Abstract
Background and purpose
Previous studies have shown that physical inactivity and obesity are risk factors for the development of colorectal cancer. However, controversy exists regarding the influence of these factors on survival in colorectal cancer patients. We evaluated the impact of recreational physical activity and body mass index (BMI) before and after colorectal cancer diagnosis on disease-specific mortality and all-cause mortality.
Patients and Methods
This prospective cohort study included 1339 women enrolled in the Women’s Health Initiative study who were diagnosed with colorectal cancer subsequent to study enrolment. BMI and recreational physical activity were measured before cancer diagnosis at study entry (pre-diagnostic) and after diagnosis at study follow-up interviews (post-diagnostic). We used Cox regression to estimate the association between pre- and post-diagnostic exposures and survival after colorectal cancer diagnosis.
Results
Among women diagnosed with colorectal cancer, 265 (13%) deaths occurred during a median study follow-up of 11.9 years, of which 171 (65%) were attributed to colorectal cancer. Compared with women reporting no pre-diagnostic recreational physical activity, those reporting activity levels of ≥18 MET-hours/week had significantly lower colorectal cancer-specific mortality (hazard ratio (HR)=0.68; 95% confidence interval (CI): 0.41–1.13) and all-cause mortality (HR=0.63; 95% CI: 0.42–0.96). Similar inverse associations were seen for post-diagnostic recreational physical activity. Neither pre- nor post-diagnostic BMI were associated with mortality after colorectal cancer diagnosis.
Conclusion
Recreational physical activity before and after CR colorectal cancer C diagnosis, but not BMI, is associated with more favourable survival.
Keywords: Physical activity, body mass index, colorectal cancer, survival, postmenopausal
Introduction
Colorectal cancer is the third most common type of cancer among women in the United States and has a five-year relative survival of approximately 65% (1). Previous studies have shown that modifiable lifestyle-related factors, including low levels of physical activity (2–5) and obesity (6–9), are associated with colorectal cancer risk in women. The relationship between these factors and survival after diagnosis has also been explored by some studies, with mixed results (10–31).
The few studies that have assessed the association between physical activity and colorectal cancer survival suggest that patients who engage in regular physical activity after diagnosis experience more favourable survival than those who do not (10–13). However, most studies evaluating the association between physical activity and colorectal cancer outcomes have focused on post-diagnostic physical activity (12, 13). Results of such analyses could reflect reverse causality if women with a more favourable prognosis to begin with are better able to participate in physical activity or, conversely, women with poor underlying health or a poor prognosis are unable to exercise.
The association between body mass index (BMI) and survival among colorectal cancer patients is less clear (10, 14–31). There is some evidence that elevated BMI is adversely related to survival (10, 14–22). However, other studies that assessed the association between BMI and survival have reported no association (23–28) or an inverse association (29–31). These conflicting results may reflect differences in sample size, patient and tumor characteristics, or the timing or mode of BMI assessment.
In this study, we assessed the association between recreational physical activity, BMI and survival in postmenopausal women with colorectal cancer using data from the Women’s Health Initiative. Baseline and follow-up questionnaire information were used to evaluate associations of pre-diagnostic physical activity and BMI with colorectal cancer-specific and all-cause mortality in colorectal cancer patients. We also evaluated associations between post-diagnostic measures of these exposures with survival outcomes.
Materials and Methods
Study population
The Women’s Health Initiative (WHI) is a large, multi-center longitudinal study consisting of four overlapping clinical trials (n=68,132 women) of hormone therapy, dietary modification, and/or calcium and vitamin D supplementation and an observational study (n=93,676 women). The WHI was designed to study major causes of morbidity and mortality in postmenopausal women. A total of 161,808 women were enrolled at 40 U.S. clinical centers between October 1993 and December 1998. Women eligible to participate were postmenopausal, aged 50 to 79 years, and likely to be accessible for follow-up for three years or more. Details of the study protocols have been described elsewhere (32). Participants provided written informed consent, and protocols were approved by institutional review boards at all participating institutions.
The present analysis included 2093 women enrolled in the observational study (n=1151) or clinical trials (n=942), who were diagnosed with an invasive, histologically-confirmed colorectal cancer during the study period. Women with a history of cancer (with the exception of non-melanoma skin cancer) prior to the WHI enrolment (n=280), women with a BMI <18.5 kg/m2 (n=8), and women diagnosed with unknown- or distant-stage disease (n=316) were excluded from all analyses. The latter exclusion was made based on concerns that occult disease could have influenced body size and physical activity patterns. Women with a BMI <18.5 kg/m2 were excluded because of concerns of underlying health problems associated with survival. For analyses of pre-diagnostic physical activity and BMI, we also excluded women with incomplete or missing data on physical activity, BMI or covariates at baseline, as well as women who died within a year after the collection of baseline exposure information (n=150). Thus, 1339 (64%) women with incident colorectal cancer were included in the analyses of pre-diagnostic physical activity and BMI.
We assessed associations with post-diagnostic physical activity and BMI in a subset of women included in analyses of pre-diagnostic exposures. Women were excluded from analyses of post-diagnostic exposures if they had missing data on post-diagnostic exposures (n=668 for physical activity analysis and n=564 for BMI analysis), were diagnosed with colorectal cancer after the 6-year study follow-up interview (n=29 for physical activity analysis and n=159 for BMI analysis), died within 1 year of post-diagnostic exposure measurement (n=30 for physical activity and n=25 for BMI analysis) or had a post-diagnostic BMI <18.5 kg/m2 (n=6 for physical activity and n=4 for BMI analysis). Thus, 606 (45%) women were included in analyses of post-diagnostic physical activity and 587 (44%) in analyses of post-diagnostic BMI.
Data collection
Information on demographic factors, medical history, and family history of colorectal cancer was collected through self-administered questionnaires completed at the time of study enrolment (baseline). Information on previous postmenopausal hormone therapy use was collected through in-person interviews.
Certified staff collected anthropometric measurements, including height and weight, at a baseline clinic visit. Weight was measured on a calibrated balance-beam scale or digital scale with shoes, heavy clothing, and pocket contents removed. Height was measured using a wall-mounted stadiometer. BMI was calculated as weight in kilograms divided by the square of the height in meters (kg/m2). Weight and height were also measured annually for women participating in the clinical trial and at year three for women in the observational study during a clinic visit. For women in the observational study, weight and height measurements after year three were based on self-report and collected on an annual basis.
Self-reported recreational physical activity was assessed by a validated questionnaire at baseline and at 3-year and 6-year study follow-up visits (33). Women were asked about their usual frequency and duration of mild recreational exercise (including slow dancing, bowling and golf), moderate exercise (including outdoor biking, exercise machines, calisthenics, easy swimming, and popular or folk dancing) and strenuous exercise (including aerobics, jogging, tennis, and swimming laps). To characterize the duration of recreational physical activity, women chose 1 out of 5 frequency categories of exercise ranging from never to 5 or more days per week. Participants were also asked how often they walked outside for more than 10 minutes (ranging from never to 7 or more times each week), the usual duration of their walking episodes and their usual walking speed (casual strolling, normal, fairly fast or very fast). Recreational physical activity was expressed as total MET-hours (metabolic equivalents) per week. MET-scores are defined as the ratio of the metabolic rate associated with specific activities divided by the resting metabolic rate; one MET is the energy expenditure for sitting quietly. MET values were assigned for strenuous-, moderate-, and mild-intensity activities (7, 4, and 3, respectively) and multiplied by the hours exercised at that intensity level per week to obtain composite MET-hours per week variables for each intensity level and for all intensity levels combined (34). The validity of the physical activity questionnaire was previously examined by comparing the questionnaire with accelerometer data, indicating a correlation of 0.73 with 100% sensitivity for meeting the current physical activity guidelines (33). The reproducibility of the physical activity questionnaire was also assessed, and ranged from 0.53 to 0.72 with an intraclass correlation coefficient for total physical activity equal to 0.77 (35).
Ascertainment of cancer mortality
The primary endpoints for the present analysis were colorectal cancer-specific mortality (defined as death attributed to colorectal cancer) and all-cause mortality (defined as death from any cause). Vital status was ascertained through follow-up with study participants and surrogates, and through regular linkage to the National Death Index. Cause of death was determined based on centralized review of death certificates and medical records by trained physician adjudicators (36).
Statistical analysis
We conducted separate analyses assessing the association between pre-diagnostic physical activity, BMI, and survival after colorectal cancer diagnosis, and the association between post-diagnostic physical activity, BMI, and survival. For analyses of pre-diagnostic exposures, we used physical activity and BMI measurements collected at baseline study visits. For post-diagnostic analyses, the physical activity and BMI measurement collected at the study interview most closely following colorectal cancer diagnosis was used. Follow-up ended at death or September 30, 2010, whichever came first.
For pre- and post-diagnostic physical activity analyses, the total MET-hours/week from recreational physical activity and walking were combined into five different categories corresponding to no recreational physical activity (i.e. 0 MET-hours/week) and the quartile distribution of MET-hours/week among those who reported recreational physical activity (>0–2.9 MET-hours/week, 3.0–8.9 MET-hours/week, 9.0–17.9 MET-hours/week and ≥18.0 MET-hours/week), consistent with a prior analysis conducted within the WHI (37). In addition, we explored the association with different classes and intensity of physical activity including mild-, moderate- and strenuous-intensity physical activity. Separate analyses were conducted with and without walking included in the calculation of physical activity variables.
For analyses of pre- and post-diagnostic BMI, women were categorized according to established classifications of normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2).
Cox proportional hazards regression with time from diagnosis as the underlying time variable was used to obtain multivariate HRs and corresponding 95% CIs. A bivariate analysis was performed to identify factors associated with the study outcomes and exposures of interest that altered the effect estimates by at least 10%. This resulted in the inclusion of the following variables in the final model: baseline age (50–54, 55–69, 70–79), study arm (observational study, clinical study), tumor stage (localized, regional), ethnicity (white, nonwhite), education (less than high school, high school graduate, college degree or higher), alcohol (non-drinker, former drinker, current drinker: <7 drinks per week, ≥7 drinks per week), and smoking (current smoker, former smoker, never smoker at baseline). We also adjusted for self-reported current use of postmenopausal hormone therapy at baseline (no current use, current use of estrogen only or combined estrogen-progestin hormone therapy), including all women assigned to one of the active arms of the WHI hormone therapy trials as current hormone therapy users. Post-diagnostic physical activity and BMI analyses were further adjusted for time between baseline measurement and diagnosis. In addition to the above variables, tumor grade, income, first-degree relative with colorectal cancer, diabetes, total energy intake, percent energy from fat, fruit and vegetable intake, and history of colonoscopy, and/or blood test screening were also assessed as potential confounders. However, inclusion of these variables did not change parameter estimates by more than 10% and, thus, these variables were not included in the final model. Tests for linear trend across categories were calculated by modeling categorical variables of MET-hours/week or BMI as ordinal variables. Proportional hazards assumptions were verified for all analyses. The Kaplan-meier method was used to analyze survival, and differences were assessed by the log-rank test.
All analyses were carried out in SAS version 9.2 (SAS Institute Inc.).
Results
Baseline characteristics
Of 1339 women with colorectal cancer eligible for analyses of pre-diagnostic physical activity and BMI, 265 (13%) died during a median study follow-up of 11.9 years (interquartile range 10.9–12.9); of those who died, 171 (65%) died due to colorectal cancer. Baseline characteristics across pre-diagnostic physical activity and BMI measurement are shown in Table 1. Compared with women in the lowest category of pre-diagnostic recreational physical activity, women who were more active were more educated, and were more likely to be white, and to be alcohol drinkers. Obese women were less likely to be current hormone therapy users or current drinkers.
Table 1.
Baseline characteristics of women developing colorectal cancer during follow-up in the WHI across levels of pre-diagnostic recreational physical activity and BMI
| Recreational physical activity, MET-hours per week |
Body mass index, kg/m2 | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 (n=234) |
>0–2.9 (n=166) |
3–8.9 (n=350) |
9.0–17.9 (n=312) |
≥18.0 (n=277) |
18.5–24.9 (n=412) |
25.0–29.9 (n=472) |
≥30.0 (n=455) |
|
| Demographic and clinical characteristics | ||||||||
| Age, years (mean±SD) | 65.6±6.9 | 65.1±6.9 | 65.7±6.5 | 65.9±6.5 | 66.3±7.0 | 66.1±6.9 | 66.5±6.6 | 64.8±6.6 |
| BMI, kg/m2 (mean±SD) | 30.2±6.2 | 30.6±6.6 | 28.8±5.7 | 27.7±4.8 | 26.8±5.3 | 22.8±1.6 | 27.5±1.5 | 35.0±4.6 |
| MET-hours/wk (mean±SD) | 0 | 1.6±0.7 | 5.8±1.8 | 12.9±2.6 | 31.1±13.0 | 13.9±14.2 | 11.3±12.1 | 8.5±11.2 |
| Observational study, % | 47 | 46 | 49 | 53 | 58 | 58 | 49 | 47 |
| Tumor stage, % | ||||||||
| Localized | 52 | 52 | 50 | 52 | 52 | 50 | 51 | 53 |
| Regional | 48 | 48 | 50 | 48 | 48 | 50 | 49 | 47 |
| Tumor location, % | ||||||||
| Colon | 76 | 83 | 83 | 78 | 84 | 79 | 82 | 81 |
| Rectum | 24 | 17 | 17 | 22 | 16 | 21 | 18 | 19 |
| Education, %a | ||||||||
| Less than high school | 6 | 8 | 7 | 4 | 3 | 4 | 5 | 7 |
| High school graduate | 32 | 40 | 26 | 29 | 19 | 23 | 28 | 33 |
| College degree or higher | 62 | 52 | 67 | 68 | 77 | 73 | 67 | 60 |
| Race/ethnicity, %a | ||||||||
| White, not Hispanic | 77 | 81 | 83 | 85 | 90 | 89 | 83 | 80 |
| Black, not Hispanic | 15 | 12 | 12 | 8 | 6 | 4 | 11 | 16 |
| Hispanic | 3 | 3 | 1 | 3 | 1 | 2 | 3 | 2 |
| Asian/Other | 5 | 4 | 4 | 4 | 3 | 5 | 4 | 2 |
| Current smoker, % | 9 | 9 | 6 | 6 | 5 | 7 | 8 | 5 |
| Current hormone therapy user, % | 36 | 36 | 45 | 44 | 46 | 46 | 44 | 34 |
| Alcohol consumption, %a | 15 | 10 | 13 | 8 | 9 | 10 | 11 | 12 |
| Non drinker | 22 | 29 | 18 | 17 | 16 | 17 | 18 | 24 |
| Past drinker | 37 | 34 | 36 | 35 | 32 | 30 | 34 | 40 |
| Current drinker <7 drinks/week | 27 | 27 | 33 | 41 | 43 | 44 | 38 | 24 |
| Current heavy drinker ≥7 drinks/week | ||||||||
P-value <0.05 by Chi-square test
Pre-Diagnostic Exposures
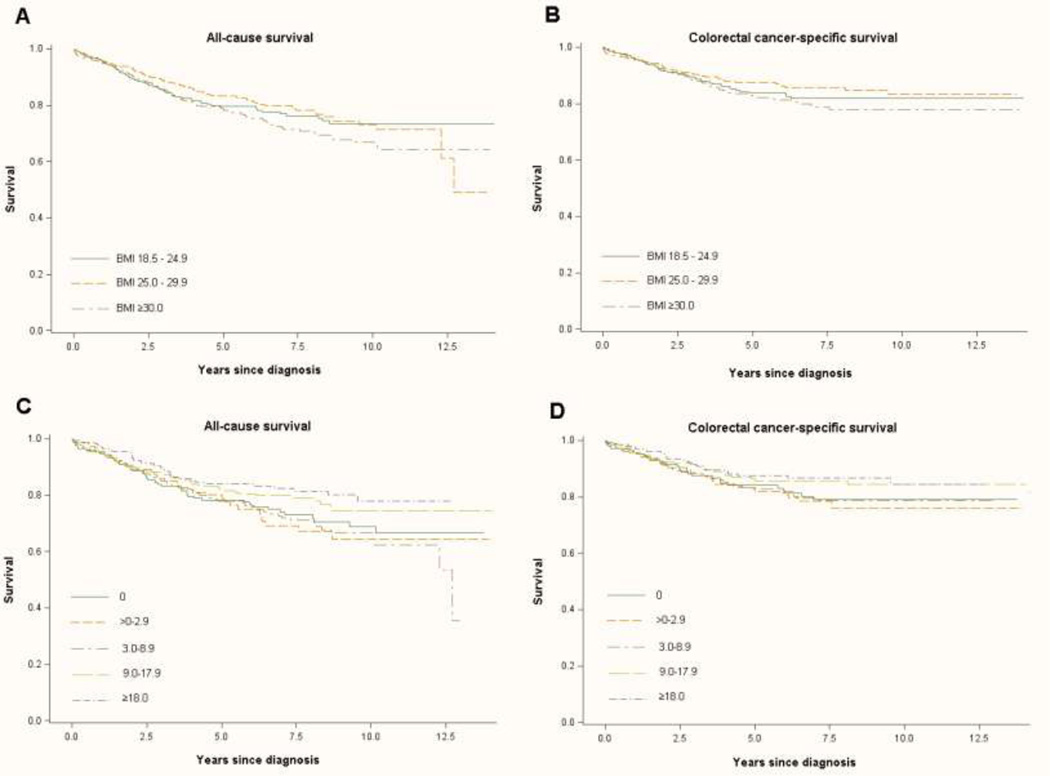
The median time between pre-diagnostic physical activity measurement and colorectal cancer diagnosis was 5.6 years (interquartile range: 2.9–8.4). The Kaplan–Meier curves showed significant differences between the groups of recreational physical activity in all-cause survival (log-rank p=0.05), but a non-significant difference in colorectal cancer-specific survival (log-rank p=0.001) (Figure 1C and 1D). In multivariate analyses, women with a pre-diagnostic physical activity level ≥18.0 MET-hours/week had a lower colorectal cancer-specific mortality (HR=0.68; 95% CI 0.41–1.13, ptrend=0.08) and all-cause mortality (HR=0.63; 95% CI 0.42–0.96, ptrend=0.02) compared to women who reported no physical activity (Table 2). This association was most prominent in women with a normal BMI (18.5–24.9 kg/m2) (HR=0.42; 95% CI 0.20–0.87; ptrend=0.02). There was no evidence of effect modification by age or tumor stage (results not shown). Among women who reported a physical activity level of 9–17.9 MET-hours/week there was a modest, although non-significant, decrease in colorectal cancer-specific mortality (HR=0.74; 95% CI 0.46–1.20) and all-cause mortality (HR=0.77; 95% CI 0.52–1.12). To address the potential impact of occult disease on physical activity level, we excluded patients who were diagnosed with colorectal cancer within 6 months of pre-diagnostic physical activity measurement; this exclusion had little impact on observed associations (data not shown). No difference in the associations was seen when using different classes and intensities of physical activity or when adjusting associations further by stage at diagnosis (results not shown).
Figure 1.
Kaplan-Meier estimates of survival functions among women with colorectal cancer. A, all-cause survival across pre-diagnostic BM categories. B, colorectal cancer-specific survival across pre-diagnostic BMI categories. C, all-cause survival across pre-diagnostic recreational physical activity categories. D, colorectal cancer-specific survival across pre-diagnostic recreational physical activity categories.
Table 2.
Association between colorectal cancer-specific mortality and all-cause mortality across pre-diagnostic levels of total recreational physical activity and body mass index
| Colorectal Cancer-Specific Mortality | All-cause Mortality | |||||
|---|---|---|---|---|---|---|
| Deaths/ Cases |
Crude adjusteda HR (95% CI) |
Multivariable- adjustedb HR (95% CI) |
Deaths/ Cases |
Crude adjusteda HR (95% CI) |
Multivariable- adjustedb HR (95% CI) |
|
| Total MET-hours/week physical activity from recreational activities and walking at baseline** | ||||||
| 0 | 34/234 | 1.00 | 1.00 | 53/234 | 1.00 | 1.00 |
| >0–2.9 | 24/166 | 1.06 (0.63–1.78) | 0.98 (0.58–1.66) | 36/166 | 1.02 (0.67–1.56) | 0.93 (0.61–1.43) |
| 3.0–8.9 | 50/350 | 1.03 (0.67–1.60) | 1.01 (0.65–1.57) | 81/350 | 1.04 (0.73–1.47) | 1.01 (0.71–1.43) |
| 9.0–17.9 | 34/312 | 0.85 (0.54–1.34) | 0.74 (0.46–1.20) | 54/312 | 0.76 (0.52–1.11) | 0.77 (0.52–1.12) |
| ≥18.0 | 29/277 | 0.69 (0.42–1.12) | 0.68 (0.41–1.13) | 41/277 | 0.61 (0.41–0.92) | 0.63 (0.42–0.96) |
| Ptrend | 0.08 | 0.08 | 0.006 | 0.02 | ||
| BMI, kg/m2 at baselinec | ||||||
| 18.5–24.9 | 53/412 | 1.00 | 1.00 | 77/412 | 1.00 | 1.00 |
| 25.0–29.9 | 51/472 | 0.82 (0.56–1.21) | 0.77 (0.52–1.13) | 84/472 | 0.90 (0.66–1.23) | 0.84 (0.61–1.14) |
| ≥30.0 | 67/455 | 1.21 (0.84–1.74) | 1.17 (0.80–1.70) | 104/455 | 1.28 (0.95–1.73) | 1.19 (0.88–1.62) |
| Ptrend | 0.27 | 0.39 | 0.08 | 0.22 | ||
Adjusted for age and study arm
Adjusted for age at diagnosis, study arm, BMI, tumor stage, ethnicity, education, alcohol, smoking, and hormone therapy use
Also adjusted for pre-diagnostic physical activity
The median time between baseline BMI measurement and colorectal cancer diagnosis was 5.8 years (interquartile range: 3.0–8.5). No significant association was seen between increasing BMI and colorectal cancer-specific or all-cause mortality (ptrend=0.39; and ptrend=0.22, respectively). In the Kaplan-meier method, the non-signifcant effect of BMI and all-cause survival and colorectal cancer-specific mortality was also evident (log-rank p=0.17; and log-rank p=0.21, respectively) (Figure 1A and 1B). Results were similar when evaluating associations with the BMI measurement most closely preceding diagnosis instead of baseline BMI and when further adjusting for stage at diagnosis (data not shown).
Post-Diagnostic Exposures
The median time from diagnosis to post-diagnostic physical activity measurement was 1.5 years (interquartile range: 0.8–2.3). There was a statistically significant trend towards lower colorectal cancer-specific mortality (ptrend=0.02) and all-cause mortality (ptrend=0.005) with increasing levels of physical activity (Table 3). After adjustment for potential confounders, women with a physical activity level at or above 18 MET-hours/week had a 71% lower risk of colorectal cancer-specific mortality (95% CI: 0.11–0.77) and a 59% lower risk of all-cause mortality (95% CI: 0.21–0.81) compared with physically inactive patients (0 MET-hours/week).
Table 3.
Association between colorectal cancer-specific mortality and all-cause mortality across post-diagnostic levels of total recreational physical activity and body mass index
| Colorectal Cancer-Specific Mortality | All-cause Mortality | |||||
|---|---|---|---|---|---|---|
| Deaths/ Cases |
Crude adjusteda HR (95% CI) |
Multivariable- adjustedb HR (95% CI) |
Deaths/ Cases |
Crude adjusteda HR (95% CI) |
Multivariable- adjustedb HR (95% CI) |
|
| Total MET-hours/week physical activity from recreational activities and walking at follow-up** | ||||||
| 0 | 15/132 | 1.00 | 1.00 | 29/132 | 1.00 | 1.00 |
| >0–2.9 | 10/110 | 0.78 (0.35–1.74) | 0.49 (0.21–1.14) | 22/110 | 0.84 (0.47–1.51) | 0.71 (0.40–1.30) |
| 3.0–8.9 | 8/156 | 0.35 (0.14–0.87) | 0.30 (0.12–0.73) | 20/156 | 0.47 (0.25–0.86) | 0.42 (0.23–0.77) |
| 9.0–17.9 | 12/142 | 0.48 (0.21–1.10) | 0.53 (0.22–1.25) | 22/142 | 0.51 (0.28–0.93) | 0.57 (0.31–1.07) |
| ≥18.0 | 6/136 | 0.33 (0.13–0.85) | 0.29 (0.11–0.77) | 15/136 | 0.39 (0.20–0.76) | 0.41 (0.21–0.81) |
| Ptrend | 0.008 | 0.02 | 0.001 | 0.005 | ||
| BMI, kg/m2 at follow-upc | ||||||
| 18.5–24.9 | 20/184 | 1.00 | 1.00 | 32/184 | 1.00 | 1.00 |
| 25.0–29.9 | 13/223 | 0.51 (0.25–1.02) | 0.45 (0.22–0.92) | 33/223 | 0.84 (0.52–1.37) | 0.77 (0.47–1.27) |
| ≥30.0 | 21/180 | 0.96 (0.51–1.79) | 0.95 (0.49–1.85) | 36/180 | 1.13 (0.70–1.84) | 1.09 (0.65–1.83) |
| Ptrend | 0.92 | 0.86 | 0.61 | 0.75 | ||
Adjusted for age and study arm
Adjusted for age at diagnosis, study arm, time from diagnosis to measurement, pre-diagnostic BMI, tumor stage, ethnicity, education, alcohol, smoking, and hormone therapy use
Also adjusted for pre-diagnostic physical activity
The median time between colorectal cancer diagnosis and follow-up BMI measurement was 0.8 years (interquartile range: 0.4–1.7). As with pre-diagnostic BMI, we observed no significant trend between post-diagnostic BMI and colorectal cancer-specific (ptrend=0.86) or all-cause mortality (ptrend=0.75) (Table 3). In analyses with colorectal cancer-specific mortality, there was a suggested reduced risk in overweight women relative to normal weight women (HR=0.45; 95% CI: 0.22–0.92). However, no such association was seen between BMI and all-cause mortality, and there was no association with obesity for either outcome of interest.
Discussion
The present data suggest that recreational physical activity, before and after colorectal cancer diagnosis, is associated with lower colorectal cancer-specific mortality and all-cause mortality in women with colorectal cancer. Women with a recreational physical activity level of ≥9 MET-hours/week before colorectal cancer diagnosis had a 32% lower risk of death from colorectal cancer and a 37% lower risk of death from any cause compared with women reporting no recreational physical activity (0 MET-hours/week). A recreational activity level of 9 MET-hours/week is equivalent to three hours per week of moderate-intensity physical activity. A similar significant trend towards lower colorectal cancer-specific mortality (ptrend = 0.02) and all-cause mortality (ptrend = 0.005) with increasing recreational physical activity was also observed with respect to post-diagnostic physical activity. We did not observe any trend of increasing or decreasing risk of colorectal cancer-specific mortality or all-cause mortality with BMI measured pre- or post-diagnostically.
Few studies have investigated physical activity in relation with colorectal cancer-specific mortality or all-cause mortality in individuals with colorectal cancer (10–13). Consistent with the current results, one prospective observational study reported lower colorectal-cancer specific mortality among physically active patients compared with physically inactive patients (HR=0.73; 95% CI 0.54–1.00) (10) six months before diagnosis. In another study (11) among women with stage I-III colorectal cancer, no association with survival was seen comparing women in the highest versus the lowest quartile of physical activity (≥18 MET-hours/week versus <3 MET-hours/week) before diagnosis; however, women with a post-diagnostic physical activity level of ≥18 MET-hours/wk had a significantly lower risk of colorectal cancer-specific mortality (HR=0.39; 95% CI: 0.18–0.82) and all-cause mortality (HR=0.43; 95% CI: 0.25–0.74) compared to women with a physical activity level of <3 MET-hours/week. The latter study also found that women who increased their physical activity level after diagnosis had a 52% lower risk of colorectal cancer-specific mortality (95% CI: 0.24–0.97) and a 49% lower risk of all-cause mortality (95% CI: 0.30–0.85). Although small numbers precluded us from assessing associations with change in physical activity after diagnosis, our findings with respect to post-diagnostic physical activity are consistent with this prior report. A randomized controlled intervention program to examine the role of physical activity in survival in colorectal cancer patients is ongoing (38) and should provide greater evidence as to the utility of physical activity in cancer patients.
Several studies have investigated the association between BMI before diagnosis with colorectal cancer-specific mortality and all-cause mortality in individuals with colorectal cancer, but results have been inconsistent (16, 17, 24, 27, 30). Meyerhardt et al. (17) found that obese women with colorectal cancer had a significantly worse overall survival (HR=1.34; 95% CI: 1.07–1.67) compared with normal weight women with colorectal cancer. Our results are comparable with another large cohort study of patients with Dukes B and C colon cancer in which there was no association between obesity and colorectal cancer-specific (HR: 1.08; 95% CI 0.90–1.30) or all-cause mortality (HR=1.11; 95% CI: 0.96–1.28) (24). This latter study was similar to ours with respect to the length of follow-up, and the distribution of age at diagnosis and race/ethnicity but, unlike our study, included men as well as women. One additional study has reported on the association between BMI following the completion of chemotherapy and subsequent survival in patients with stage III colorectal cancer (31). In that study, obese patients were not at a higher risk of all-cause mortality compared with normal weight patients (HR=0.90; 95% CI: 0.61–1.34), possibly related to the overall poorer prognosis among those diagnosed at an advanced stage that would be less modifiable with lifestyle factors.
It is possible that observed null findings for the association between pre-diagnostic BMI and survival outcomes may be explained by the timing of weight and height measurements if BMI closer to diagnosis is more important to survival. The median time between baseline BMI assessment and diagnosis was 5.8 years. In sensitivity analysis, we did examine whether the association between pre-diagnostic BMI and outcome would change when using BMI measurements taken 1–2 years before diagnosis, but found no change in our results.
There is some suggestion that the association between BMI and survival is limited to patients with colorectal cancers exhibiting specific molecular signatures (39–43). One study reported an improved survival in patients with STNMI1-postive tumors (stathmin or oncoprotein-18); however, a reduced risk of colorectal cancer-specific mortality was found in obese (BMI ≥ 30 kg/m2) patients with STMN1-negative tumors (HR=0.51; 95% CI: 0.24–1.07) compared to patients with a BMI <30.0 kg/m2 (40). Another study found that obese patients (BMI ≥30 kg/m2) with nuclear CTNNB1-positive colorectal cancer had a significantly better colorectal cancer-specific survival (HR=0.24; 95%CI: 0.12–0.49) compared with obese patients (BMI ≥30 kg/m2) with nuclear CTNNB1-negative colorectal cancer; for patients with high levels of post-diagnostic physical activity, a better colorectal cancer-specific survival (HR=0.33; 95%CI: 0.13–0.81) was seen for patient with negative CTNNB1 colorectal cancer (39). Moreover, it is suggested that overexpression of fatty acid synthase, p27 (cyclin-dependent kinase inhibitor 1B), p21 (cyclin-dependent kinase inhibitor 1A) in obese patients is associated with lower survival (41–43). This findings may imply a potential mechanism by which excess energy balance affect tumor behavior and cancer mortality. We cannot completely exclude the possibility that BMI is associated with survival in certain case groups as such molecular characterization was not conducted within the WHI.
Although we did not find an association between pre-diagnostic BMI and mortality after colorectal cancer diagnosis, we did find that the inverse association between pre-diagnostic physical activity and mortality was most prominent in women with a BMI of 18.5–24.9 kg/m2. This finding suggests that, although BMI may not be directly associated with colorectal cancer survival, it may play a more indirect role in mediating associations with other prognostic factors.
Potential biological mechanisms by which physical activity influences CRC survival are likely related, at least in part, to BMI. Although the specific mechanisms by which physical activity influences survival have not been extensively studied, it has been hypothesized that physical activity has an impact on insulin metabolism (44–46). Increased plasma levels of insulin-like growth factors (IGF) and C-peptide are both predictors of decreased colorectal cancer-specific survival and overall survival. Physically active people may have decreased plasma levels of both IGF and C-peptide and may, therefore, increase their relative survival.
It is also plausible that reverse causality may have influenced the observed associations. That is, women with a more favourable prognosis to begin with may have been better able to engage in recreational physical activity post-diagnostically, and women who had occult disease at the time of the pre-diagnostic physical activity measurement may have been less active as a result of their undiagnosed disease. To account for the latter possibility, we excluded women with metastatic disease (stage IV); in sensitivity analyses we also excluded women who were diagnosed ≤6-months after physical activity measurement, however, this exclusion did not markedly alter the results.
Limitations of this study should be mentioned. Data on cancer treatment was not available. It is unlikely that the received treatment would differ according to pre-diagnostic physical activity or BMI; however, it is plausible that response to treatment could differ by these exposures. It is also plausible that receipt of more aggressive treatment in women with more advanced disease could limit a woman’s ability to engage in physical activity after diagnosis and could impact post-diagnostic BMI to a greater extent than in women who received less aggressive treatment. Additionally, women who are more active may be better able to withstand and complete treatment, which may improve their survival (47). However, we observed no marked difference in the association between pre-diagnostic physical activity and all-cause mortality when analyses were stratified by stage at diagnosis, a strong predictor of treatment course. In addition, results were not changed when further adjusting for stage at diagnosis. Due to the self-reported nature of data collection for physical activity, misclassification is possible; however, prior validation studies have demonstrated a high degree of correlation between these data and accelerometer data in a sample of cancer patients (33). Furthermore, although occupational and household activity may also have an effect on survival in women with colorectal cancer, we only assessed recreational physical activity and walking. Also, a high proportion of the study population had no follow-up measurement of physical activity or BMI. As such, selection bias might have been introduced in analyses of post-diagnostic exposures; however, the mean pre-diagnostic physical activity or BMI did not differ between women with post-diagnostic measurements (11.4 MET-hours/week and 28.6 kg/m2) and without post-diagnostic measurements (11.1 MET-hours/week and 28.5 kg/m2). In addition, no significant differences were seen between women who were included versus excluded from the analyses based on their clinical and demographic characteristics. Furthermore, follow-up weight for women in the observational study was self-reported after year three, while women in the clinical trial had their weight measured. We found that excluding women with self-reported weight and height after diagnosis did not alter results. Lastly, it is plausible that the association between physical activity, BMI and survival might differ for women diagnosed with colon versus rectal cancer. Due to the limited number of patients diagnosed with rectal cancer a separate analysis was not possible; however, adjustment for tumor site had little impact on results.
There are also several important strengths of the present analysis, including the large overall sample size, adjudication of outcomes, and the use of prospective ascertainment in the WHI. The prospective design of this study has the advantage of reducing the likelihood of recall bias as exposures were assessed before diagnosis, and also has the advantage of enrolling women prior to diagnosis such that inclusion in the study population is not influenced by the duration of survival after diagnosis. Other main strengths of this study are the accurate BMI measurements collected by trained staff and the comprehensive list of potential confounding factors. To our review, this study is one of the few studies investigating the relationship between both pre-diagnostic and post-diagnostic physical activity, BMI, and colorectal cancer-specific mortality and all-cause mortality.
In conclusion, these findings, along with previous studies, suggest that physical activity before and after colorectal cancer diagnosis may enhance survival, but indicate no association between BMI and survival. These data support the need for randomized trials to elucidate the effect of recreational physical activity and BMI on survival in women with colorectal cancer.
Acknowledgments
Funding
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services (contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221). This publication was also supported by the National Cancer Institute (R25-CA94880 and K05152715).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.American Cancer Society. Cancer Facts and Figures, 2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 2.Chao A, Connell CJ, Jacobs EJ, et al. Amount, type, and timing of recreational physical activity in relation to colon and rectal cancer in older adults: the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:2187–2195. [PubMed] [Google Scholar]
- 3.Friedenreich C, Norat T, Steindorf K, et al. Physical activity and risk of colon and rectal cancers: the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2006;15:2398–2407. doi: 10.1158/1055-9965.EPI-06-0595. [DOI] [PubMed] [Google Scholar]
- 4.Larsson SC, Rutegard J, Bergkvist L, Wolk A. Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. Eur J Cancer. 2006;42:2590–2597. doi: 10.1016/j.ejca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Mai PL, Sullivan-Halley J, Ursin G, et al. Physical activity and colon cancer risk among women in the California Teachers Study. Cancer Epidemiol Biomarkers Prev. 2007;16:517–525. doi: 10.1158/1055-9965.EPI-06-0747. [DOI] [PubMed] [Google Scholar]
- 6.Campbell PT, Jacobs ET, Ulrich CM, et al. Case-control study of overweight, obesity, and colorectal cancer risk, overall and by tumor microsatellite instability status. J Natl Cancer Inst. 2010;102:391–400. doi: 10.1093/jnci/djq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin J, Zhang SM, Cook NR, Rexrode KM, Lee IM, Buring JE. Body mass index and risk of colorectal cancer in women (United States) Cancer causes & control : CCC. 2004;15:581–589. doi: 10.1023/B:CACO.0000036168.23351.f1. [DOI] [PubMed] [Google Scholar]
- 8.Slattery ML, Ballard-Barbash R, Edwards S, Caan BJ, Potter JD. Body mass index and colon cancer: an evaluation of the modifying effects of estrogen (United States) Cancer causes & control : CCC. 2003;14:75–84. doi: 10.1023/a:1022545017867. [DOI] [PubMed] [Google Scholar]
- 9.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. 2007;16:2533–2547. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 10.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 12.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 13.Meyerhardt JA, Ogino S, Kirkner GJ, et al. Interaction of molecular markers and physical activity on mortality in patients with colon cancer. Clin Cancer Res. 2009;15:5931–5936. doi: 10.1158/1078-0432.CCR-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batty GD, Shipley MJ, Jarrett RJ, Breeze E, Marmot MG, Smith GD. Obesity and overweight in relation to organ-specific cancer mortality in London (UK): findings from the original Whitehall study. International Journal of Obesity. 2005;29:1267–1274. doi: 10.1038/sj.ijo.0803020. [DOI] [PubMed] [Google Scholar]
- 15.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England journal of medicine. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 16.Doria-Rose VP, Newcomb PA, Morimoto LM, Hampton JM, Trentham-Dietz A. Body mass index and the risk of death following the diagnosis of colorectal cancer in postmenopausal women (United States) Cancer causes & control : CCC. 2006;17:63–70. doi: 10.1007/s10552-005-0360-0. [DOI] [PubMed] [Google Scholar]
- 17.Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 18.Moghimi-Dehkordi B, Safaee A, Zali MR. Prognostic factors in 1,138 Iranian colorectal cancer patients. International journal of colorectal disease. 2008;23:683–688. doi: 10.1007/s00384-008-0463-7. [DOI] [PubMed] [Google Scholar]
- 19.Murphy TK, Calle EE, Rodriguez C, Kahn HS, Thun MJ. Body mass index and colon cancer mortality in a large prospective study. American Journal of Epidemiology. 2000;152:847–854. doi: 10.1093/aje/152.9.847. [DOI] [PubMed] [Google Scholar]
- 20.Prizment AE, Flood A, Anderson KE, Folsom AR. Survival of women with colon cancer in relation to precancer anthropometric characteristics: the Iowa Women's Health Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:2229–2237. doi: 10.1158/1055-9965.EPI-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinicrope FA, Foster NR, Sargent DJ, O'Connell MJ, Rankin C. Obesity Is an Independent Prognostic Variable in Colon Cancer Survivors. Clin Cancer Res. 2010;16:1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Wayenburg CA, van der Schouw YT, van Noord PA, Peeters PH. Age at menopause, body mass index, and the risk of colorectal cancer mortality in the Dutch Diagnostisch Onderzoek Mammacarcinoom (DOM) cohort. Epidemiology. 2000;11:304–308. doi: 10.1097/00001648-200005000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Ballian N, Yamane B, Leverson G, et al. Body mass index does not affect postoperative morbidity and oncologic outcomes of total mesorectal excision for rectal adenocarcinoma. Annals of surgical oncology. 2010;17:1606–1613. doi: 10.1245/s10434-010-0908-4. [DOI] [PubMed] [Google Scholar]
- 24.Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98:1647–1654. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 25.Dray X, Boutron-Ruault MC, Bertrais S, Sapinho D, Benhamiche-Bouvier AM, Faivre J. Influence of dietary factors on colorectal cancer survival. Gut. 2003;52:868–873. doi: 10.1136/gut.52.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Oria Serrano MJ, Armengol Carrasco M, Caballero Millan A, Ching CD, Codina Cazador A. [Is body mass index a prognostic factor of survival in colonic cancer? A multivariate analysis] Cirugia espanola. 2011;89:152–158. doi: 10.1016/j.ciresp.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol. 2004;22:648–657. doi: 10.1200/JCO.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 28.Park SM, Lim MK, Shin SA, Yun YH. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:5017–5024. doi: 10.1200/JCO.2006.07.0243. [DOI] [PubMed] [Google Scholar]
- 29.Asghari-Jafarabadi M, Hajizadeh E, Kazemnejad A, Fatemi SR. Site-specific evaluation of prognostic factors on survival in Iranian colorectal cancer patients: a competing risks survival analysis. Asian Pacific journal of cancer prevention : APJCP. 2009;10:815–821. [PubMed] [Google Scholar]
- 30.Hines RB, Shanmugam C, Waterbor JW, et al. Effect of comorbidity and body mass index on the survival of African-American and Caucasian patients with colon cancer. Cancer. 2009;115:5798–5806. doi: 10.1002/cncr.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26:4109–4115. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 33.Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31:193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- 34.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test-retest reliability of the Women's Health Initiative physical activity questionnaire. Med Sci Sports Exerc. 2009;41:530–538. doi: 10.1249/MSS.0b013e31818ace55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 37.Irwin ML, McTiernan A, Manson JE, et al. Physical Activity and Survival in Postmenopausal Women with Breast Cancer: Results from the Women's Health Initiative. Cancer Prev Res (Phila) 2011;4:522–529. doi: 10.1158/1940-6207.CAPR-10-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Courneya KS, Booth CM, Gill S, et al. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Current oncology. 2008;15:279–285. doi: 10.3747/co.v15i6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morikawa T, Kuchiba A, Yamauchi M, et al. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA : the journal of the American Medical Association. 2011;305:1685–1694. doi: 10.1001/jama.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogino S, Nosho K, Baba Y, et al. A cohort study of STMN1 expression in colorectal cancer: body mass index and prognosis. Am J Gastroenterol. 2009;104:2047–2056. doi: 10.1038/ajg.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogino S, Nosho K, Meyerhardt JA, et al. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol. 2008;26:5713–5720. doi: 10.1200/JCO.2008.18.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogino S, Nosho K, Shima K, et al. p21 expression in colon cancer and modifying effects of patient age and body mass index on prognosis. Cancer Epidemiol Biomarkers Prev. 2009;18:2513–2521. doi: 10.1158/1055-9965.EPI-09-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogino S, Shima K, Nosho K, et al. A cohort study of p27 localization in colon cancer, body mass index, and patient survival. Cancer Epidemiol Biomarkers Prev. 2009;18:1849–1858. doi: 10.1158/1055-9965.EPI-09-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuchs CS, Goldberg RM, Sargent DJ, et al. Plasma insulin-like growth factors, insulin-like binding protein-3, and outcome in metastatic colorectal cancer: results from intergroup trial N9741. Clinical Cancer Res. 2008;14:8263–8269. doi: 10.1158/1078-0432.CCR-08-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haydon AM, Macinnis RJ, English DR, Morris H, Giles GG. Physical activity, insulin-like growth factor 1, insulin-like growth factor binding protein 3, and survival from colorectal cancer. Gut. 2006;55:689–694. doi: 10.1136/gut.2005.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volkova E, Willis JA, Wells JE, Robinson BA, Dachs GU, Currie MJ. Association of angiopoietin-2, C-reactive protein and markers of obesity and insulin resistance with survival outcome in colorectal cancer. Br J Cancer. 2011;104:51–59. doi: 10.1038/sj.bjc.6606005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]