Abstract
Objectives
To compare the prevalence of periodontal disease between two randomly selected population-based studies (the Oral Infections and Vascular Disease Epidemiology Study (INVEST) and the Study of Health in Pomerania (SHIP)) and address relevant methodological issues.
Methods
Comparison was restricted to 55–81-years-olds. Attachment loss (AL), probing depth (PD), and tooth count were assessed in INVEST (full-mouth, six sites) and SHIP (half-mouth, four sites). Subjects were classified according to the CDC/AAP case definition. Recording protocols were standardized. Mixed linear or logistic models were used to compare INVEST with SHIP.
Results
Mean half-mouth AL was lower in INVEST vs. SHIP (INVEST: 2.9mm versus SHIP: 4.0mm, p<0.05). Findings were similar across multiple periodontal disease definitions. After equalisation of recording protocols and adjustment for periodontal risk factors, mean AL and PD were 1.2mm and 0.3mm lower in INVEST vs. SHIP (p<0.001). The odds for severe periodontitis (CDC/AAP) was 0.2-fold in INVEST vs. SHIP (p<0.001). Confounding effects of age, gender, race/ethnicity, education, and use of interdental care devices were highest as indicated by change-in-estimate for study.
Conclusion
Implementation of the proposed method for comparison of epidemiological studies revealed that periodontitis was less prevalent in INVEST compared to SHIP, even after extensive risk-factor adjustment.
Keywords: Cross-cultural comparison, Periodontal disease, Attachment loss, Probing depth, Periodontal risk factors
Introduction
Periodontal diseases are highly prevalent and substantial variation in periodontal disease prevalence both between and within country has been reported (Demmer and Papapanou 2010). These observations arise from several recent reviews, which compared the periodontal status between epidemiological studies stratified by age and/or gender (Crocombe et al. 2009, Demmer and Papapanou 2010, Konig et al. 2010). For example, compared with the United States (Dye et al. 2007), periodontal diseases are apparently more prevalent in Europe (Konig et al. 2010), especially in Germany (Holtfreter et al. 2009, Holtfreter et al. 2010). However, the lack of standardized clinical periodontal examination protocols and standardized periodontal disease definitions (Preshaw 2009), inferences regarding the true global variation in disease prevalence are limited. Moreover, the lack of adjustment for inherent differences in risk factor profiles (age, diabetes mellitus, smoking status, health care availability, oral hygiene behaviours, etc.) (Savage et al. 2009) prevents a better understanding of the reasons for global prevalence variation.
We are unaware of any systematic comparisons of periodontal disease prevalence using large population-based epidemiological studies, standardized definitions of periodontal disease, harmonised clinical periodontal assessments, and direct adjustment for variations in periodontal disease risk factors. Studies of this nature are important to inform the magnitude of variation in disease prevalence as well as the degree to which traditional risk factors explain the observed global variation. The latter point is important because it informs the importance of currently unidentified causal risk factors for periodontal disease.
For this purpose we evaluated data from the Study of Health in Pomerania (SHIP, Germany) and the Oral Infections and Vascular Disease Epidemiology Study (INVEST, New York, United States). A comparison of these two studies was of specific interest, because they both sampled unique populations within their countries. Both populations are characterised by high rates of cardiovascular diseases and its risk factors (Meisinger et al. 2006, Sacco et al. 1998, Volzke et al. 2009, Janhsen et al. 2008). Because both are regional studies, whose populations cannot be considered as being nationally representative, the major focus of this study is to provide guidance with respect to the analytical methodologies involved in the comparison of periodontal disease prevalence between the two studies.
The aim of this study was to i) analyse differences in periodontal status between INVEST and SHIP using standardized definitions of periodontal disease arising from harmonized clinical periodontal protocols, ii) to evaluate the relationship between study (INVEST versus SHIP) and periodontal disease adjusting for established periodontal risk factors, and iii) to determine whether or not adjustment for established periodontal risk factors removed study effects.
Material and methods
Study of Health in Pomerania (SHIP)
The Study of Health in Pomerania (SHIP) is a prospective population-based cohort study in Pomerania with baseline examinations conducted in 1997–2001 as previously described (John et al. 2001). Briefly, a two-stage cluster sampling design was adopted (Keil et al. 1988). From the entire population of 212,157 inhabitants, 7008 adults aged 20–79 years, with German citizenship and main residency within the target region, were randomly selected, proportional to each community population size, and stratified by age and gender. After 746 subjects were excluded (126 died, 615 moved away, five had severe medical problems), 6262 inhabitants were invited. The net random sample included 4308 individuals. The response rate was 68.8%.
Oral Infections and Vascular Disease Epidemiology Study (INVEST)
The Oral Infections and Vascular Disease Epidemiology Study (INVEST) is a randomly sampled prospective population-based cohort study in New York with baseline examinations conducted in 1999–2003 (Desvarieux et al. 2010). Briefly, 1056 subjects were randomly selected from Northern Manhattan, an area between 145th Street and 218th Street, bordered westward by the Hudson River, and separated eastward from the Bronx by the Harlem River. Hispanics, Black, and Whites live together in this area and have similar access to medical care. The selection process was derived from the Northern Manhattan Study (NOMAS) in which patients are also enrolled (Sacco et al. 1998).
Eligibility criteria for INVEST are as follows: 1) Hispanic, Black, or White resident (>3 months) of Northern Manhattan (zip-codes 10031, 10032, 10033, 10034 and 10040); 2) contacted by random digit dialing among households with a telephone (one adult from an eligible household was randomly chosen for the telephone interview, telephone response rate was 94%); 3) age 55 or older at time of first in-person assessment; 4) no baseline history of stroke, myocardial infarction, or chronic inflammatory conditions such as systemic lupus erythematosus, Lyme’s disease, gonococcal arthritis or bacterial endocarditis; 5) ability to come to the clinic. About 70% of those who completed the telephone interview and were called by the INVEST/NOMAS staff completed an in-person assessment.
Details on oral examination and assessment of covariate data for SHIP and INVEST are provided in the Online Supplement.
Comparison
Derivation of data
Both studies were restricted to subjects aged 55–81 years with dental recordings, leaving 1824 subjects in SHIP and 948 subjects in INVEST. Details on derivation of final sample sizes in SHIP and INVEST are given in the Online Supplement (Suppl. Fig. 1).
To avoid problems associated with diverse distribution of race/ethnicity in both samples and to enhance comparability between both studies, analyses were also performed in Whites only. Because of small sample sizes of Whites in INVEST, we present results in the Online Supplement.
Probe correction
The use of different manual periodontal probes in SHIP-0 (PCP-11) and INVEST (UNC-15) introduced a bias on periodontal measures through digit-preference of probe markings (Holtfreter et al.). Thus, we corrected periodontal measures on site level from SHIP according to correction factors (basic probe: PCP-11, target probe: UNC-15, Suppl. Table 1) retrieved from a cross-over study conducted at the University of Greifswald. Mean PD and AL were recalculated after probe correction of periodontal site measurements.
Assessment of periodontal status
To reduce bias of AL, PD, and BOP measures due to periodontal protocols, periodontal disease severity in INVEST subjects was recalculated based on the recording protocol used in SHIP (half-mouth, four sites). Prevalence was defined as the percentage of subjects having at least one site with a given condition, e.g. AL≥3 mm and extent was defined as the percentage of sites exhibiting that condition (Holtfreter et al. 2009). Mean AL/PD were calculated. No restriction according to the number of teeth was imposed. Edentulous subjects were excluded from analyses (Tables 2–4). To visualize periodontal situations in both populations, we showed percentile plots which are based on extent measures of AL or PD (e.g. ≥3 mm).
Statistical analyses
Chi-square tests or Mann-Whitney-U-tests were used to compare variable distributions between INVEST and SHIP subjects. Differences in periodontal variables between full-mouth (six sites) and half-mouth (four sites) recordings in INVEST subjects were compared using the Wilcoxon matched-pairs signed-ranks test or the McNemar test. Respective p values were adjusted for multiple testing controlling the false discovery rate (Benjamini and Hochberg 1995).
Mixed regression models were used to evaluate differences in periodontal variables according to study (INVEST versus SHIP). Study and covariates (gender, age, race/ethnicity, education, smoking status, pack years, diabetic status, BMI, last dental visit was within last 12 months, tooth brushing frequency, and use of dental care devices) were included as fixed effects. To account for the clustering of subjects within both studies, variation within studies was described by random intercepts. Analyses were model-based; for SHIP sampling weights and design-based variables were not considered.
Linear and logistic models were used depending on whether the dependent variable was continuous (extent, mean) or dichotomous (prevalence, CDC/AAP classification). Accordingly, linear regression coefficients (B) or Odds Ratios (OR) with their respective 95% confidence intervals (CI) were reported. Adjusted means or mean predicted probabilities with their standard errors (SE) were reported, respectively.
To determine how study effects depend on putative and established risk factors, change-in-estimate for ‘study’ was evaluated. Age and gender were included first, followed by race/ethnicity. The order in which remaining risk factors were entered into the model was based on the expected effect based on Table 2.
In addition, the added predictive ability of ‘study’ was evaluated. The change in R2 from the model including all periodontal risk factors was compared with the R2 from the model including additionally ‘study’. Linear regression models were evaluated.
To exclude bias by subjects with few teeth, study effects were recalculated including only subjects with ≥12 measurement sites. Analyses were also stratified by number of teeth.
Data analysis was performed with STATA/SE 12.0 (StataCorp 2011) and R 2.15.0 (R Development Core Team 2012). Unless stated otherwise, the statistical significance level was set at p<0.05.
Results
Crude comparison of SHIP and INVEST
When comparing full-mouth estimates for INVEST with half-mouth estimates for SHIP (Suppl. Table 2), INVEST participants presented significantly lower estimates for various periodontal disease definitions than SHIP participants.
Comparison of half-mouth results from both studies (Table 1) showed that INVEST subjects revealed significantly lower prevalence and extent values for AL than SHIP subjects (p<0.05). Prevalence of AL≥5 mm was 73.3% in INVEST vs. 85.1% in SHIP; 16.6% fewer sites were affected by AL≥5 mm in INVEST subjects. Mean AL was 2.9 mm in INVEST vs. 4.0 mm in SHIP (p<0.05). Probe corrected results were unchanged (p<0.05, Table 1).
Table 1.
Crude prevalence and extent values (%) according to varying thresholds for attachment loss (AL) and probing depth (PD) for 55–81-year-old subjects from SHIP and INVEST (all subjects and Whites only) using half-mouth protocols with four sites.
| SHIP | INVEST | INVEST Whites | |
|---|---|---|---|
| N-AL/N-PD | 1182/1317 | 756/775 | 127/133 |
| Prevalence of subjects with, % | |||
| AL≥4 mm | 94.4 (0.7) | 89.9 (1.1) * | 83.5 (3.3) * |
| AL≥5 mm | 85.1 (1.0) | 73.3 (1.6) * | 63.0 (4.3) * |
| AL≥6 mm | 69.8 (1.3) | 55.0 (1.8) * | 49.6 (4.5) * |
| Mean percentage of sites/subject with, % | |||
| AL≥4 mm | 51.4 (1.0) | 31.2 (1.0) * | 23.9 (2.1) * |
| AL≥5 mm | 35.4 (0.9) | 18.9 (0.9) * | 12.9 (1.7) * |
| AL≥6 mm | 23.6 (0.9) | 10.1 (0.6) * | 6.6 (1.1) * |
| Mean AL, mm | 4.0 (0.06) | 2.9 (0.06) * | 2.5 (0.11) * |
| Mean AL, mm # | 3.8 (0.05) | 2.9 (0.06) * | 2.5 (0.11) * |
| Prevalence of subjects with, % | |||
| PD≥4 mm | 78.1 (1.1) | 61.5 (1.7) * | 59.4 (4.3) * |
| PD≥5 mm | 54.0 (1.4) | 39.1 (1.8) * | 30.1 (4.0) * |
| PD≥6 mm | 35.7 (1.3) | 16.6 (1.3) * | 12.0 (2.8) * |
| Mean percentage of sites/subject with, % | |||
| PD≥4 mm | 18.2 (0.6) | 10.9 (0.6) * | 6.5 (0.9) * |
| PD≥5 mm | 9.5 (0.4) | 5.4 (0.4) * | 2.5 (0.7) * |
| PD≥6 mm | 5.4 (0.3) | 1.5 (0.2) * | 1.0 (0.5) * |
| Mean PD, mm | 2.8 (0.02) | 2.3 (0.03) * | 2.2 (0.05) * |
| Mean PD, mm # | 2.6 (0.02) | 2.3 (0.03) * | 2.2 (0.05) * |
| CDC/AAP classification, % | |||
| No or mild | 22.3 (1.2) | 34.6 (1.8) | 42.1 (4.4) |
| Moderate | 45.5 (1.5) | 48.6 (1.8) | 43.7 (4.4) |
| Severe | 32.2 (1.4) | 16.8 (1.4) * | 14.3 (3.1) * |
| Tooth count, incl. edentulous subjects † | 10.6 (0.2) | 14.4 (0.3) * | 19.0 (0.8) * |
| Tooth count, dentates † | 14.3 (0.2) | 17.4 (0.3) * | 21.1 (0.6) * |
| Edentulous, % † | 25.8 (1.0) | 17.3 (1.2) * | 10.1 (2.5) * |
| BOP, % | 43.3 (1.2) | 28.5 (1.1) * | 22.1 (2.6) * |
Data are presented as Mean (SE) or percentage (SE).
N-AL, number of subjects with available attachment loss data; N-PD, number of subjects with available probing depth data; BOP, Bleeding on probing.
p<0.05 for Mann-Whitney-U-test or Chi-square test comparing SHIP with INVEST or INVEST Whites.
probe corrected for SHIP
including all subjects with dental data
Similarly, prevalence and extent values for PD were significantly lower in INVEST compared with SHIP (p<0.05, Table 1).
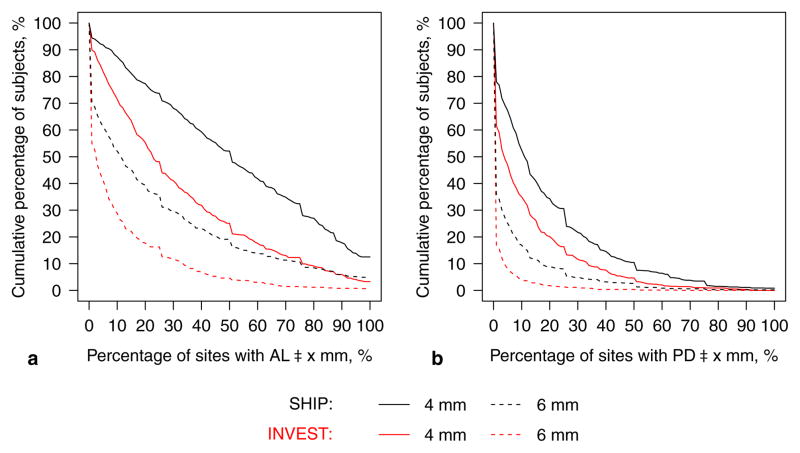
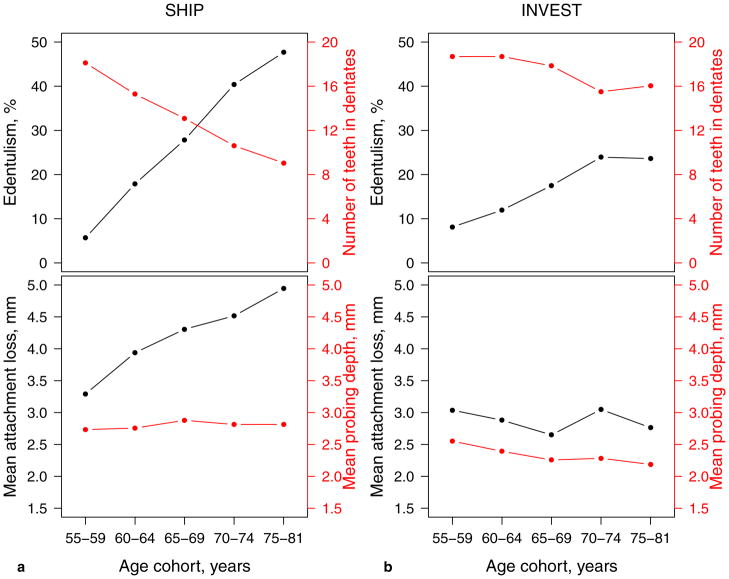
In both studies, the likelihood of multi-site involvement decreased as thresholds increased from ≥4 to ≥6 mm (Fig. 1). Differences in disease extent estimates between INVEST and SHIP were more pronounced for AL (Fig. 1a) than for PD (Fig. 1b). The percentage of subjects presenting PD≥4 mm at ≥30% of sites was 11.6% in INVEST and 21.7% in SHIP. The age-dependent change in tooth count, mean AL, and mean PD was significantly different between both studies (p for interaction=0.03, <0.001, and 0.02, respectively, Fig. 2). The main factor leading to markedly higher mean AL in SHIP compared to INVEST was gingival recession rather that periodontal pocket formation, irrespective of age. In INVEST, both mean AL and mean PD were constant across age groups. Further, a steeper increase in the percentage of edentulous subjects and a more progressively decreasing number of missing teeth in dentate subjects was observed in SHIP compared to INVEST.
Figure 1.
Extent of a) attachment loss (AL) and b) probing depth (PD) for SHIP (in black) and INVEST (in red), age 55–81 years. The percentage of affected sites was reported on the x-axis such that subjects most severely affected are located to the right; i.e., each point refers to the percentage of subjects (y-axis) exhibiting AL≥x mm on at least x% of the examined sites (x-axis). E.g., the percentage of subjects presenting AL≥4 mm at ≥30% of sites was 68.0% in SHIP and 40.4% in INVEST.
Figure 2.
For SHIP (a) and INVEST (b): Top figures present percentage of edentulous subjects (in black) and number of present teeth in dentate subjects (in red); while bottom figures present mean attachment loss (in black), and mean probing depth (in red) according to age cohorts (x-axis). p<0.001, p=0.02, and p=0.03 for interaction of study with age group for mean attachment loss, mean probing depth, and tooth count, respectively.
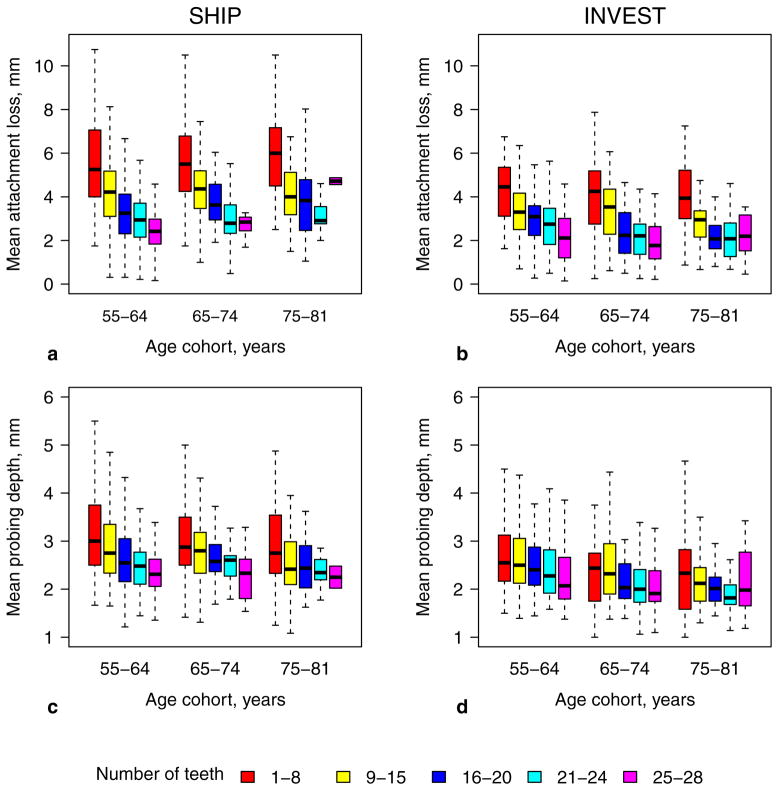
Moreover, in both studies mean AL increased as tooth count decreased within each 10-year age cohort (Fig. 3a–c). Though less distinct, the same pattern was found for PD (Fig. 3d–f).
Figure 3.
Mean attachment loss (a, b) and mean probing depth (c, d) according to age cohorts and number of present teeth categories (colouring) for SHIP (left) and INVEST (right). Outliers were excluded.
Characteristics for subjects with complete risk factor data
Table 2 describes the characteristics of subjects with available AL and covariate data in SHIP (n=1158), INVEST (n=670), and after restriction to INVEST Whites (n=108). Both study populations differed significantly with respect to race/ethnicity, age, gender, education, pack years, and BMI (p<0.05). Oral health care was significantly better in INVEST subjects compared with SHIP subjects (p<0.05). INVEST Whites were highly educated, less often smokers, had a better metabolic status and better oral hygiene patterns compared to the total INVEST sample.
Table 2.
Characteristics for dentate subjects with available attachment loss data and complete covariate data in SHIP and INVEST.
| SHIP | INVEST | INVEST Whites | |
|---|---|---|---|
| N | 1158 | 670 | 108 |
| Age (years) | 64.5 ± 6.7 | 67.3 ± 7.3 *** | 70.8 ± 6.9 *** |
| Gender (male) | 609 (52.6%) | 277 (41.3%) *** | 46 (42.6%) ** |
| Education (SHIP/INVEST) | |||
| ≤9 years/≤8th grade | 770 (66.5%) | 243 (36.3%) | 9 (8.3%) |
| 10–11 years/some high school | 216 (18.7%) | 91 (13.6%) | 3 (2.8%) |
| 12 years/completed high school or some college | 41 (3.5%) | 210 (31.3%) | 45 (41.7%) |
| Completed university/completed college | 131 (11.3%) | 126 (18.8%) *** | 51 (47.2%) *** |
| Race/ethnicity | |||
| White | 1158 (100.0%) | 108 (16.1%) | 108 (100.0%) |
| Black | - | 142 (21.2%) | - |
| Hispanic | - | 406 (60.6%) | - |
| Other | - | 14 (2.1%) | - |
| Smoking status | |||
| Never | 523 (45.2%) | 339 (50.6%) | 47 (43.5%) |
| Former | 476 (41.1%) | 246 (36.7%) | 53 (49.1%) |
| Current | 159 (13.7%) | 85 (12.7%) | 8 (7.4%) |
| Pack years (packs/day × years) | 9.6 ± 17.2 | 11.6 ± 23.4 * | 14.9 ± 26.2 ** |
| Body Mass Index (kg/m2) | |||
| <25 | 224 (19.3%) | 172 (25.7%) | 37 (34.3%) |
| 25–<30 | 551 (47.6%) | 274 (40.9%) | 44 (40.7%) |
| ≥30 | 383 (33.1%) | 224 (33.4%) ** | 27 (25.0%) |
| Diabetes mellitus/Diabetic (yes) # | 211 (18.2%) | 127 (19.0%) | 15 (13.9%) |
| Last dental visit was within last 12 months (yes) † | 1044 (90.2%) | 479 (71.5%) *** | 87 (80.6%) ** |
| Tooth brushing frequency ‡ | |||
| <2 times/day | 246 (21.2%) | 109 (16.3%) | 29 (26.8%) |
| 2 times/day | 779 (67.3%) | 419 (62.5%) | 64 (59.3%) |
| >2 times/day | 133 (11.5%) | 142 (21.2%) *** | 15 (13.9%) |
| Use of interdental care devices (yes) $ | 365 (31.5%) | 304 (45.4%) *** | 60 (55.6%) *** |
In addition, INVEST subjects were also restricted to Whites only (last column). Data are presented as mean ± standard deviation (SD) or numbers (percentages).
p<0.05,
p<0.01,
p<0.001, Chi-square test or Mann-Whitney-U-test comparing variable distributions between INVEST/INVEST Whites and SHIP.
INVEST: self-reported and/or fasting plasma glucose >126 mg/dl; SHIP: self-reported, anti-diabetic medication (ATC-Code A10), and/or HbA1c ≥6.5%
INVEST: ‘When was the last time you went to the dentist?’; SHIP: ‘When was the last time you went to the dentist?’
INVEST: ‘How many times did you brush your teeth yesterday?’; SHIP: ‘How many times do you normally brush your teeth?’
INVEST: ‘How many times did you floss your teeth over the last six days?’; SHIP: ‘Do you use other dental care devices?’
Mixed model evaluating the study effect
All further analyses were performed in all INVEST subjects (Main document) and in Whites only (see Online Supplement).
To account for differential periodontal risk factor distributions between studies, adjusted effects for ‘study’ were determined for the full sample (Table 3) or INVEST Whites vs. SHIP only (Suppl. Table 3). For prevalences of AL, significant ORs for ‘study’ ranged between 0.4 (≥4 mm) and 0.6 (≥6 mm). Adjusted probabilities of having at least one site with AL≥4 mm were 96.1% in SHIP and 90.1% in INVEST. For extent values of AL, differences between both studies were highest for AL≥4 mm (B=−22.7 (−29.1; −16.3)) and lowest for AL≥6 mm (B=−13.6 (−18.9; −8.4)). Mean AL was 1.2 mm lower in INVEST subjects (for full model see Suppl. Table 4). After probe correction in SHIP, the difference in mean AL was 1.0 mm. Similarly, prevalences and extent measures of PD and risk for severe periodontitis were significantly lower in INVEST compared with SHIP (Table 3).
Table 3.
Mixed regression models evaluating study effects (exposure: INVEST versus SHIP (ref.), half-mouth with four sites, regression coefficients (B) or Odds Ratios (OR) listed in the last column) on periodontal variables adjusting for remaining covariates. Model predicted values according to study are given as mean (SE). Subjects were 55–81 years old.
| SHIP | INVEST | Study effect | |
|---|---|---|---|
| N-AL/N-PD | 1158/1285 | 670/688 | |
| Prevalence of subjects with, % | OR (95% CI) | ||
| AL≥4 mm | 96.1 (0.2) | 90.1 (0.2) | 0.4 (0.2; 0.8) ** |
| AL≥5 mm | 86.8 (0.1) | 74.6 (0.2) | 0.4 (0.3; 0.8) ** |
| AL≥6 mm | 69.5 (0.1) | 58.4 (0.2) | 0.6 (0.4; 1.0) * |
| Mean percentage of sites/subject with, % | B (95% CI) | ||
| AL≥4 mm | 52.2 (1.4) | 29.5 (2.2) | −22.7 (−29.1; −16.3) *** |
| AL≥5 mm | 35.7 (1.3) | 17.8 (2.1) | −18.0 (−24.0; −11.9) *** |
| AL≥6 mm | 23.4 (1.1) | 9.8 (1.8) | −13.6 (−18.9; −8.4) *** |
| Mean AL, mm | 4.0 (0.08) | 2.8 (0.12) | −1.2 (−1.5; −0.8) *** |
| Mean AL, mm # | 3.9 (0.08) | 2.8 (0.12) | −1.0 (−1.4; −0.7) *** |
| Prevalence of subjects with, % | OR (95% CI) | ||
| PD≥4 mm | 75.6 (0.1) | 70.5 (0.2) | 0.8 (0.5; 1.2) |
| PD≥5 mm | 53.1 (0.1) | 40.0 (0.2) | 0.6 (0.4; 0.9) * |
| PD≥6 mm | 34.3 (0.1) | 16.5 (0.2) | 0.4 (0.2; 0.7) ** |
| Mean percentage of sites/subject with, % | B (95% CI) | ||
| PD≥4 mm | 17.7 (0.8) | 11.6 (1.4) | −6.1 (−10.2; −2.1) ** |
| PD≥5 mm | 9.2 (0.6) | 5.8 (1.1) | −3.3 (−6.4; −0.2) * |
| PD≥6 mm | 4.8 (0.5) | 2.4 (0.8) | −2.4 (−4.7; −0.2) * |
| Mean PD, mm | 2.7 (0.04) | 2.4 (0.06) | −0.3 (−0.5; −0.2) *** |
| Mean PD, mm $ | 2.6 (0.04) | 2.4 (0.06) | −0.2 (−0.4; −0.05) * |
| CDC/AAP classification, % | OR (95% CI) | ||
| moderate/severe versus no or mild | 70.0 (0.1) | 62.3 (0.2) | 0.7 (0.4; 1.1) |
| severe versus no or mild | 67.1 (0.1) | 32.6 (0.2) | 0.2 (0.1; 0.5) *** |
p<0.05,
p<0.01,
p<0.001,
before calculation of mean values, periodontal site measurements were corrected using probe correction values (see Online Supplement).
Mixed linear (for mean and extent values) or logistic models (for prevalences and CDC/AAP classification) with ‘study’ as the exposure variable adjusting for age, gender, race/ethnicity, smoking status, pack years, education, diabetic status, BMI, last dental visit was within last 12 months, tooth brushing frequency, and use of interdental care devices.
N-AL, number of subjects in models where attachment loss (AL) was the dependent variable; N-PD, number of subjects in models where probing depth (PD) was the dependent variable.
For dichotomous periodontal variables (prevalence, CDC/AAP classification), adjusted predicted probabilities with standard errors (SE) for linear predictions are given. For continuous periodontal variables (mean, mean percentage of sites/subject) adjusted linear predictions with their standard errors (SE) are given.
Comparing only Whites, INVEST subjects had significantly lower prevalences and extent measures of AL and PD and lower prevalences of moderate to severe or severe periodontitis (CDC/AAP) compared with SHIP subjects (p<0.05, Suppl. Table 3).
In sensitivity analyses, subjects were stratified by number of teeth (Suppl. Table 5). In both subgroups, study effects for prevalences and extent measures of AL were still statistically significant, while study effects for extent measures of PD and probe corrected mean PD lost significance. Further, results were confirmed if subjects were restricted to those with ≥12 measurement sites (mean AL: B= −1.0 (−1.3; −0.6); mean PD: B=−0.3 (−0.4; −0.1)).
Dependency of the study effect on putative and established periodontal risk factors
To evaluate how the study effect depends on periodontal risk factor distributions, regression models were created to assess change-in-estimate for ‘study’ corresponding to the sequential, stepwise addition of periodontal risk factors (Table 4). For mean AL, the coefficient for ‘study’ from the crude model changed by 5.5% after simultaneous inclusion of age and gender. Further, inclusion of ethnicity (47.4%), education (28.6%), and use of interdental cleaning devices (8.0%) lead to relevant changes in the coefficient for ‘study’ of more than 5%.
Table 4.
Change in regression coefficient estimates (B, with 95% confidence interval (CI)) for study (INVEST vs. SHIP) for stepwise inclusion of periodontal risk factors into mixed regression models. Risk factors were included according to their association with study in Table 2.
| B (95%-CI) for study | Relative change in B | |
|---|---|---|
| Dependent variable: Mean attachment loss | ||
| Study | −1.10 (−1.27; −0.93) | |
| +Age and gender | −1.16 (−1.33; −1.00) | 5.5% |
| +Race/ethnicity | −1.71 (−2.05; −1.37) | 47.4% |
| +Last dental visit was within last 12 months | −1.75 (−2.09; −1.42) | 2.3% |
| +Education | −1.25 (−1.62; −0.89) | 28.6% |
| +Use of interdental care devices | −1.15 (−1.51; −0.78) | 8.0% |
| +Tooth brushing frequency | −1.15 (−1.51; −0.78) | 0% |
| +Body Mass Index | −1.14 (−1.50; −0.77) | 0.9% |
| +Smoking status and pack years | −1.19 (−1.55; −0.83) | 4.4% |
| +Diabetes mellitus/Diabetic | −1.18 (−1.54; −0.82) | 0.8% |
|
| ||
| Dependent variable: Mean probing depth | ||
| Study | −0.45 (−0.52; −0.38) | |
| +Age and gender | −0.42 (−0.50; −0.34) | 6.7% |
| +Race/ethnicity | −0.57 (−0.73; −0.42) | 35.7% |
| +Last dental visit was within last 12 months | −0.58 (−0.75; −0.42) | 1.8% |
| +Education | −0.40 (−0.57; −0.23) | 31.0% |
| +Use of interdental care devices | −0.35 (−0.51; −0.18) | 12.5% |
| +Tooth brushing frequency | −0.35 (−0.51; −0.18) | 0% |
| +Body Mass Index | −0.34 (−0.50; −0.17) | 2.9% |
| +Smoking status and pack years | −0.35 (−0.52; −0.17) | 2.9% |
| +Diabetes mellitus/Diabetic | −0.34 (−0.51; −0.18) | 2.9% |
Multivariable adjustment for putative AL risk factors did not remove study effects but rather enhanced the between study disparity in periodontal disease severity (Table 4). Conversely, multivariable adjustment attenuated the differences in mean PD between studies, although differences remained statistically significant (Table 4).
We additionally evaluated the added predictive ability of ‘study’ comparing R2 from the model including all periodontal risk factors with the model including additionally ‘study’. ‘Study’ additionally explained 1.8% of total variation for mean AL and 0.7% for mean PD, resulting in a total R2 of 23.6% and 15.4%, respectively.
Discussion
This is the first study to directly compare the prevalence of periodontal disease between two randomly selected population-based samples, while concurrently harmonising periodontal recording protocols and definitions in addition to adjusting for differences in periodontal risk factors. In doing so we have found the prevalence of periodontal disease in INVEST to be half that of SHIP and these results were sustained after multivariable risk factor adjustment. Especially in case of mean AL, unmeasured factors related to study population explained a sizable portion of variation in periodontal disease (1.8%).
A comparison of these two studies was of specific interest, because they both sampled unique populations within their countries. Both studies represent higher-risk populations with an underlying profile of unhealthy habits and a specific cohort history. Most SHIP subjects had been living their whole life in the former German Democratic Republic. East Germans had higher prevalences of risk markers, chronic diseases and increased mortality compared to West Germans (Gaber and Wildner 2011, Janhsen et al. 2008), with highest rates of cardiovascular diseases being reported for subjects living in West Pomerania, the catchment area of SHIP (Gaber and Wildner 2011, Volzke et al. 2009, Meisinger et al. 2006). INVEST subjects had a specific ethnic composition and known higher incidence rates of cardiovascular diseases among Hispanics (Sacco et al. 1998). Importantly, INVEST subjects lived in the same environment, presenting homogeneity with regard to SES and access to care. While neither study is nationally representative, both studies are representative of their respective source populations.
In agreement with this study, a crude comparison of previously published national studies supports the notion that periodontal disease prevalence is lower in the US populations when compared to Germany. Periodontal disease prevalence was markedly lower in NHANES (Dye et al. 2007) compared with DMS (Holtfreter et al. 2010). Even if some methodological bias cannot be avoided, this study presents a thorough and valid comparison of periodontal status in two geographically distinct populations.
To a large part, ‘study’ is a surrogate for differences in potential periodontal risk factor distributions and different cohort life time experience between SHIP and INVEST. Thus, adjustment for periodontal risk factors is important to better understand the reasons for the observed inter-study differences. In other words, differential disease prevalences between SHIP and INVEST are explained by measured and unmeasured periodontal disease risk factors. In this study, differences in mean AL/PD between SHIP and INVEST were most explained by differences in age, gender, ethnicity, education, and use of interdental care devices.
In addition to confounding by traditional risk factors, the role of cohort effects are also likely to play a role but could not be accounted for presently. Cohort effects comprise historical differences in social and physical environments between cohorts. SHIP and INVEST subjects were born between 1916 and 1946 - during and after the time of the First and Second World War. Poor social conditions, reduced public utility infrastructure, and poor health care during both World Wars and, particularly, an economy of scarcity after the World Wars in the former German Democratic Republic might have aggravated general and oral health care differentially across cohorts. The diversity of race/ethnicity in INVEST is also important to note. Hispanics and Blacks amount to 79% of INVEST participants. They quite possibly experienced historical hardships that led to great socioeconomic (Williams 1996, Williams 1999) and health disparities (Franzini et al. 2001, Markides and Coreil 1986), including periodontitis (Borrell and Crawford 2008, Borrell et al. 2006). In this context, several exposures like racial discrimination, neighbourhood socioeconomic conditions, limited access to and quality of care may act directly or indirectly on periodontal diseases (Borrell and Crawford 2008, Williams 1999). The race/ethnicity differential was also apparent in the INVEST data (Suppl. Table 3, 6 and Suppl. Fig. 2, 3). Regarding periodontal prevalences and extent measures, Whites were better off than Hispanics (p<0.05, Suppl. Table 6). Lower periodontal prevalences in Whites as compared to Hispanics might be partially attributed to higher percentages of subjects having ≥12 years of education (89% vs. 29%, respectively). Thus, data support the notion that race/ethnicity is a major determinant of one’s socioeconomic status and, consequently, one’s periodontal status. Further, the fact that adjustment for race/ethnicity enhanced the observed disparities in periodontal health between the two studies (Table 4) bolsters the notion that historical hardships are likely to be meaningful contributors to these disparities.
Differences in educational background explained the second largest amount of differences in periodontal status between both samples. Changes-in-estimates for ‘study’ were 21.0% for mean AL and 24.1% for mean PD, respectively. Socio-economic status is considered to be an important risk factor for periodontitis (Borrell and Papapanou 2005). It attributes to socially patterned lifestyles, unequal access to and quality of health care, and differences in the psychosocial environment over the life course (Mundt et al. 2009). Education was associated with periodontal disease (Borrell et al. 2006, Borrell and Crawford 2008) and the number of missing teeth and incident tooth loss both in the United States (Gilbert et al. 2003) and Germany (Mundt et al. 2011, Mundt et al. 2007). The higher educational status in INVEST subjects compared with SHIP subjects, in combination with better access to and quality of dental care appears to have contributed to the observed lower prevalence of periodontitis in INVEST.
Health care utilisation and motivation for and performance of oral hygiene are important factors affecting periodontal health (van der Weijden and Slot 2011). Here, study effects might depend on different distributions of oral hygiene and care in both studies as indicated by change-in-estimate for mean PD/AL. INVEST subjects appeared to be more motivated to carry out effective oral hygiene, which was also reflected in a higher mean tooth brushing frequency and a higher percentage of subjects using interdental care devices (see Table 2). Indeed, bleeding on probing, a clinical sign of gingival inflammation, was less prevalent in INVEST. Less gingival bleeding, in turn, was associated with less attachment loss and pocket formation (Schatzle et al. 2003).
In SHIP (Holtfreter et al. 2009) and in other studies (Beck and Koch 1994, Schurch and Lang 2004) AL increased across age groups, whereas PD remained constant after the age of 40. However, in contrast to SHIP, INVEST subjects presented a nearly constant slope for both AL and PD, reflecting less gingival recession in INVEST subjects. In addition, it is well known that edentulism and tooth loss increase with age with higher numbers in Germany (Mundt et al. 2007, Holtfreter et al. 2010) compared to US American populations (Dye et al. 2007, Elter et al. 2004). However, tooth retention did not explain the differences observed between samples. After stratification by number of teeth, periodontal status still differed significantly between samples (Suppl. Table 5). Thus, oral hygiene and care should be encouraged as a means to promote periodontal health.
While smoking status was similarly distributed in both studies, the number of pack years was significantly higher in INVEST subjects. However, the higher number of pack years in INVEST subjects did not result in relevant confounding by smoking status on the association between ‘study’ and periodontal disease.
Though diabetic status and obesity are considered as risk factors for periodontitis (Suvan et al. 2011, Lalla and Papapanou 2011), inclusion of both variables did not change the coefficient for ‘study’. The missing confounding effect was well explained by the comparable percentage of diabetic subjects in both studies (Table 2). Moreover, recent evidence from SHIP suggests that the influence of diabetes, while important, might have been overstated in previous studies (Demmer et al. 2012). Though obesity was differently distributed in both studies, it did not affect the association between ‘study’ and periodontal disease.
An important strength of this study was the use of direct standardisation of age range and recording protocols, including a between-study probe standardisation. Thus periodontal status in SHIP subjects was not only compared to full-mouth but also to half-mouth estimates for INVEST subjects, strengthening conclusions on disparities in periodontal status. Mean values of AL/PD correcting for probe-associated bias were calculated, providing also an estimate of probe-caused differences in mean AL/PD between both studies. Third, potential periodontal risk factors (age, gender, ethnicity, smoking, pack years, education, diabetes, BMI, dental care variables) were adjusted for in mixed linear or logistic models. Thus, study effects were directly estimated, while effects of periodontal risk factors were filtered out. Further, contributions of each periodontal risk factor in explaining study effects on mean AL/PD were determined.
The main limitation of this study was residual confounding in mixed models evaluating study effects of SHIP versus INVEST, one aspect being insufficient adjustment for social determinants including socio-economic status (SES). The SES attributes to socially patterned lifestyles, unequal access to and quality of health care, and differences in the psychosocial environment over the life course (Mundt et al. 2009). Previous studies have reported differences in periodontal health according to socioeconomic indicators (i.e., income and education) (Borrell et al. 2002, Borrell and Crawford 2008, Boillot et al. 2011). In this study, information on school education and college education were combined into four categories. Information on income was not available for INVEST. Occupational status was not comparable between the two studies and was afflicted with a high number of missing data. Thus, neither variable was accounted for in this study. However, it can be assumed that educational status correlates well with occupation, reducing residual confounding. Although the INVEST sample is very homogenous in regard to SES, the possibility for minor residual confounding of the association between ‘study’ and periodontal disease prevalence by income must be acknowledged.
Further, risk factor definitions were not identical in SHIP and INVEST. Diabetic status was defined differently due to unavailability of fasting glucose measurements in SHIP. HbA1c has recently been recommended by the American Diabetes Association (American Diabetes Association 2010) to diagnose diabetes. Using a 6.5% cut-off, HbA1c well reflects diabetic diagnoses using fasting glucose as the gold-standard (Selvin et al. 2011). Thus, it can be assumed that the percentage of false negative diabetic subjects in SHIP is low. Also, use of interdental cleaning devices included only flossing in INVEST, but use of ‘other’ interdental cleaning aids, including dental floss, tooth sticks, interdental brushes, etc. in SHIP.
The comparison of SHIP data with half-mouth estimates for INVEST is based on the assumption a partial recording protocol results in a similar underestimation of prevalence and extent of periodontitis irrespective of the study population it is applied on. However, it is possible that the degree of underestimation due to partial recordings may also depend on various properties of the study population. Finally, we need to point out that analyses were model-based. As a consequence, standard errors in SHIP might be underestimated, though to a minor degree (Holtfreter et al. 2009).
Inter-study differences may have occurred because of selection or methodological biases. In SHIP, the response rate was 68% with responders being healthier, younger, and less often male than non-responders. In INVEST, about 70% of those who completed the telephone interview and were called by the INVEST/NOMAS staff completed an in-person assessment; non-response analyses were not available. Because response rates were comparable in both studies, periodontal disease might be similarly underestimated. Item non-response was 2% for SHIP and 10% for INVEST. Assuming that subjects with worse item values refused to answer, periodontal disease might additionally be underestimated in INVEST. However, results in Table 1 were not severely affected by item non-response (data not shown) making it unlikely that selection bias was a major reason for inter-study differences.
SHIP and INVEST examiners were well calibrated and bias related to different examiners within studies might be low. However, because examiners in SHIP and INVEST were not cross-trained and there was no common reference examiner, it cannot be ruled out that clinical examinations were performed differently between studies. Thus, the bias introduced by different groups of examiners was not assessable. Different rounding schemes were unlikely to explain differences in periodontal measurements. In SHIP, measurements were rounded to the whole millimetre (up or down). In INVEST no specific formal rounding protocol was present. Other issues implicating bias when comparing two studies, i.e. the recording protocol and the periodontal probe, were adequately addressed in this study.
In summary, we have conducted a comprehensive inter-study comparison of periodontal disease prevalence among adult men and women living in either New York City or Northeast Germany. We have consistently found the prevalence of periodontal disease to be lower in INVEST compared with SHIP participants, even after accounting for periodontal risk factor distributions and differential recording protocols. Restricted to Whites only, differences were even stronger. Importantly, established periodontal risk factors did not fully explain the observed inter-study disease differences. Though unmeasured risk factors may partly account for residual inter-study differences, the results suggest that future research might uncover novel periodontal disease risk factors.
Supplementary Material
Clinical Relevance.
Scientific rationale for the study
To compare periodontal disease prevalence between SHIP and INVEST and to evaluate whether or not putative and established periodontal risk factors explain observed differences.
Principal findings
Periodontal disease severity was significantly higher in SHIP versus INVEST, even after equalisation of recording protocols and adjustment for periodontal risk factors. Differences in prevalences of periodontal disease between studies were confounded by disparities in age, gender, ethnicity, education, and use of interdental care devices.
Practical implications
There is a need for motivation in oral hygiene, oral health knowledge promotion and improved health care access in both sample populations.
Footnotes
Conflict of Interests and Source of Funding Statement
There are no conflicts of interest associated with this work.
SHIP is part of the Community Medicine Research net (CMR) of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (grant no. ZZ9603) and the Ministry of Cultural Affairs as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania (http://www.community-medicine.de). B.H. was financed by an unlimited educational grant by GABA, Switzerland. INVEST is financed by R01 DE-13094 (M.D.) and supported in part by Columbia University’s CTSA grant No. UL1 RR024156 from NCRR/NIH. This work has also been partly funded by an INSERM Chair of Excellence award (M.D.) by the French Agency for Research (ANR-R05115DD). M.D. is also the recipient of the Chair in Chronic Disease from the École des Hautes Études en Santé Publique, France. R.T.D. is supported by NIH grant #R00 DE-018739 and P.N.P. by NIH grants #DE-015649 and #DE-021820, CTSA Award #RR-025158, and by a grant from Colgate-Palmolive, USA.
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–69. doi: 10.2337/dc10-S062. 33/Supplement_1/S62 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JD, Koch GG. Characteristics of older adults experiencing periodontal attachment loss as gingival recession or probing depth. J Periodontal Res. 1994;29:290–298. doi: 10.1111/j.1600-0765.1994.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57:289–300. [Google Scholar]
- Boillot A, El Halabi B, Batty GD, Range H, Czernichow S, Bouchard P. Education as a predictor of chronic periodontitis: a systematic review with meta-analysis population-based studies. PLoS One. 2011;6:e21508. doi: 10.1371/journal.pone.0021508. PONE-D-11-01044 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell LN, Beck JD, Heiss G. Socioeconomic disadvantage and periodontal disease: the Dental Atherosclerosis Risk in Communities study. Am J Public Health. 2006;96:332–339. doi: 10.2105/AJPH.2004.055277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell LN, Burt BA, Gillespie BW, Lynch J, Neighbors H. Periodontitis in the United States: beyond black and white. J Public Health Dent. 2002;62:92–101. doi: 10.1111/j.1752-7325.2002.tb03428.x. [DOI] [PubMed] [Google Scholar]
- Borrell LN, Crawford ND. Social disparities in periodontitis among United States adults 1999–2004. Community Dent Oral Epidemiol. 2008;36:383–391. doi: 10.1111/j.1600-0528.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- Borrell LN, Papapanou PN. Analytical epidemiology of periodontitis. J Clin Periodontol. 2005;32(Suppl 6):132–158. doi: 10.1111/j.1600-051X.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- Crocombe LA, Mejia GC, Koster CR, Slade GD. Comparison of adult oral health in Australia, the USA, Germany and the UK. Aust Dent J. 2009;54:147–153. doi: 10.1111/j.1834-7819.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- Demmer RT, Holtfreter B, Desvarieux M, Jacobs DRJ, Kerner W, Nauck M, Völzke H, Kocher T. The Influence of Type 1 and Type 2 Diabetes on Periodontal Disease Progression: Prospective Results from the Study of Health in Pomerania (SHIP) Diabetes Care. 2012 doi: 10.2337/dc11-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer RT, Papapanou PN. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol 2000. 2010;53:28–44. doi: 10.1111/j.1600-0757.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvarieux M, Demmer RT, Jacobs DR, Jr, Rundek T, Boden-Albala B, Sacco RL, Papapanou PN. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST) J Hypertens. 2010;28:1413–1421. doi: 10.1097/HJH.0b013e328338cd36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, Eke PI, Beltran-Aguilar ED, Horowitz AM, Li CH. Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital Health Stat. 2007;11:1–92. [PubMed] [Google Scholar]
- Elter JR, Champagne CM, Offenbacher S, Beck JD. Relationship of periodontal disease and tooth loss to prevalence of coronary heart disease. J Periodontol. 2004;75:782–790. doi: 10.1902/jop.2004.75.6.782. [DOI] [PubMed] [Google Scholar]
- Franzini L, Ribble JC, Keddie AM. Understanding the Hispanic paradox. Ethn Dis. 2001;11:496–518. [PubMed] [Google Scholar]
- Gaber E, Wildner M. Robert Koch Institut and Statistisches Bundesamt. Gesundheitsberichterstattung des Bundes. Berlin: 2011. Mortality, causes of death, and regional differences [Sterblichkeit, Todesursachen und regionale Unterschiede] p. 51. [Google Scholar]
- Gilbert GH, Duncan RP, Shelton BJ. Social determinants of tooth loss. Health Serv Res. 2003;38:1843–1862. doi: 10.1111/j.1475-6773.2003.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtfreter B, Alte D, Schwahn C, Desvarieux M, Kocher T. Effects of different manual periodontal probes on periodontal measurements. doi: 10.1111/j.1600-051X.2012.01941.x. submitted to J Clin Periodontol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtfreter B, Kocher T, Hoffmann T, Desvarieux M, Micheelis W. Prevalence of periodontal disease and treatment demands based on a German dental survey (DMS IV) J Clin Periodontol. 2010;37:211–219. doi: 10.1111/j.1600-051X.2009.01517.x. [DOI] [PubMed] [Google Scholar]
- Holtfreter B, Schwahn C, Biffar R, Kocher T. Epidemiology of periodontal diseases in the Study of Health in Pomerania. J Clin Periodontol. 2009;36:114–123. doi: 10.1111/j.1600-051X.2008.01361.x. [DOI] [PubMed] [Google Scholar]
- Janhsen K, Strube H, Starker A. Robert Koch Institut and Statistisches Bundesamt. Gesundheitsberichterstattung des Bundes. Berlin: 2008. Hypertension [Hypertonie] p. 35. [Google Scholar]
- John U, Greiner B, Hensel E, Ludemann J, Piek M, Sauer S, Adam C, Born G, Alte D, Greiser E, Haertel U, Hense HW, Haerting J, Willich S, Kessler C. Study of Health In Pomerania (SHIP): a health examination survey in an east German region: objectives and design. Soz Praventivmed. 2001;46:186–194. doi: 10.1007/BF01324255. [DOI] [PubMed] [Google Scholar]
- Keil U, Stieber J, Doring A, Chambless L, Hartel U, Filipiak B, Hense HW, Tietze M, Gostomzyk JG. The cardiovascular risk factor profile in the study area Augsburg. Results from the first MONICA survey 1984/85. Acta Med Scand Suppl. 1988;728:119–128. doi: 10.1111/j.0954-6820.1988.tb05563.x. [DOI] [PubMed] [Google Scholar]
- Konig J, Holtfreter B, Kocher T. Periodontal health in Europe: future trends based on treatment needs and the provision of periodontal services--position paper 1. Eur J Dent Educ. 2010;14(Suppl 1):4–24. doi: 10.1111/j.1600-0579.2010.00620.x. [DOI] [PubMed] [Google Scholar]
- Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. nrendo.2011.106 [pii] [DOI] [PubMed] [Google Scholar]
- Markides KS, Coreil J. The health of Hispanics in the southwestern United States: an epidemiologic paradox. Public Health Rep. 1986;101:253–265. [PMC free article] [PubMed] [Google Scholar]
- Meisinger C, Heier M, Volzke H, Lowel H, Mitusch R, Hense HW, Ludemann J. Regional disparities of hypertension prevalence and management within Germany. J Hypertens. 2006;24:293–299. doi: 10.1097/01.hjh.0000200508.10324.8e. 00004872-200602000-00015 [pii] [DOI] [PubMed] [Google Scholar]
- Mundt T, Polzer I, Samietz S, Grabe HJ, Doren M, Schwarz S, Kocher T, Biffar R, Schwahn C. Gender-dependent associations between socioeconomic status and tooth loss in working age people in the Study of Health in Pomerania (SHIP), Germany. Community Dent Oral Epidemiol. 2011 doi: 10.1111/j.1600-0528.2010.00607.x. [DOI] [PubMed] [Google Scholar]
- Mundt T, Polzer I, Samietz S, Grabe HJ, Messerschmidt H, Doren M, Schwarz S, Kocher T, Biffar R, Schwahn C. Socioeconomic indicators and prosthetic replacement of missing teeth in a working-age population--results of the Study of Health in Pomerania (SHIP) Community Dent Oral Epidemiol. 2009;37:104–115. doi: 10.1111/j.1600-0528.2009.00463.x. [DOI] [PubMed] [Google Scholar]
- Mundt T, Schwahn C, Mack F, Polzer I, Samietz S, Kocher T, Biffar R. Risk indicators for missing teeth in working-age Pomeranians--an evaluation of high-risk populations. J Public Health Dent. 2007;67:243–249. doi: 10.1111/j.1752-7325.2007.00041.x. [DOI] [PubMed] [Google Scholar]
- Preshaw PM. Definitions of periodontal disease in research. J Clin Periodontol. 2009;36:1–2. doi: 10.1111/j.1600-051X.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing} Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147:259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- Savage A, Eaton KA, Moles DR, Needleman I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol. 2009;36:458–467. doi: 10.1111/j.1600-051X.2009.01408.x. [DOI] [PubMed] [Google Scholar]
- Schatzle M, Loe H, Burgin W, Anerud A, Boysen H, Lang NP. Clinical course of chronic periodontitis. I. Role of gingivitis. J Clin Periodontol. 2003;30:887–901. doi: 10.1034/j.1600-051x.2003.00414.x. [DOI] [PubMed] [Google Scholar]
- Schurch E, Jr, Lang NP. Periodontal conditions in Switzerland at the end of the 20th century. Oral Health Prev Dent. 2004;2:359–368. [PubMed] [Google Scholar]
- Selvin E, Steffes MW, Gregg E, Brancati FL, Coresh J. Performance of A1C for the classification and prediction of diabetes. Diabetes Care. 2011;34:84–89. doi: 10.2337/dc10-1235. dc10-1235 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- Suvan J, D’Aiuto F, Moles DR, Petrie A, Donos N. Association between overweight/obesity and periodontitis in adults. A systematic review. Obes Rev. 2011;12:e381–404. doi: 10.1111/j.1467-789X.2010.00808.x. [DOI] [PubMed] [Google Scholar]
- van der Weijden F, Slot DE. Oral hygiene in the prevention of periodontal diseases: the evidence. Periodontol 2000. 2011;55:104–123. doi: 10.1111/j.1600-0757.2009.00337.x. [DOI] [PubMed] [Google Scholar]
- Volzke H, Stritzke J, Kuch B, Schmidt CO, Ludemann J, Doring A, Schunkert H, Hense HW. Regional differences in the prevalence of left ventricular hypertrophy within Germany. Eur J Cardiovasc Prev Rehabil. 2009;16:392–400. doi: 10.1097/HJR.0b013e32832a4dc1. [DOI] [PubMed] [Google Scholar]
- Williams DR. Race/ethnicity and socioeconomic status: measurement and methodological issues. Int J Health Serv. 1996;26:483–505. doi: 10.2190/U9QT-7B7Y-HQ15-JT14. [DOI] [PubMed] [Google Scholar]
- Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann N Y Acad Sci. 1999;896:173–188. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.