Abstract
Aims
Histomorphometric analysis is a widely used technique to assess changes in tissue structure and function. Commercially-available programs that measure histomorphometric parameters can be cost prohibitive. In this study, we compared an inexpensive method of histomorphometry to a current proprietary software program.
Methods and results
Image J and Adobe Photoshop® were used to measure static and kinetic bone histomorphometric parameters. Photomicrographs of Goldner’s Trichrome stained femurs were used to generate black and white image masks, representing bone and non-bone tissue, respectively, in Adobe Photoshop®. The masks were used to quantify histomorphometric parameters (bone volume, tissue volume, osteoid volume, mineralizing surface, and interlabel width) in Image J. The resultant values obtained using Image J and the proprietary software were compared and found to be statistically non-significant.
Conclusions
The wide ranging use of histomorphometric analysis for assessing the basic morphology of tissue components makes it important to have affordable and accurate measurement options that are available for a diverse range of applications. Here we have developed and validated an approach to histomorphometry using commonly and freely available software that is comparable to a much more costly, commercially-available software program.
Keywords: histomorphometry, bone, image analysis, Image J
Introduction
Histomorphometric examination of tissues is based on quantitative measurements of microscopic organization and structure and has been used to provide information on cellular responses (e.g., migration1, inflammation2), tissue pathology (atherosclerotic lesions4,5, tumor growth6–8) as well as metabolic bone disturbances9. Here we focus on bone histomorphometric analysis since parameters of bone structure and function are well established10. These parameters require three types of measurements: area, perimeter, and distance between defined segments of interest. These basic measurements translate into primary histomorphometric indices including tissue volume, bone volume, osteoid volume, mineralizing surface, and interlabel width11.
Advances in histomorphometric analyses have been made possible with the use of computer-assisted image recognition software and the development of sophisticated approaches to assess the microstructure of bone. There are commercially-available programs that allow for increased automation of histomorphometric analyses. However, these programs can be prohibitively expensive, requiring both the purchase of proprietary software and dedicated cameras and computers. Therefore, the availability of substantially less costly alternative methods that maintain comparable accuracy would make histomorphometric analyses more readily accessible to researchers across diverse fields of investigation.
Materials and methods
TISSUE SAMPLES
Human bone samples were obtained from elective hip arthroplasties in accordance with protocols approved by the Institutional Review Board of the University of Pennsylvania. Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC), University of Pennsylvania.
HISTOLOGICAL ANALYSIS
To measure dynamic bone formation parameters, mice (wild-type) were injected subcutaneously with calcein (Sigma, St Louis, MO, USA) [30mg/kg body weight] on day 9 before tissue harvest and xylenol orange (Sigma, St Louis, MO, USA) [90mg/kg body weight] on day 2 before tissue harvest.
Both human core bone samples and mouse hind limbs were excised, cleaned of soft tissue, and fixed in 3.7% formaldehyde for 72 hours. Isolated bone tissue were dehydrated in graded alcohols (70 to 100%), cleared in xylene and embedded in methyl methacrylate. Plastic tissue blocks were cut into 5µm sections using a Polycut-S motorized microtome(Reichert-Jung, Nossloch, Germany).
After the mouse bone sections were used to measure the fluorochrome labeled surface and interlabel width, they were deplasticized in xylene and then stained with Goldner’s Trichrome.
Randomly selected regions of interest (ROIs) within three sections per limb were visualized for fluorochrome labeling using a Nikon Eclipse 90i microscope and Nikon Plan Fluor 10X objective. ROIs from the same sections were visualized using a Nikon Eclipse 90i microscope and 4X and 20X objectives for Goldner’s Trichrome staining. Image capture was performed using NIS Elements Imaging Software 3.10 Sp2 and a Photometrics Coolsnap EZ camera. The Bioquant Osteo II digitizing system (R&M Biometrics, Nashville, TN) according to the manufacturer’s instructions, or sequentially Adobe Photoshop® and Image J software, were used for image analysis. The following primary measurements for dynamic parameters of bone formation were collected from the trabecular surface in defined ROIs (100 µm distal to the growth plate and 50 µm in from the endosteal cortical bone) at 100X magnification: single-label perimeter (sL.PM), double-labeled perimeter measured along the first label (dL.Pm) and interlabel distance. The same sections were then evaluated under brightfield microscopy after Goldner’s Trichrome staining to determine static parameters of bone formation including: tissue volume (TV), bone volume (BV) and osteoid volume (OV).
PREPARATION OF IMAGE MASKS
Before evaluation of bone sections in Image J, black and white image masks were created using Adobe Photoshop® Version CS2 (9.0).
In Adobe Photoshop®, the following steps were taken for each image captured from tissue sections stained with Goldner’s Trichrome:
Click File>Open
- Once the image is open make a copy of this original image:
-
◦Click the rectangular marquee tool
-
◦Outline the entire image with the rectangle tool
-
◦Click Edit>Copy
-
◦Click File>New
-
◦Click Edit>Paste
-
◦
-
On the copied image
-
◦Use the wand tool (
 ) to select the areas of bone.
) to select the areas of bone.
-
▪To optimize the wand tool for the purposes of making the black and white mask it is important to optimize the wand color tolerance. The tolerance determines the similarity or differences in the color of pixels selected. The scale for tolerance ranges from 0 to 255. The higher the tolerance value, the more colors (wider pixel range) will be incorporated into the wand selections. Conversely, the lower the tolerance value, the fewer the colors (shorter pixel range) that will be included in the wand selection. We determined the optimum tolerance value to be 128.
-
▪
-
◦After selection of all relevant areas, choose Edit>Fill.
-
◦In the pop-up window
-
▪Click in the pull down menu
-
▪Click ”Black”
-
▪Click “OK”
-
▪
-
◦Click Select>Inverse.
-
◦Click Edit>Fill.
-
◦In the pop-up window
-
▪Click in the pull down menu
-
▪Click “White”
-
▪Click “OK”
-
▪
Note: The image will now be black (bone) and white (all other tissue).
-
◦
Click File>Save as
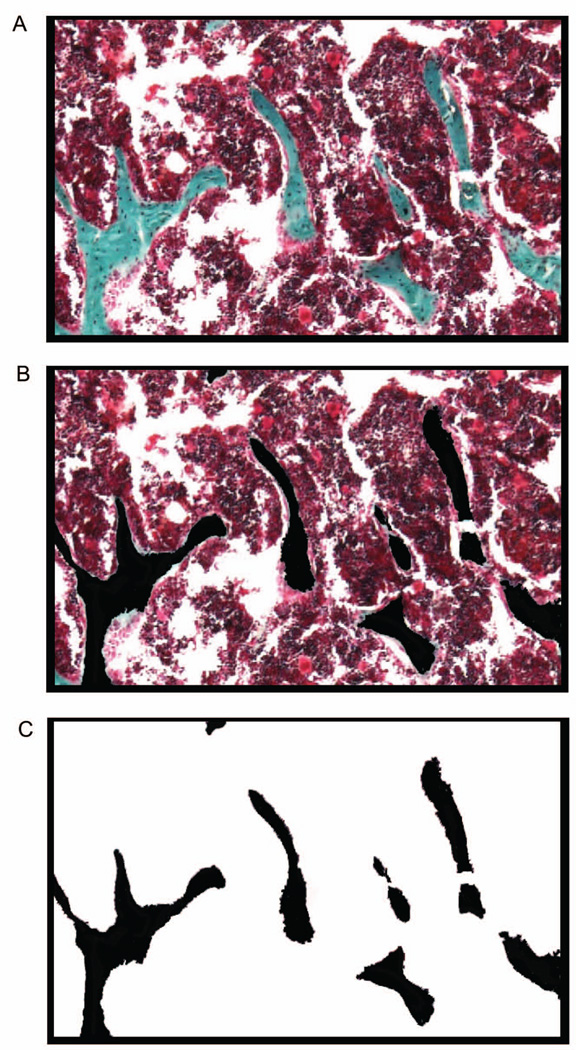
Save this image as a .TIFF file named “Bone Volume Mask” (Figure. 1).
Figure 1.
Construction of the black and white image mask. (A) A region of interest was selected at 40X magnification from a Goldner’s Trichrome stained section. This captured image was then opened in Adobe Photoshop® to prepare the black and white mask. (B) Within the selected region of interest, bone was identified and represented in black. (C) The remaining, non-bone tissue, was selected to be white.
CALIBRATING IMAGE J
Image J was calibrated for each magnification used in the analysis.
If the calibrations for the microscope are known:
At the end of the Image J menu, select the last icon (“>>”)
Click “Scale bar tools for Microscopes”
Click the icon Microscope Profiles Manger Menu (
 )> Create a New Microscope Profile
)> Create a New Microscope Profile- A new window appears: “Editing of the Microscope Profile”
-
◦Click “Microscope Profile Name”
-
◦Name the Profile (e.g., “My Microscope Calibrations”)
-
◦Enter the known calibrations for the microscope used to capture the images
-
◦Click “OK”
-
◦
- Once the image is open
-
◦Click the microscope icon “Available Microscope Profiles Menu” (
 )> “My Microscope Calibration”
)> “My Microscope Calibration” -
◦Click the “Scale Bar” icon(
 )
) -
◦In pop-up window, select the magnification that is appropriate for the image being analyzed (>OK>OK)
-
◦
If the calibration of the microscope is unknown:
To calibrate Image J, a scale bar must be placed on one image for each magnification.
- Click File>Open
-
◦Open the file with an image containing a scale bar inserted by the microscope or camera software that acquired the image.
-
◦
Click Image >Type: 8-bit (image will become black and white)
Click the line icon (
 ) and draw a line measuring the length of the scale bar.
) and draw a line measuring the length of the scale bar.- Click Analyze>Set Scale
-
◦The length measured for the scale bar is entered as Distance in pixels.
-
◦The length of the scale bar as labeled by the microscope is entered as Known distance.
-
◦Enter the unit of length for the scale bar.
-
◦Check Global. (Subsequent analysis will be measured on this scale. When the magnification changes the calibration must be changed accordingly to the new magnification.)
-
◦
QUANTIFYING THE BONE AND OSTEOID VOLUME
In Image J:
Click File> Open Bone Volume Mask or Osteoid Mask
Click Edit> Selection>Select All
- Click Analyze>Measure
-
◦The area is the Tissue Area.
-
◦
Click the “wand tool” (
 ) and shift key to select all the black areas.
) and shift key to select all the black areas.- Click Analyze>Measure
-
◦The area is the Bone Area (or the area is the Osteoid Area, if osteoid is being quantified)
-
◦
QUANTIFYING DISTANCES AFTER FLUORESCENT LABELING
Images of fluorescent labeling can be opened directly in Image J.
Select File: Open
Upload the image of the fluorescently labeled sections.
Right click the line icon (
 ) and select a segmented line.
) and select a segmented line.Draw a line tracing the single labeled dye fronts.
Select Analyze> Measure the line.
Draw a line tracing the length of the double labeled dye fronts along the front facing the bone marrow (Figure. 2).
Select Analyze> Measure the line.
This is repeated for the entire ROI until all of the single and double labeled tracular bone has been measured. The mineralizing surface (MS) is calculated by the formula MS= dL.Pm + (0.5 × sL.Pm), where sL.Pm is the length of the single labeled surfaces and dL.Pm the length of double labeled surface.
Right click the line icon (
 )and select a straight line.
)and select a straight line.Measure the distance between the inner and outer fluorescent labels at increments of approximately 5µm part. These measurements are averaged as the interlabel width (Figure 2).
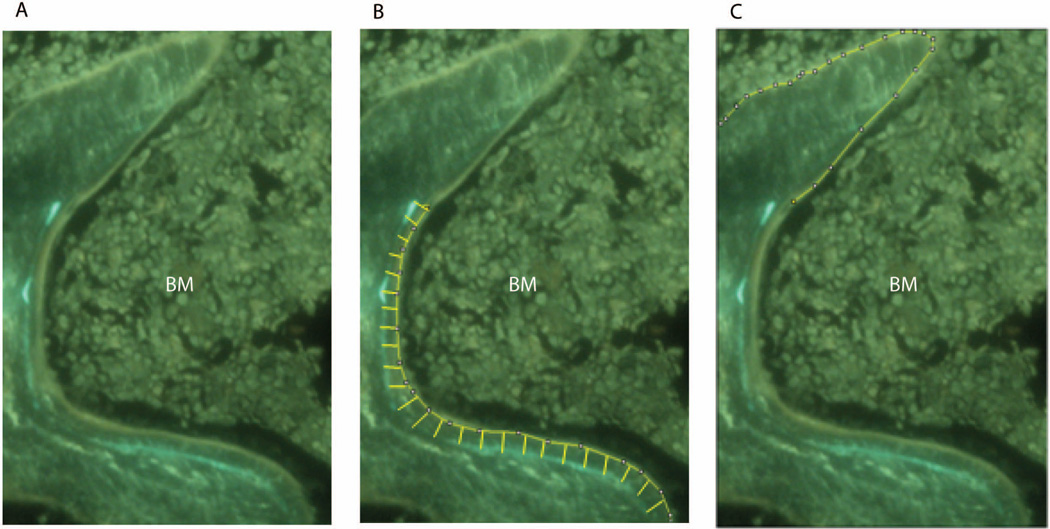
Figure 2.
Measurement of mineral apposition. (A) A region of interest was selected at 100X magnification showing incorporation of the fluorochromes calcein and xylenol orange. (B) Demarcation of interlabel width between double labeled fronts. (C) Demarcation of single labeling. BM, Bone marrow.
STATISTICS
To determine if measurements made by BioQuant and Image J were statistically different, the paired Student’s t-test (two-tailed) was used for all measurements. All statistics were generated using GraphPad (GraphPad Software, Inc La Jolla, CA). The differences were considered statistically significant at p <0.05. Associated error is reported as ± standard deviation.
Results
Tissue sections from mouse and human femurs were stained with Goldner’s Trichrome and used to assess bone histomorphometric parameters. Images were visualized under light microscopy and regions of interest were captured for analysis. Adobe Photoshop® was used to prepare the images for analysis. Color images were converted to black and white masks using the wand tool in Adobe Photoshop® to highlight areas of bone. All bone tissue was designated in black color. The remaining tissue was designated in white color, creating a black and white mask for analysis by Image J software (http://imagej.nih.gov/ij/) (Figure 1). Similarly, a mask was created for analysis of osteoid, with osteoid selected using the wand tool and colored black; the remaining tissue being colored white.
The tissue volume, bone volume, and osteoid volume were quantified using freely-available Image J software and commercially-available BioQuant imaging software. As shown in Table 1, the values for tissue volume, bone volume and osteoid volume determined by each program were not statistically significantly different.
Table 1.
Comparison of static parameters of bone formation using commercially and freely-available software programs.
|
Bone Volume (mm2) |
Tissue Volume (mm2) |
Osteoid Volume (µm2) |
||||
|---|---|---|---|---|---|---|
| BioQuant | Image J | BioQuant | Image J | BioQuant | Image J | |
| WT (n = 10) |
0.068±0.019 | 0.069±0.021 | 0.55±0.00 | 0.55±0.00 | 137.02±48.81 | 141.57±48.99 |
| p=0.34 | p=0.36 | |||||
| Human Core (n = 10) |
0.082±0.011 | 0.088±0.028 | 0.55±0.00 | 0.55±0.00 | 0.0082±0.006 | 0.010±0.008 |
| p=0.63 | p=0.09 | |||||
The bone volume, tissue volume, and osteoid volume were measured using 10 sections from each sample. The numbers shown are averaged results. Images for quantitating tissue volume were taken at 4X magnification and standardized to be the same size (statistical analysis was not applicable). The other two parameters were shown to be statistically similar by paired student t-tests.
Dynamic histomorphometry makes use of fluorochromes, such as calcein, that are incorporated into bone at the front of mineralization. When two sequential fluorochromes are administered over a defined time interval, mineralization and the rates of bone formation can be calculated from the distance between the two labeled fronts (interlabel width; Figure. 2). The mineralizing surface (MS) was quantified by measuring the length of the single labeled surfaces (sL.Pm), and double labeled surface (dL.Pm), according to the formula MS= dL.Pm + (0.5 × sL.Pm) 11. Calculated measurements were determined using Image J and BioQuant software and differences between derived parameters were not found to be statistically significantly different (Table 2).
Table 2.
Comparison of dynamic parameters of bone formation using commercially and freely-available software programs.
| Mineralizing Surface (mm) |
Interlabel Width (µm) |
|||
|---|---|---|---|---|
| BioQuant | Image J | BioQuant | Image J | |
| WT Sample 1 | 1.21±0.32 | 1.22±0.32 | 11.94±2.17 | 11.43±2.44 |
| p=0.26 | p=0.23 | |||
| WT Sample 2 | 0.93±0.25 | 0.92±0.23 | 14.11±1.20 | 14.27±1.55 |
| p=0.74 | p=0.72 | |||
| WT Sample 3 | 0.82±0.38 | 0.84±0.37 | 12.04±0.28 | 11.84±0.28 |
| p=0.75 | p=0.72 | |||
| WT Sample 4 | 0.70±0.37 | 0.71±0.38 | 12.86±1.01 | 12.79±0.72 |
| p=0.63 | p=0.76 | |||
| WT Sample 5 | 0.77±0.09 | 0.77±0.09 | 12.80±1.76 | 12.56±2.41 |
| p=0.24 | p=0.69 | |||
| WT Sample 6 | 0.35±0.24 | 0.36±0.24 | 16.63±0.99 | 16.51±1.05 |
| p=0.42 | p=0.64 | |||
| WT Sample 7 | 1.17±0.52 | 1.17±0.53 | 12.29±0.95 | 12.29±0.05 |
| p=0.67 | p=0.94 | |||
| WT Sample 8 | 0.75±0.00 | 0.75±0.00 | 12.27±0.29 | 12.30±0.38 |
| p=0.86 | p=0.75 | |||
| WT Sample 9 | 0.87±0.35 | 0.87±0.35 | 13.21±1.72 | 13.12±1.87 |
| p=0.29 | p=0.39 | |||
| WT Sample 10 | 0.14±0.05 | 0.15±0.06 | 4.52±5.64 | 4.52±5.65 |
| p=0.60 | p=0.90 | |||
The mineralizing surface and the interlabel width were measured using multiple regions of interest from sections of each sample. The numbers shown are averaged results. Parameters were shown to be statistically similar by paired student t-tests.
Discussion
The wide ranging use of histomorphometric analysis for assessing the basic morphology of tissue components makes it important to have affordable and accurate measurement options that are available for a diverse range of applications. Here we have developed an approach to histomorphometry using commonly and freely available software that is comparable to a much more costly, commercially-available software program.
Histomorphometric analyses are based on the identification of cells and extracellular matrix by chemical or fluorescent staining within a region of interest and defined by primary parameters of areas they occupy, their boundary perimeters, or their distances from other points of reference. Commercially-available programs allow assignment of cells or extracellular matrix components by color, followed by the calculation of primary (or secondary) parameters by image analysis. In the approach described here, the former is achieved using commonly-available software such as Adobe Photoshop, while the latter is performed using the freely-available program Image J.
Image J is available as a no-cost download from the internet and is frequently updated. It allows freedom to work on any computer, adding flexibility to when and where the analysis of sections is performed. All measurements in Image J are made on archived images and so determination of histomorphometric parameters are semi-automated rather than live (i.e., in real time from tissue sections).
The approach described in this paper offers an alternative possibility for quantitative analysis of tissue sections that is both accessible and as accurate as more cost prohibitive approaches requiring proprietary software.
Acknowledgements
This work is supported by National Institute of Health/National Institute on Aging grant R01AG028873 (R.J.P.) and a National Institute of Health/National Institute of Arthritits, Musculoskeletal, and Skin Diseases grant P30-AR050950 sub-project (R.J.P.)
Footnotes
Declarations of interest
The authors declare that they have no competing interests.
References
- 1.Daniel TO, Liu H, Morrow JD, Crews BC, Marnett LJ. Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogensis. Cancer Res. 1999;59:4574–4577. [PubMed] [Google Scholar]
- 2.Blano MV, Vega HR, Giuliano R, et al. Histomorphometry of umbilical cord blood vessels in preeclampsia. J. Clin. Hypertens. 2010;50:260–265. doi: 10.1111/j.1751-7176.2010.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naïve T and immature dendritic cells. J. Leukocyte Biol. 2000;68 9-1. [PubMed] [Google Scholar]
- 4.De Urgate DA, Puapong D, Roostaeian J, et al. Surgisis patch tracheoplasty in a rodent model of tracheal stenosis. J. Surg. Res. 2003;112:65–69. doi: 10.1016/s0022-4804(03)00107-0. [DOI] [PubMed] [Google Scholar]
- 5.Davis HR, Hoos LM, Tetzloff G, et al. Deficiency of Niemann-Pick C1 like 1 prevents atherosclerosis in ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:841–849. doi: 10.1161/01.ATV.0000257627.40486.46. [DOI] [PubMed] [Google Scholar]
- 6.McEntee MF, Chiu C, Whelan J. Relationship of β-catenin and Bcl-2 expression to sulindac-induced regression of intestinal tumors in Min mice. Carcinogenesis. 1999;20:635–640. doi: 10.1093/carcin/20.4.635. [DOI] [PubMed] [Google Scholar]
- 7.Kumar MS, Erkeland SJ, Pester RE, et al. Suppression of non-small cell lung tumor development by the let-7 microRNA family. PNAS. 2008;105:3903–3908. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson EL, Olive KP, Tuveson DA, et al. The differential effects of mutant p53 alleles on advances murine lung cancer. Cancer Research. 2005;65:10280–10288. doi: 10.1158/0008-5472.CAN-05-2193. [DOI] [PubMed] [Google Scholar]
- 9.Brennan TA, Adapala NS, Barbe MF, Yingling V, Sanjay A. Abrogation of Cbl-PI3K increases bone formation and osteoblast proliferation. Calcified Tissue Int. 2011;89:396–410. doi: 10.1007/s00223-011-9531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parfitt MA, Drezner MK, Glorieux FH, et al. Bone histomorphometry: Standardization of nomenclature, symbols, and units. J Bone Miner Res. 1987;2:595–609. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 11.Vendi S, Compton J. Bone Histomorphometry. In: Helfich M, Ralston S, editors. Methods in Molecular Medicine: Bone Research Protocols. Totowa: Humana Press; 2003. pp. 345–351. [Google Scholar]