.c. immunized controls (Fig. 4b). Accordingly, mice treated with the chemokine pull had a 100% survival rate compared to the 36.3% survival rate of the s.c. immunized control (Fig. 4c). Upon challenge with WT HSV-2 four weeks post-pull, mice immunized and chemokine-treated in the absence of TCR Tg CD8 T cells were also significantly protected from weight loss (Supplementary Fig. 5a) and clinical disease (Supplementary Fig. 5b), although we did not observe a significant difference in survival rate (Supplementary Fig. 5c). Anti-HSV antibody titers in the vagina were not significantly different between s.c. immunized controls and chemokine treated mice (Supplementary Fig. 6), suggesting that the control of viral challenge was likely T-cell mediated. These results demonstrate that the addition of a chemokine pull to parenteral immunization could greatly enhance protective immunity against genital HSV-2 infection.
Figure 4. Prime and pull protects mice from lethal genital HSV-2 challenge.
Mice were immunized as shown in Figure 1a and challenged vaginally with a lethal dose of HSV-2 four weeks post-pull. Weight loss (a), disease severity (b), and survival (c) were assessed. d, HSV-2 was titered from vaginal washes collected at the indicated time points. Dashed line indicates limit of detection. ND = none detected. n = 11 (unimmunized), n = 9 (ivag), n = 12 (s.c., s.c. + pull). e, Viral titers were measured in the dorsal root ganglia 6–7 days post-challenge. # × N.D. = number of mice in which no virus was detected. n = 6–11 per group. Statistical significance was measured by two-way ANOVA (a-b, d), log-rank (Mantel-Cox) test (c) or two-tailed unpaired Student’s t-test (e). Data are pooled from 3–5 independent experiments.
To more stringently test the long-term protection afforded by prime and pull, we also challenged mice 10–12 weeks post-pull. At this late time point, the prime and pull group lost less weight compared to s.c. immunized controls (Supplementary Fig. 7a), and were significantly protected from development of disease (Supplementary Fig. 7b). Furthermore, at two weeks post-challenge, the prime and pull group had a survival rate of 100%, while s.c. immunized controls had a survival rate of 57% (Supplementary Fig. 7c). Thus, our results show that the protection provided by prime and pull lasts over time and remains robust up to 12 weeks after chemokine treatment.
To determine the mechanism by which the prime and pull strategy mediates protection from HSV-2 disease, we measured viral replication within the genital mucosa. Surprisingly, we found no difference in virus titers from the vaginal secretion of s.c. immunized vs. chemokine treated (s.c. + pull) mice (Fig. 4d, Supplementary Fig. 5d and 7d), indicating that protection was likely being mediated at a different location. As the severer symptoms of clinical disease in mice are associated with viral replication in the peripheral nervous system18, we next investigated whether prime and pull could protect the DRG against infection. When viral replication within the DRG was measured, we found mice treated with the chemokine pull had significantly lower virus titers than unimmunized mice (Fig. 4e). Furthermore, viral titers in the DRG of prime and pull group were significantly lower than that of s.c. immunized mice (Fig. 4e). Together, these data suggest that prime and pull strategy greatly reduces disease by controlling neuronal infection with HSV-2 rather than by controlling mucosal viral replication.
Our study demonstrates that after conventional vaccination to generate a systemic T cell population (prime), a single topical treatment with chemokines applied vaginally (pull) can provide superior protection against genital herpes by decreasing the spread of virus from the mucosal epithelia into the neurons. Importantly, protection of neurons from HSV-2 infection by prime and pull may decrease reactivation and viral shedding, which may reduce disease and transmission. While the exact role of T cells in controlling neuronal HSV-2 infection after prime and pull is not yet clear, we speculate that the local HSV-specific T cells may help control entry of virus at the neuronal endings, or promote blockade of viral replication once inside the neurons. Furthermore, other studies have demonstrated that T cells recruited to the genital tract by inflammation alone can decrease viral replication at the mucosal surface19, suggesting that control of infection at the site of entry may be possible by optimizing prime and pull. Thus, in addition to preventing reactivation of latent HSV20, virus-specific memory T cells may be mobilized to control neuronal viral infection during primary infection. While topical application in the genital tract of TLR ligands such as imiquimod have been shown to be effective as a therapeutic approach21, they may not be ideal vaccine candidates as they appear to be effective for only a short time after application and function through the induction of pro-inflammatory cytokines22. The novel prime and pull vaccine strategy described here provides an alternative to direct immunization of the genital tract, and establishes robust, long-term immunity with minimal local inflammation.
Cellular immunity is critical in mediating protection against viral STIs such as HSV-2 and HIV-1 (Ref.23). Both viruses enter through the genital mucosa, begin local replication and then spread to other tissues23. While our data highlight the role of prime and pull in controlling viral spread to the peripheral nervous system, this vaccination approach is not necessarily restricted to neurotropic viruses. HIV-1 enters the genital mucosa and invades the draining lymph node, from which systemic dissemination of the virus occurs23. In its current incarnation, prime and pull establishes tissue-resident memory CD8 T cells but not CD4 T cells. Given that a single HIV-1 virion can establish infection in humans23, local memory CD8 T cells may be key to protection against HIV-1 (Ref. 23) by reducing replication and dissemination of the founder virus, while the absence of local CD4 T cells could limit the availability of immediate target cells. Beyond viral infections, prime and pull could be applied to improve recruitment of immune cells to other restrictive microenvironments such as solid tumors. Effective immunotherapy can be hindered by either decreased or inappropriate expression of chemokines at the tumor tissue, leading to minimal trafficking of immune cells24. Delivery of appropriate chemokines to the tumor tissue after immunization could enhance recruitment of tumor-specific T cells and augment the efficacy of immunotherapies. While the protocol we present pairs the “pull” with a suboptimal subcutaneous immunization, we propose that the prime and pull strategy could be used in junction with more effective immunizations5 in order to enhance protection. Ultimately, the ability to boost recruitment of T cells and establish resident T cell populations in peripheral tissues restrictive for lymphocyte homing will aid not only in the prevention but also in treatment of a wide variety of diseases.
Methods
Mice
Female 6-week old C57BL/6 mice were purchased from NCI. gBT-I TCR Tg mice specific for the glycoprotein B epitope gB(498–505) were provided by F.R. Carbone and W.R. Heath and bred in our facility to C57BL/6-Ly5.2Cr mice (CD45.1+) (NCI). All procedures used in this study complied with federal and institutional policies of the Yale Animal Care and Use Committee.
Adoptive transfers and infections
Spleens were harvested from naïve CD45.1+ gBT-I TCR Tg mice and CD8 T cells were magnetically purified by CD8α microbeads or CD8α+ T cell isolation kits (Miltenyi Biotec). Donor cells (105) gBT-I CD8 T cells were adoptively transferred into Depo-Provera-treated (GE Healthcare), 7–8 week old C57BL/6 recipients retro-orbitally. Mice were then immunized intravaginally (ivag) or subcutaneously (s.c.) with 105 or 106 plaque forming units (PFU) of 186TKΔkpn HSV-2 (TK- HSV-2) respectively. At 5 days post-infection (p.i.), the vaginal cavity of mice was swabbed with a Calginate swab (Fisher) and either PBS or a solution of CXCL9 and CXCL10 (3 µg each, Peprotech) in PBS was delivered via pipet tip into the vagina. Where indicated, C57BL/6 mice that did not receive gBT-I cells were primed and pulled in similar manner. Some s.c. immunized mice were intraperitoneally injected with 200 µg αCD4 (GK1.5) antibody at day 3 and 5 p.i. to deplete CD4 T cells. For the 4 week challenge, unimmunized or previously immunized mice at the indicated time points were infected ivag with 5,000 PFU WT HSV-2 186 syn+. Challenges given at 10 – 12 weeks post-pull were treated with Depo-Provera for a second time 1 – 2 weeks post-pull (9–10 weeks prior to challenge) before infection with 5,000 PFU WT HSV-2 186 syn+.
Flow cytometry
At various time points, single cell suspensions from the spleen, lungs, vagina and iliac lymph nodes were prepared for analysis as described17. Briefly, lungs were digested with collagenase D (Roche). Vaginas were treated with Dispase II (Roche) for 15 mins and then collagenase D for 30 mins. Cells from the spleen and iliac lymph node were counted by hemacytometer. Lung and vagina cell numbers were quantified using CountBright absolute counting beads from Invitrogen. Dead cells were excluded from analysis using the LIVE/DEAD Fixable Aqua Dead Cell Stain kit (Invitrogen). All samples were acquired on an LSRII equipped with a 532 nm green laser (BD Biosciences). All data were analyzed with FlowJo (Treestar).
Antibodies
The following antibodies were used for this study: CD3 (17A2), CD4 (RM4-4, RM4-5), CD8 (53-6.7), CD44 (1M7), CD45.1 (A20), CXCR3 (CXCR3-173), CD11c (N418), CD11b (M1/70), MHC class II (M5/114.15.2), Ly6C (AL-21), Ly6G (1A8), F4/80 (BM8), B220 (RA3-6B2), CD19 (ebio1D3), and NK1.1 (PD136). Antibodies were purchased from BD Biosciences, Biolegend, eBioscience and Invitrogen. H-2Kb-gB498–505 tetramer was obtained from the NIH tetramer core facility.
Measurement of viral titers, weight and disease scores
Vaginal secretions were collected days 5 post-challenge using PBS and Calginate swabs. Lumbar and sacral dorsal root ganglia (DRG) were harvested at days 6–7 post-challenge as described27. DRG were homogenized using a motorized pestle (VWR). Titers from vaginal and DRG samples were measured on Vero cell monolayers as previously described17. Weight loss was measured daily and normalized to body weight on day 0 of challenge. Disease was monitored daily and scored as follows: 0) no disease, 1) genital inflammation, 2) genital lesions and hair loss, 3) hunched posture and ruffled fur, 4) hind limb paralysis and 5) premoribund28. Mice were euthanized prior to the moribund state due to humane concerns.
Detection of HSV-2 antigen by quantitative PCR
Mice were immunized s.c. or ivag were sacrificed at day 5 post-infection. Vaginal tissue was harvested and genomic DNA was extracted as previously described29. Briefly, tissue was homogenized in a salt homogenizing buffer using a motorized pestle. Proteinase K and SDS were added to samples and incubated overnight at 55°C. After addition of a sodium chloride solution, samples were centrifuged and supernatants were transferred to new tubes. Isopropanol was added to the supernatants and incubated at 20°C for 1 hr. DNA was pelleted by centrifugation, cleaned with ethanol and resuspended in H20. HSV was measured with primers detecting gB (Forward, agaccagggccgctgatc; Reverse, gcgctggacctccgtgtag) with quantitative PCR (Stratagene). DNA purified from TK− HSV-2 was used as standard to calculate PFU equivalents.
Measurement of HSV-specific antibody titers
Vaginal secretions were collected from mice with PBS and Calginate swabs 4 weeks post-pull. HSV-specific IgG was measured by ELISA as previously described30. Known quantities of anti-HSV gB mAb (SS10 mouse IgG) kindly provided by G. Cohen and R. Eisenberg (University of Pennsylvania, Philadelphia, PA) was used as a standard.
Supplementary Material
Acknowledgements
We thank E. Foxman and R. Medzhitov for critical reading of the manuscript, and N. Iijima, H. Dong and B. Yordy for technical support. H. S. is supported by F32AI091024. This work is supported by NIH grants to A. I. AI054359 and AI062428.
Footnotes
‘Supplementary Information accompanies the paper on www.nature.com/nature.’
Author Contributions
Experiments were conceived and designed by H.S. and A.I. Experiments were performed by H.S. Data were analyzed by H.S. and A.I. Paper was written by H.S. and A.I.
Author Information
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
References
- 1.McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity. 2010;33:542–554. doi: 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature immunology. 2009 doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 3.Jiang X, et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koelle DM, Corey L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med. 2008;59:381–395. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- 6.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 7.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. The Journal of experimental medicine. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klonowski KD, et al. Dynamics of Blood-Borne CD8 Memory T Cell Migration In Vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 9.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller SN, Heath W, McLain JD, Carbone FR, Jones CM. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol Cell Biol. 2002;80:156–163. doi: 10.1046/j.1440-1711.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- 11.Jones CA, Taylor TJ, Knipe DM. Biological properties of herpes simplex virus 2 replication-defective mutant strains in a murine nasal infection model. Virology. 2000;278:137–150. doi: 10.1006/viro.2000.0628. [DOI] [PubMed] [Google Scholar]
- 12.Smith CM, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 13.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. The Journal of experimental medicine. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gebhardt T, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nature medicine. 2009;15:886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iijima N, et al. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. The Journal of experimental medicine. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parr MB, Parr EL. Intravaginal administration of herpes simplex virus type 2 to mice leads to infection of several neural and extraneural sites. J Neurovirol. 2003;9:594–602. doi: 10.1080/13550280390246499. [DOI] [PubMed] [Google Scholar]
- 19.Mackay LK, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khanna KM, Lepisto AJ, Hendricks RL. Immunity to latent viral infection: many skirmishes but few fatalities. Trends Immunol. 2004;25:230–234. doi: 10.1016/j.it.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Perkins N, Nisbet M, Thomas M. Topical imiquimod treatment of aciclovir-resistant herpes simplex disease: case series and literature review. Sex Transm Infect. 2011;87:292–295. doi: 10.1136/sti.2010.047431. [DOI] [PubMed] [Google Scholar]
- 22.Gill N, Davies EJ, Ashkar AA. The role of toll-like receptor ligands/agonists in protection against genital HSV-2 infection. Am J Reprod Immunol. 2008;59:35–43. doi: 10.1111/j.1600-0897.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol. 2010;10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajewski TF, Fuertes M, Spaapen R, Zheng Y, Kline J. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr Opin Immunol. 2011;23:286–292. doi: 10.1016/j.coi.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parr MB, et al. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369–380. [PubMed] [Google Scholar]
- 26.Spang AE, Godowski PJ, Knipe DM. Characterization of herpes simplex virus 2 temperature-sensitive mutants whose lesions map in or near the coding sequences for the major DNA-binding protein. Journal of virology. 1983;45:332–342. doi: 10.1128/jvi.45.1.332-342.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2:152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- 28.Morrison LA, Da Costa XJ, Knipe DM. Influence of mucosal and parenteral immunization with a replication- defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology. 1998;243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- 29.Aljanabi SM, Martinez I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Research. 1997;25:4692–4693. doi: 10.1093/nar/25.22.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soderberg KA, Linehan MM, Ruddle NH, Iwasaki A. MAdCAM-1 Expressing Sacral Lymph Node in the Lymphotoxin OE≤-Deficient Mouse Provides a Site for Immune Generation Following Vaginal Herpes Simplex Virus-2 Infection. The Journal of Immunology. 2004;173:1908–1913. doi: 10.4049/jimmunol.173.3.1908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.