Abstract
Angiotensin II (AngII) contributes to the pathogenesis of hypertension and other cardiovascular diseases. AngII induces a pro-oxidative, pro-inflammatory, and pro-thrombogenic phenotype in vascular endothelial cells. Although the peptide promotes the recruitment of leukocytes and platelets and induces oxidative stress in the microvasculature, it remains unclear whether and how the blood cell recruitment is linked to the production of reactive oxygen species (ROS). In this study, we addressed the contributions of AngII type-1 receptors (AT1r), and gp91phox to the recruitment of leukocytes and platelets and ROS production in venules during chronic (2 wks) infusion of AngII in wild type (WT) and mutant mice. Intravital video microscopy was used to measure the adhesion and emigration of leukocytes, the adhesion of fluorescently labeled platelets, and dihydrorhodamine oxidation (a measure of oxidative stress) in cremaster muscle post-capillary venules. In WT mice, AngII infusion induced a time-dependent increase in the adhesion of leukocytes and platelets and enhanced ROS production in venules. These changes in blood cell adhesion and ROS production were not observed in AT1r−/− mice, in AT1r−/− bone marrow chimeras (blood cells deficient in AT1r), gp91phox−/−, gp91phox−/− chimeras (blood cells or endothelial cells deficient in gp91phox) and in WT mice rendered granulocytopenic via i.p injection of anti-mouse Gr-1 antibody. Thrombocytopenic WT mice (platelets depleted by i.p. injection of rabbit anti-mouse thrombocyte antiserum) responded similar to WT mice. These findings implicate leukocyte-associated AT1r and gp91phox in the induction of the pro-oxidative, proinflammatory and prothrombogenic phenotype assumed by microvessels that are chronically exposed to elevated AngII.
Keywords: Angiotensin II, neutrophils, oxidative stress, blood vessels, microcirculation
Introduction
Hypertension (HTN) is one of several risk factors for cardiovascular disease (CVD) that can lead to structural and functional alterations in both large and microscopic blood vessels.1, 2 It is now widely appreciated that the blood vessels responses to HTN result from physical and chemical factors that exert an influence on the endothelial and/or smooth muscle components of the vessel wall.3 Angiotensin II (AngII), a product of the renin-angiotensin system (RAS), has received considerable attention as a circulating soluble factor that is capable of mediating the elevated blood pressure as well as other phenotypic changes in blood vessels that accompany HTN and other risk factors (e.g., hypercholesterolemia).4–6 AngII is a pleiotropic agent that has been implicated as a mediator of the oxidative stress, immune cell activation and recruitment, thrombogenesis, and the impaired vasomotor and endothelial barrier functions caused by CVD risk factors.4, 7, 8 The profile of vascular changes that are induced by AngII suggests that this vasoactive peptide may play an equally important role as an inflammatory mediator.
An important feature of the pro-inflammatory actions of AngII in the microvasculature is its ability to activate a variety of cells that normally circulate in blood (leukocytes, platelets) or that reside within the vessel wall (endothelial cells).5, 8, 9 Most of the evidence in the literature indicates that AngII mediates the activation of these different cell populations by engaging with angiotensin II type-1 receptors (AT1r).6–8, 10 An accelerated production of reactive oxygen species (ROS) is observed in microscopic vessels that are either acutely or chronically exposed to AngII.4, 11 The vascular wall oxidative stress induced by AngII has been linked to AT1r and it is generally attributed to NADPH oxidase-dependent ROS production by endothelial cells.12 However, both leukocytes and platelets, which are recruited onto the vessel wall in response to AngII, are known to express AT1r and NADPH oxidase, and can increase ROS production in response to AngII.13 This raises the possibility that some of the ROS production that is detected in microvessels exposed to elevated AngII may result from the activation of adherent leukocytes and/or platelets, perhaps via the engagement of AT1r on the circulating cells. This possibility appears tenable in view of previous reports that describe a major contribution of adherent leukocytes to the oxidative stress associated with other models of microvascular inflammation.14–16
The overall objective of this study was to determine whether the recruitment of adherent neutrophils and platelets in venules during chronic AngII exposure contributes to the oxidative stress that accompanies this condition. In addition, using bone marrow chimeras, we assessed the relative contributions of blood cell- vs endothelial cell-associated NADPH oxidase (gp91phox) and AT1r to the oxidative stress and inflammatory cell recruitment elicited by AngII. Our findings indicate that neutrophil-associated NADPH oxidase plays a major role in AngII mediated oxidative stress and implicate blood cell-associated AT1r in the increased ROS production.
Methods
All mice used in the experimental studies were on a C57BL/6 background and the mutant mice were backcrossed to C57BL/6 for at least seven generations. Male wild type (WT) C57BL/6J mice, AT1r−/−, and gp91phox−/− (B6.129S6-Cybbtm1Din/J) mice were purchased from Jackson Laboratory (Bar Harbor, ME) or derived from an established breeding colony (e.g., AT1r−/−) on our campus. All of the experimental procedures involving the use of animals were approved by the LSU Health Sciences Center Institutional Animal Care and Use Committee and were in accordance with the guidelines of the American Physiological Society.
Bone marrow chimera production
Bone marrow transfer was used to create chimeric mice (denoted as donor->recipient), i.e., WT→WT, gp91phox−/−→WT, AT1r−/−→WT, and WT→ gp91phox−/− mice, as previously described.6,9 For a more detailed method, see the online Data Supplement (available at http://hyper.ahajournals.org).
Osmotic pump implantation
Saline or AngII (1 μg/kg/min) was infused over 3, 7, 10 or 14 days using micro-osmotic pumps (Alzet, Cupertino, CA, model 1002), which were implanted subcutaneously in the intrascapular region of isofluorane-anesthetized mice, as previously described9. Blood pressure was measured by tail-cuff plethysmography (model SC-1000, Hatteras Instruments, Inc., North Carolina, USA) in non-anesthetized animals.
Surgical Protocol
Mice were anesthetized with ketamine hydrochloride (150 mg/kg body wt. IP) and xylazine (7.5 mg/kg body wt. IP). The right jugular vein was cannulated for administration of heparinized saline and platelets, and the right carotid artery was cannulated for systemic arterial pressure measurement. The cremaster muscle was prepared for intravital fluorescence microscopic observation and the adhesion of leukocytes and platelets in cremaster muscle venules was determined, as previously described.17,18 A more detailed description of the intravital microscopic methods used to evaluate blood cell-endothelial cell interactions in cremaster venules is provided in the online Data supplement (available at http://hyper.ahajournals.org).
Platelet Preparation
Male WT mice were used as platelet donors. Platelets were isolated from whole blood by a series of centrifugation steps, labeled with the fluorochrome carboxyfluorescein diacetate succinimudyl ester (CFSE; Molecular Probes, Eugene OR) as described previously 17, and resuspended in PBS at a concentration of 8.33 × 105 cells/μL. This technique does not cause platelet activation as determined by P-selectin expression using flow cytometry. 18
Dihydrorhodamine (DHR) Oxidation
The production of reactive oxygen species (ROS) in cremaster venules was determined by monitoring the oxidation of DHR, as previously described.19 DHR oxidation was monitored in cremaster preparations separate from those used to monitor blood cell adhesion. Background fluorescence (IBgrd) of the first 100 μm of every 300 μm vessel length was recorded along the length of selected postcapillary venules with a xenon light source and a SIT camera system (Hamamatsu). Then, freshly prepared dihydrorhodamine-123 (1 mmol/L, a nonfluorescent dye that is oxidized by ROS to the fluorescent compound rhodamine-123) in bicarbonate-buffered saline (BBS) was superfused over the cremaster for 15 minutes. The tissue was then washed with BBS, and the fluorescent image of each section recorded (IDHR). Images were captured onto a computer, and an area 100 μm long and 7μm wide along the vessel wall was analyzed in each vascular segment using NIH Image 1.62 software. The ratio of IDHR:IBgrd was calculated for each vascular segment and the average ratio for each animal was determined.
Blood cell analyses
Whole blood samples (20–25 μl) were obtained from each mouse for determination of leukocyte (stained with 3% acetic acid and 10% crystal violet) and platelet (stained with 1% buffered ammonium oxalate) counts using a hemocytometer. The flow-cytometric methods used to identify and quantify the different circulating leukocyte populations in control and AngII infused mice are summarized in the online Data Supplement (available at http://hyper.ahajournals.org).
Experimental Protocols
An initial study was directed to defining the time-course of changes in the adhesion of leukocytes and platelets, leukocyte emigration, and DHR oxidation (oxidative stress) in cremaster muscle venules, and blood pressure. In 5 groups of mice (n 5), all of these variables were measured on days 0, 3, 7, 10 and 14 day using the intravital video microscopic procedures described above. All subsequent experiments were performed in mice exposed to AngII infusion for 14 days. Some WT mice with AngII pumps were rendered thrombocytopenic via an intraperitoneal injection of anti-platelet serum (125 μL/kg in 200 μL PBS IP; rabbit anti-mouse thrombocyte antiserum, Accurate Chemicals, Westbury, NY) 24 h before intravital microscopy (n=5). Treatment with antiplatelet serum (APS) reduced the number of circulating platelets by ~91%, without significantly reducing the number of circulating leukocytes, as confirmed by measurements of blood cells with a hemocytometer. The effects of neutrophil depletion on AngII induced microvascular responses were examined in mice using two different antibodies, i.e., anti-granulocyte receptor-1 (Gr-1) RB6-8C5 (eBioscience, 100μl per mouse) or the anti-mouse Ly-6G antibody 1A8 (BioLegend, 150μl per mouse) Both antibodies were delivered by intraperitoneal injection 24h before intravital microscopy (n=7, n=5 respectively). Treatment with RB6-8C5 reduced the number of circulating neutrophils by ~90% while showing no effect on the number of circulating platelets, while 1A8 treatment reduced number of neutrophils by ~71%.
Spinning disk confocal microscopy
In a separate group of mice, an effort was made to define the different leukocyte populations that adhered in cremaster muscle venules on the 14th day of AngII infusion. A spinning disk confocal microscope was used to visualize different leukocyte subpopulations that were labeled with fluorescently-labeled antibodies that target specific surface proteins expressed by the different cell populations. A more detailed description of the methods used for this analysis is provided in the online Data supplement (available at http://hyper.ahajournals.org).
Statistics
Data were analyzed using standard statistical analysis, i.e., one-way ANOVA and Tukey post hoc test and/or Fisher’s least significant difference test. A Student’s t-test was used to compare differences between two groups. All values are reported as means ± SE from 5–14 mice, and statistical significance was set at P < 0.05.
Results
Ang II pump implantation yielded a significantly elevated blood pressure in WT mice (132.6 ± 2.8 mmHg) compared with WT mice with a saline-loaded pump and WT mice not subjected to pump implantation (99.4 ± 0.5 mmHg). Shear rates did not differ significantly between the experimental groups for both arterioles and venules (data not shown). The Ang II–induced hypertension was not observed in AT1r−/− mice. Table 1 summarizes the effects of AngII on systolic blood pressure in the different experimental groups. The time-course studies revealed that blood pressure was significantly elevated beginning on day 3 of AngII infusion. All mice except AT1r−/− with an implanted AngII loaded pump exhibited a significantly elevated systolic blood pressure, compared to WT mice. AT1r−/− chimeras showed no attenuation of blood pressure compared to WT mice.
Table 1.
Angiotensin II mediated changes in systolic blood pressure in wild-type (WT) (control), AT1 receptor knockout (AT1r−/−), chimeric (AT1r−/− into WT recipient; AT1rCh), neutrophil-depleted, platelet-depleted, GP91phox−/− and GP91phox−/−Ch.
| Systolic Blood Pressure (mmHg) | AngII
|
||||||
|---|---|---|---|---|---|---|---|
| WT | AT1r−/− | AT1r−/−→WT | Neutrophil-depleted | Platelet-depleted | gp91phox−/− | gp91phox−/− →WT | |
| Before pump implantation | 99.9±0.6 | 85.9±2.4* | 97.50±1.3 | 99.64±0.4 | 100±0.3 | 100.6±2 | 91.7±1.9 |
| 14th day of pump implantation | 132.6±2.8* | 96.1±3 | 130.1±3.7* | 143.4±1.4* | 151±2.9* | 144.9±5.3* | 141.3±3* |
p ≤0.05 vs Control (WT – before pump implantation).
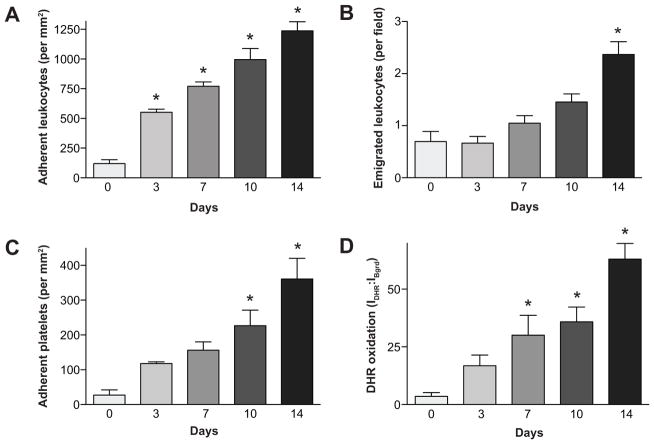
Figure 1 demonstrates that mice with an AngII pump exhibit a significant increase in the number of leukocytes adhering in postcapillary venules on days 3, 7, 10 and 14 of pump implantation, when compared with the control group (Figure 2a). Leukocyte emigration was also elevated in the AngII infused mice on day 14 versus control mice (Figure 1b). The AngII-induced adhesion of leukocytes was accompanied by substantial platelet recruitment. While platelet adhesion was elevated at all time points, only on day 10 and day 14 were the increases statistically significant, when compared with control animals (Figure 1c). The DHR oxidation measurements revealed that oxidative stress in venules is evident (statistically significant) on the 7th day of AngII pump infusion and it remains elevated for the remainder of the infusion period (Figure 1d). Figure S1 (available in the online Data Supplement) shows representative images of dihydrorhodamine oxidation within and surrounding cremaster muscle venules in the different experimental groups on day 14 of angiotensin II infusion.
Figure 1.
Time-course of changes in leukocyte adhesion and emigration (panel A, B), platelet adhesion (panel C), and DHR oxidation (panel D) over 14 days of AngII infusion. *, p≤0.05 vs Control.
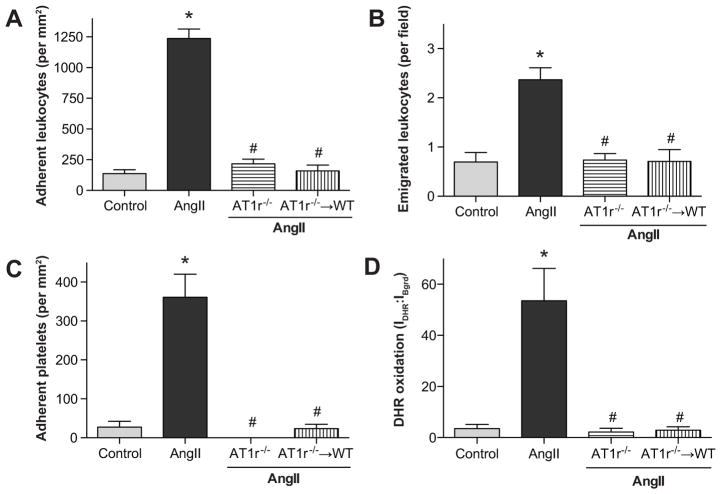
Figure 2.
Angiotensin II mediated changes in leukocyte adhesion (panel A), emigration (panel B), platelet adhesion (panel C) and DHR oxidation (panel D) in wild type (WT) and angiotensin II-type 1 receptor deficient (AT1r−/−) mice and in AT1r−/− bone marrow chimeras *, indicates p<0.05 relative to WT control, #, indicates p<0.05 relative to AngII-WT.
Figure 2 illustrates the contribution of blood cell-associated and endothelial cell-associated AT1r to the microvascular responses elicited by AngII infusion. The number of adherent leukocytes in postcapillary venules of WT + AngII mice were significantly higher than that observed in control mice (Figure 2a). The AngII-induced increase in adherent leukocytes was significantly blunted in AT1r−/− mice and in AT1r−/−→WT chimeras (Figure 2a). Leukocyte emigration was also increased in the AngII + WT mice, compared with the control group (Figure 2b). Much like the leukocyte adherence responses, a profound reduction in leukocyte emigration was noted in both AT1r−/− and AT1r−/−→WT mice. The number of adherent platelets in the WT + AngII mice was increased, compared to control mice. This response to AngII was completely abolished in AT1r−/− mice and AT1r−/−→WT mice (Figure 2c). AngII administration also increases DHR oxidation levels (Figure 2C, Panel B of Figure S1), compared to control mice (Panel A of Figure 1a) however this effect was significantly attenuated in both AT1r−/− mice (Panel C of Figure S1, Figure 2c) and AT1r−/−→WT mice (Figure 2d).
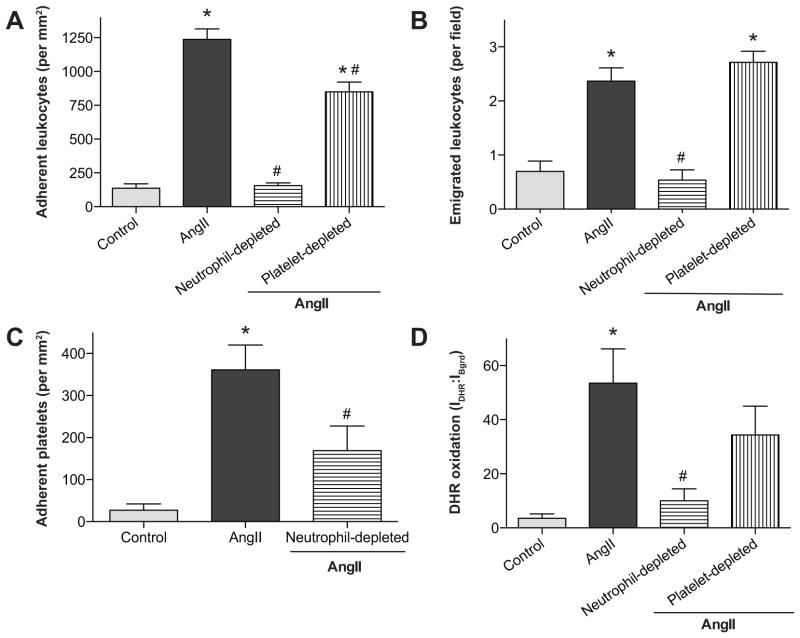
Figure 3 demonstrates the effects of short-term granulocyte (with the anti-Gr-1 antibody, RB6-8C5) or platelet depletion on AngII-induced blood cell recruitment. In mice rendered granulocytopenic (neutrophil-depleted group), leukocyte adhesion (Figure 3a) and emigration (Figure 3b) were significantly reduced to control levels. Treatment with another anti-granulocyte antibody (1A8) exerted the same level of protection for all microvascular responses (data not shown), as noted in Figure 3 for RB6-8C5 treatment. In platelet-depleted animals, leukocyte adhesion (figure 4a) was only partially reduced and emigration (Figure 4b) was not altered, compared to control animals. AngII-induced platelet recruitment into venules of granulocytopenic mice was reduced to control values (Figure cc). Rendering the mice granulocytopenic largely prevented the oxidative stress induced by chronic AngII infusion (Panel D of Figure 1, Figure 3d). However, in thrombocytopenic animals (Figure 1e), DHR oxidation did not differ from the value detected in WT + AngII mice (Panel E of Figure 1, Figure 3d).
Figure 3.
Angiotensin II mediated changes in leukocyte adhesion (panel A), leukocyte emigration (panel B), platelet adhesion (panel C) and DHR oxidation (panel D) in untreated (control) WT mice and WT mice treated with either anti-granulocyte serum or anti-platelet serum. *, indicates p<0.05 relative to WT control, # indicates p<0.05 relative to AngII-WT.
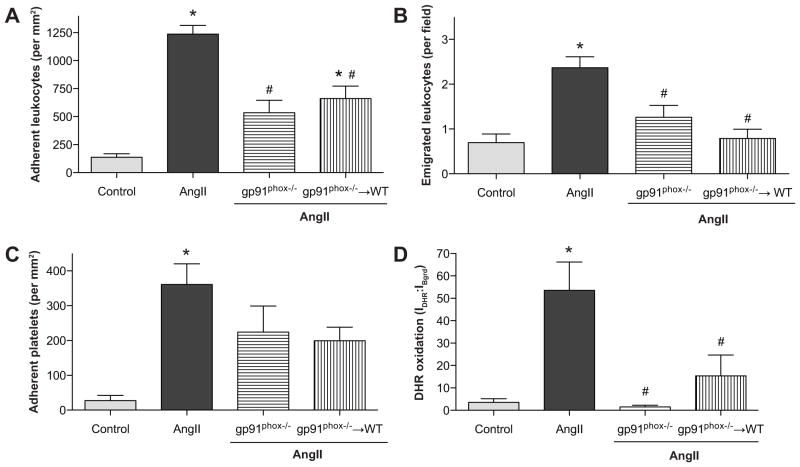
Figure 4.
Angiotensin II mediated changes in leukocyte adhesion (panel A), emigration (panel B), platelet adhesion (panel C) and DHR oxidation (panel D) in wild type (WT) and gp91phox deficient (gp91phox−/−) mice and in gp91phox−/− bone marrow chimeras *, indicates p<0.05 relative to WT control, #, indicates p<0.05 relative to AngII-WT.
Figure S2 summarizes the confocal microscopic analysis of leukocyte subpopulations that are recruited into venules in response to AngII infusion. As noted with light microscopy, AngII infusion is associated with a significant recruitment of CD45+ (total) leukocytes. While granulocytes appear to represent the largest population of recruited leukocytes, monocytes, T-lymphocytes and B-lymphocytes also appear to accumulate in the vessels as a result of AngII stimulation. The leukocyte counts in blood for the different sub-populations were not altered by AngII, except for T-lymphocytes, which exhibited a statistically significant increase (data not shown).
An analysis of the formation of platelet aggregates with different leukocyte populations in AngII-infused mice revealed a significantly increased formation of platelet-neutrophil aggregates, without any change in aggregate formation of platelets with either monocytes or lymphocytes (Figure S3).
Figure 4 summarizes the results of experiments designed to address the role of NADPH oxidase in mediating the oxidative stress and increased blood cell recruitment elicited by chronic AngII infusion. AngII-induced leukocyte adhesion was significantly attenuated in both gp91phox−/− and gp91phox−/−→WT mice (Figure 4a). The number of emigrated leukocytes was also reduced in both groups; the reduction achieved statistical significance in gp91phox−/− and gp91phox−/−→WT mice (Figure 4b). AngII-induced platelet adhesion was not altered in either gp91phox−/− or gp91phox−/−→WT mice (Figure 4c). However, the oxidative stress elicited by AngII was largely prevented by gp91phox deficiency in either all cells (gp91phox−/− mice) (Panel F of Figure 1f) or only in circulating blood cells (gp91phox−/−→WT mice) (Figure 4d). Experiments with WT→ gp91phox−/− chimeras (NADPH oxidase deficiency in the vessel wall but not in blood cells) revealed significant reductions in leukocyte adhesion and emigration, and DHR oxidation that were comparable to those observed in gp91phox−/−→WT chimeras.
Discussion
The link between oxidative stress and AngII-mediated vascular dysfunction is well established.20, 21 AngII mediated ROS production has been proposed to be a critical event in the induction of an inflammatory phenotype in the vasculature caused by CVD risk factors, including HTN and hypercholesterolemia.22 The prevailing view of AngII-mediated inflammation is largely based on the assumption that endothelial cells are the principal target of AngII action, and that the engagement of AngII with AT1r on endothelial cells leads to an accelerated production of ROS via NADPH oxidase.23 The resultant oxidative stress enhances the generation of inflammatory mediators, increases adhesion molecule expression and endothelial adhesivity, and promotes the subsequent recruitment and activation of adherent of leukocytes and platelets on the endothelial cell surface.24 While much attention has been devoted to the role of endothelial cells in the vascular oxidative stress induced by AngII, less is known about the contribution of recruited/adherent blood cells to the accelerated ROS production elicited by AngII. The results of the present study suggest that neutrophils, but not platelets, play a major role in eliciting the venular wall oxidative stress that accompanies chronically elevated AngII levels and that the neutrophil-dependent oxidative stress is mediated via an NADPH oxidase-dependent mechanism.
The findings of this study provide several lines of evidence that suggest a role for blood cells in the oxidative stress elicited in venules by chronic AngII infusion: 1) the time-course of blood cell recruitment into postcapillary venules during the 14 days of AngII infusion parallels the increased intensity of the oxidative stress exhibited in these vessels (Figure 1), 2) bone marrow chimeras that are deficient in blood cell-associated AT1r or NADPH oxidase exhibit blunted oxidative stress responses to chronic AngII exposure that are comparable to the response observed in the respective knockout (AT1r−/− or gp91phox−/−) mice (Figures 2 & 4), and 3) selective depletion of circulating neutrophils (but not platelets) effectively prevents AngII-mediated oxidative stress (Figure 3). Collectively, these lines of evidence are consistent with a mechanism whereby the elevated circulating AngII levels engage with AT1r on neutrophils to activate NADPH oxidase. The oxidative stress detected in venules may either reflect attached activated neutrophils that produce ROS and/or an activating effect of neutrophils on endothelial cells, which in turn produce ROS at an accelerated rate. The latter possibility is supported by our observation that gp91phox−/−→WT and WT→gp91phox−/− chimeras produced the same level of attenuation of oxidative stress, suggesting that the activation of NADPH oxidase in one cell population (eg, endothelial cells) is required for enzyme activation (and oxidative stress) in the other cell type (eg, leukocytes). Such a series-coupled relationship between endothelial cell and leukocyte NADPH oxidases is consistent with the results of a previous report wherein gp91phox−/−→WT and WT→ gp91phox−/− bone marrow chimeras exhibited a similar level of attenuation of leukocyte and platelet adhesion in cremaster muscle venules of hypercholesterolemic mice.22 This shared characteristic of the AngII infusion and diet-induced hypercholesterolemia may reflect the dependence of oxidative stress induction in both models on AT1 receptors.25
The importance of neutrophils in AngII mediated microvascular alterations is supported by several reports in the literature.4, 9, 10 Acute AngII exposure has been shown induce the adhesion of leukocytes to monolayers of cultured endothelial cells26 and in postcapillary venules 5, 10, with neutrophils accounting for most of the adherent leukocytes in vivo. This is consistent with our observation that most of the leukocyte adhesion elicited by chronic AngII exposure is abolished by rendering mice neutropenic. The dominant role of neutrophils in this model is also evident from our confocal imaging studies which reveal that neutrophils account a large majority of adherent leukocytes that adhere in AngII stimulated venules (Figure 3). Equally important is our observation that other leukocyte populations, including T-cells and B-cells, adhere in venules in response to AngII, albeit to a more limited extent than neutrophils. The pathophysiological importance of these smaller populations of recruited leukocytes to the oxidative stress and vascular dysfunction elicited by AngII remains unclear.
Our findings in neutropenic mice also suggest that neutrophil recruitment is an important determinant of AngII-mediated platelet recruitment. Studies in other experimental models of chronic inflammation have demonstrated a similar co-dependency of platelet and neutrophil recruitment in inflamed postcapillary venules.17,27 For example, Vowinkel and coworkers27 have reported that the adhesion of platelets in colonic venules of mice with colitis is profoundly reduced when the animals are rendered neutropenic with antineutrophil serum. Another manifestation of the neutrophil-platelet interactions that occur during chronic inflammation is the formation of platelet-leukocyte aggregates in circulating blood.28 The results of our study indicate that such aggregates are generated in blood during chronic AngII exposure and that platelets preferentially form aggregates with neutrophils, which supports that contention that neutrophils are an important target of AngII action.
While the ability of AngII to elicit oxidative stress in endothelial cells is well-documented23, 29, less is known about the effects of the peptide in promoting ROS production in leukocytes. Monocytes from hypertensive patients and neutrophils from hypercholesterolemic patients exhibit increased ROS production in response to AngII stimulation.30 Furthermore, it has been demonstrated that AngII enhances NADPH oxidase activity in human neutrophils by engaging with AT1 receptors and by activating mitogen-activated protein kinase, calcineurin, and the transcription factor NF-kappaB.31 These observations are consistent with our finding that neutrophil-associated NADPH oxidase makes a significant contribution to the oxidative stress that is manifested in the walls of postcapillary venules exposed to elevated levels of AngII.
Perspectives
AngII is known to mediate the pro-inflammatory and pro-thrombogenic phenotype that is assumed by the microvasculature in the presence of risk factors for CV disease, including hypertension and hypercholesterolemia. These responses to AngII have been linked to the ability of the peptide to induce a pro-oxidative state in endothelial cells via AT1r-mediated activation of NADPH oxidase. The findings of the present study suggest that the neutrophils adhering to the vessel wall in response to AngII also undergo an oxidative burst via AT1r. Furthermore, it appears that the neutrophil-derived ROS is of greater quantitative importance than endothelial cell-derived ROS production in eliciting the inflammatory and prothrombogenic phenotype associated with elevated AngII levels. These findings suggest that neutrophilic NADPH oxidase may be a novel therapeutic target for prevention of the inflammatory and thrombogenic responses that accompany the risk factors for cardiovascular diseases.
Supplementary Material
Novelty & Significance.
What Is New?
The link between angiotensin II receptor activation, oxidative stress, and microvascular inflammation appears to involve the activation of NADPH oxidase in circulating blood cells.
Neutrophils, but not platelets, are largely responsible for the oxidative stress associated with chronically elevated angiotensin II levels.
What Is Relevant?
Leukocyte activation is equally important as endothelial cell activation in mediating the chronic, low-grade inflammation that accompanies angiotensin II mediated hypertension.
Neutrophil-associated NADPH oxidase represents a novel therapeutic target for prevention of the inflammatory and thrombogenic responses that accompany hypertension and other risk factors for cardiovascular diseases.
Summary.
Chronic angiotensin II infusion induces oxidative stress and promotes the recruitment of adherent leukocytes and platelets in skeletal muscle venules. Neutrophils, but not platelets, appear to contribute to the oxidative stress via a mechanism that involves leukocyte-associated AT1 receptors and NAHPH oxidase. This mechanism is also critical in mediating the blood cell-endothelial cell interactions elicited by angiotensin II.
Acknowledgments
Funding Sources
Supported by a grant from the National Heart Lung and Blood Institute (HL26441). Dr. Yildirim is supported by a postdoctoral fellowship from the Malcolm Feist Cardiovascular Endowment at LSU Health Sciences Center.
Footnotes
Disclosures
None
References
- 1.Frey RS, Ushio-Fukai M, Malik AB. Nadph oxidase-dependent signaling in endothelial cells: Role in physiology and pathophysiology. Antioxid Redox Signal. 2009;11:791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigo R, Gonzalez J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res. 2011;34:431–440. doi: 10.1038/hr.2010.264. [DOI] [PubMed] [Google Scholar]
- 3.Granger DN, Rodrigues SF, Yildirim A, Senchenkova EY. Microvascular responses to cardiovascular risk factors. Microcirculation. 2010;17:192–205. doi: 10.1111/j.1549-8719.2009.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benigni A, Cassis P, Remuzzi G. Angiotensin ii revisited: New roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng ZJ, Vapaatalo H, Mervaala E. Angiotensin ii and vascular inflammation. Med Sci Monit. 2005;11:RA194–205. [PubMed] [Google Scholar]
- 6.Petnehazy T, Stokes KY, Wood KC, Russell J, Granger DN. Role of blood cell-associated at1 receptors in the microvascular responses to hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26:313–318. doi: 10.1161/01.ATV.0000193625.32499.71. [DOI] [PubMed] [Google Scholar]
- 7.Alexander RW. Leukocyte and endothelial angiotensin ii type 1 receptors and microvascular thrombotic and inflammatory responses to hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26:240–241. doi: 10.1161/01.ATV.0000199680.42737.ca. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa M, Sekizuka E, Yamaguchi N, Nakadate H, Terao S, Granger DN, Minamitani H. Angiotensin ii type 1 receptor signaling contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. Am J Physiol Heart Circ Physiol. 2007;292:H2306–2315. doi: 10.1152/ajpheart.00601.2006. [DOI] [PubMed] [Google Scholar]
- 9.Vital SA, Terao S, Nagai M, Granger DN. Mechanisms underlying the cerebral microvascular responses to angiotensin ii-induced hypertension. Microcirculation. 2010;17:641–649. doi: 10.1111/j.1549-8719.2010.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piqueras L, Kubes P, Alvarez A, O’Connor E, Issekutz AC, Esplugues JV, Sanz MJ. Angiotensin ii induces leukocyte-endothelial cell interactions in vivo via at(1) and at(2) receptor-mediated p-selectin upregulation. Circulation. 2000;102:2118–2123. doi: 10.1161/01.cir.102.17.2118. [DOI] [PubMed] [Google Scholar]
- 11.Briones AM, Touyz RM. Oxidative stress and hypertension: Current concepts. Curr Hypertens Rep. 2010;12:135–142. doi: 10.1007/s11906-010-0100-z. [DOI] [PubMed] [Google Scholar]
- 12.Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin ii in smooth muscle cells from resistance arteries of hypertensive patients: Role of phospholipase d-dependent nad(p)h oxidase-sensitive pathways. J Hypertens. 2001;19:1245–1254. doi: 10.1097/00004872-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Hunyady L, Catt KJ. Pleiotropic at1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin ii. Mol Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 14.Panes J, Granger DN. Neutrophils generate oxygen free radicals in rat mesenteric microcirculation after abdominal irradiation. Gastroenterology. 1996;111:981–989. doi: 10.1016/s0016-5085(96)70065-3. [DOI] [PubMed] [Google Scholar]
- 15.Salas A, Panes J, Elizalde JI, Granger DN, Pique JM. Reperfusion-induced oxidative stress in diabetes: Cellular and enzymatic sources. J Leukoc Biol. 1999;66:59–66. doi: 10.1002/jlb.66.1.59. [DOI] [PubMed] [Google Scholar]
- 16.Suematsu M, Schmid-Schonbein GW, Chavez-Chavez RH, Yee TT, Tamatani T, Miyasaka M, Delano FA, Zweifach BW. In vivo visualization of oxidative changes in microvessels during neutrophil activation. Am J Physiol. 1993;264:H881–891. doi: 10.1152/ajpheart.1993.264.3.H881. [DOI] [PubMed] [Google Scholar]
- 17.Cooper D, Russell J, Chitman KD, Williams MC, Wolf RE, Granger DN. Leukocyte dependence of platelet adhesion in postcapillary venules. Am J Physiol Heart Circ Physiol. 2004;286:H1895–1900. doi: 10.1152/ajpheart.01000.2003. [DOI] [PubMed] [Google Scholar]
- 18.Tailor A, Granger DN. Hypercholesterolemia promotes p-selectin-dependent platelet-endothelial cell adhesion in postcapillary venules. Arterioscler Thromb Vasc Biol. 2003;23:675–680. doi: 10.1161/01.ATV.0000056742.97580.79. [DOI] [PubMed] [Google Scholar]
- 19.Stokes KY, Clanton EC, Clements KP, Granger DN. Role of interferon-gamma in hypercholesterolemia-induced leukocyte-endothelial cell adhesion. Circulation. 2003;107:2140–2145. doi: 10.1161/01.CIR.0000062687.80186.A0. [DOI] [PubMed] [Google Scholar]
- 20.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension. 2009;54:619–624. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Touyz RM, Briones AM. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens Res. 2011;34:5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 22.Stokes KY, Clanton EC, Russell JM, Ross CR, Granger DN. Nad(p)h oxidase-derived superoxide mediates hypercholesterolemia-induced leukocyte-endothelial cell adhesion. Circ Res. 2001;88:499–505. doi: 10.1161/01.res.88.5.499. [DOI] [PubMed] [Google Scholar]
- 23.KKG Hua Cai, Harrison David G. The vascular nad(p)h oxidases as therapeutic targets in cardiovascular diseases. Trands in Pharmacological Sciences. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues SF, Granger DN. Role of blood cells in ischaemia-reperfusion induced endothelial barrier failure. Cardiovasc Res. 2010;87:291–299. doi: 10.1093/cvr/cvq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petnehazy T, Stokes KY, Russell JM, Granger DN. Angiotensin II type-1 receptor antagonism attenuates the inflammatory and thrombogenic responses to hypercholesterolemia in venules. Hypertension. 2005;45:209–215. doi: 10.1161/01.HYP.0000154085.27868.93. [DOI] [PubMed] [Google Scholar]
- 26.Hahn AW, Jonas U, Buhler FR, Resink TJ. Activation of human peripheral monocytes by angiotensin ii. FEBS Lett. 1994;347:178–180. doi: 10.1016/0014-5793(94)00531-1. [DOI] [PubMed] [Google Scholar]
- 27.Vowinkel T, Wood KC, Stokes KY, Russell J, Tailor A, Anthoni C, Senninger N, Krieglstein CF, Granger DN. Mechanisms of platelet and leukocyte recruitment in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1054–1060. doi: 10.1152/ajpgi.00350.2007. [DOI] [PubMed] [Google Scholar]
- 28.McGregor L, Martin J, McGregor JL. Platelet-leukocyte aggregates and derived microparticles in inflammation, vascular remodelling and thrombosis. Front Biosci. 2006;11:830–837. doi: 10.2741/1840. [DOI] [PubMed] [Google Scholar]
- 29.Paravicini TM, Touyz RM. Nadph oxidases, reactive oxygen species, and hypertension: Clinical implications and therapeutic possibilities. Diabetes Care. 2008;31 (Suppl 2):S170–180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez A, Cerda-Nicolas M, Naim Abu Nabah Y, Mata M, Issekutz AC, Panes J, Lobb RR, Sanz MJ. Direct evidence of leukocyte adhesion in arterioles by angiotensin ii. Blood. 2004;104:402–408. doi: 10.1182/blood-2003-08-2974. [DOI] [PubMed] [Google Scholar]
- 31.Hazan-Halevy I, Levy T, Wolak T, Lubarsky I, Levy R, Paran E. Stimulation of nadph oxidase by angiotensin ii in human neutrophils is mediated by erk, p38 map-kinase and cytosolic phospholipase a2. J Hypertens. 2005;23:1183–1190. doi: 10.1097/01.hjh.0000170381.53955.68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.