Abstract
Toll-like receptor 4 (TLR4), a receptor forDamage Associated Molecular Pattern Molecules and also the lipopolysaccharide receptor, is required for early endothelial activation leading to maximal inflammation and injury during murine ischemic acute kidney injury. DNA microarray analysis of ischemic kidneys from TLR4-sufficient and deficient mice showed that pentraxin 3 (PTX3) was upregulated only on the former while transgenic knockout of PTX3 ameliorated acute kidney injury. PTX3 was expressed predominantly on peritubular endothelia of the outer medulla of the kidney in control mice. Acute kidney injury increased PTX3 protein in the kidney and the plasma where it may be a biomarker of the injury. Stimulation by hydrogen peroxide, or the TLR4 ligands recombinant human High-Mobility Group protein B1 or lipopolysaccharide, induced PTX3 expression in the Mile Sven 1 endothelial cell line and in primary renal endothelial cells suggesting that endothelial PTX3 was induced by pathways involving TLR4 and reactive oxygen species. This increase was inhibited by conditional endothelial knockout of Myeloid differentiation primary response gene 88, a mediator of a TLR4 intracellular signaling pathway. Compared to wild type mice, PTX3 knockout mice had decreased endothelial expression of cell adhesion molecules at 4 hours of reperfusion possibly contributing to a decreased early maladaptive inflammation in the kidneys of knockout mice. At 24 hours of reperfusion, PTX3 knockout increased expression of endothelial adhesion molecules when regulatory and reparative leukocytes enter the kidney. Thus, endothelial PTX3 plays a pivotal role in the pathogenesis of ischemic acute kidney injury.
INTRODUCTION
Although ischemic acute kidney injury (AKI) continues to have a high incidence and mortality despite modern supportive therapy, and leads to progressive kidney disease which has its own high mortality (1–3), the pathogenesis remains poorly understood.
TLR4 is required for the inflammatory response that exacerbates the initial ischemic injury (4). We previously showed that HMGB1 released by injured renal cells bound endothelial TLR4 and this increased expression of proinflammatory adhesion molecules (5). In the absence of endothelial TLR4, these adhesion molecules were not expressed, inflammation was decreased, and injury ameliorated. This data incriminates TLR4 as the trigger for the initial endothelial activation necessary for inflammation and maximal injury during ischemic injury. To better understand the maladaptive role of TLR4, we compared genome-wide gene expression at 4 hr reperfusion in kidneys from WT C57BL/10 mice versus TLR4 null C57BL/10ScNJ mice using Affymetrix GeneChip Mouse Genome 430 2.0 Array chips. One of the most differentially expressed genes was pentraxin 3(PTX3)1.
We now report that knockout (KO) of PTX3 ameliorates ischemic AKI. PTX3 is the prototypic member of the long pentraxin family that is produced in peripheral tissues. It is conserved from arachnids to humans. In particular its gene organization, structure, and promoter are highly conserved in human and mouse. This not only suggests the fundamental importance of PTX3 in biology and disease, but also that translation of murine studies to human disease should be possible (6). Although PTX3 increases in human plasma after acute ischemia reperfusion injury (IRI) to the brain and heart; and although this increase is proposed as a clinically useful and early reliable prognostic marker for bad outcomes (7, 8), if and how PTX3 contributes to pathophysiology of IRI is not well established.
Altogether the above suggests fundamental important links between TLR4, endothelium, and PTX3 in the pathophysiology of renal AKI. We now explore these links for the first time experimentally. We localize PTX3 to renal endothelium, explore its regulation by ROS, TLR4, and the Myd88 dependent signaling pathway of TLR4, and determine the effect of PTX3 KO on endothelial functions. Our data establish a maladaptive role for PTX3 during ischemic AKI.
RESULTS
PTX3 knockout attenuates ischemic AKI
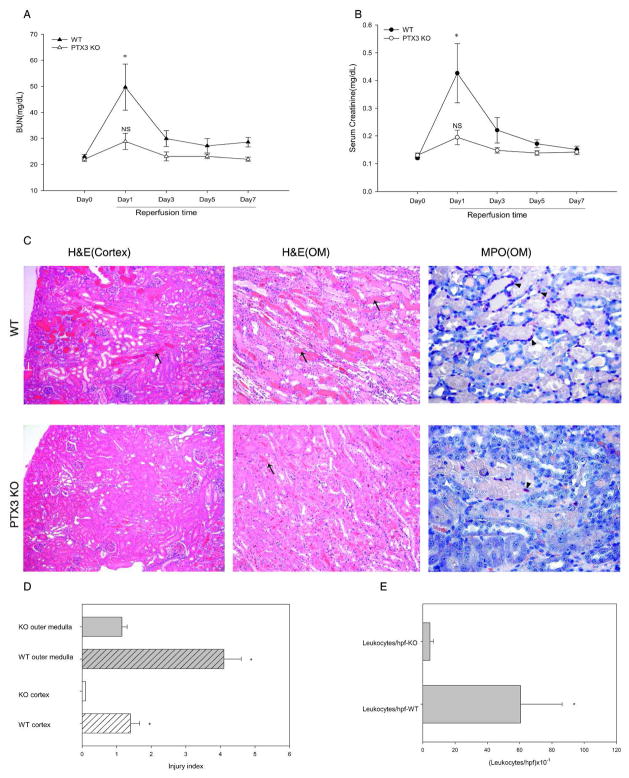
We compared the response of wildtype (WT) versus PTX3 homozygous knockout (PTX3 KO) kidneys to IRI caused by temporarily occluding the renal pedicle (see “Methods”). At 24hr reperfusion, both measures of renal function increased significantly in WT mice compared to PTX3 KO mice (Figure 1A and B). For sham-operated mice, the renal function remained similar and close to baseline in both strains (data not shown).
Figure 1.
PTX3 deficiency ameliorates ischemic AKI. Renal pedicles of PTX3 KO mice and WT littermates were clamped for 16min. Blood samples were collected on day 1, 3, 5, and 7 reperfusion. Day 0 were samples from mice without operation. (A) BUN was measured using a colorimetric method. (B) Serum creatinine was measured using a capillary electrophoresis method. Error bars represent mean ±SEM, n=5 per group, * P< 0.05 WT Day 1 versus Day 0; NS, not significant PTX3 KO Day 1 versus Day 0. (C) H&E and MPO staining: at 24hr reperfusion, kidneys were harvested and fixed in formalin. H&E and MPO staining was performed on paraffin sections. Arrow shows one of many damaged tubules. Original magnification, x10. Arrowhead indicates one of many positively staining cells for MPO(x20). (D) Injury index at 24hr reperfusion: tissue damage was scored in cortex and outer medulla. (E) Leukocytes infiltration at 24hr reperfusion. The number of inflammatory cells in cortex and outer medulla was counted. In both “D” and “E”, error bars represent mean ± SEM, n=5 per group, *P< 0.05 WT versus KO.
Note that we used capillary electrophoresis to measure the serum creatinine (Scr) (9). This is more accurate than the usual Jaffe method which is confounded by extraneous chromophores in murine serum. Thus, values obtained by direct chemical measurements (HPLC and capillary electrophoresis) are 1/6th of the values obtained by the Jaffe type measurements (10, 11). A rise of the Scr from 0.1 to 0.4 mg/dL in our assay represents significant renal injury. In Figure 1, we used an ischemia time of 16 minutes that allowed all the control mice to survive for the entire 7 day experiment. We observed the same protection by PTX3 knockout after a 23 minute ischemia time, but some wildtype mice died. In most experiments where kidneys were harvested at 4 hr and survival of the wildtype beyond 24 hr was not important, we used the 23 minute ischemia time because this gave a larger signal for PTX3 and the other proinflammatory molecules in the wildtype groups.
We also analyzed the histology of the kidneys by scoring the tubular damage and inflammation. After IRI, we observed less injury and inflammation in PTX3 KO ischemic kidneys. Kidney injury scores were significantly more severe in the WT compared to PTX3 KO kidneys (Figure 1C and D). To study leukocyte infiltration, we stained kidney sections with myeloperoxidase (MPO) and counted positively staining cells per 10 high-power fields (hpf). WT kidneys had significantly more inflammation (Figure 1C and E).
PTX3 is increased in ischemic WT kidneys but not in TLR4 KO kidneys
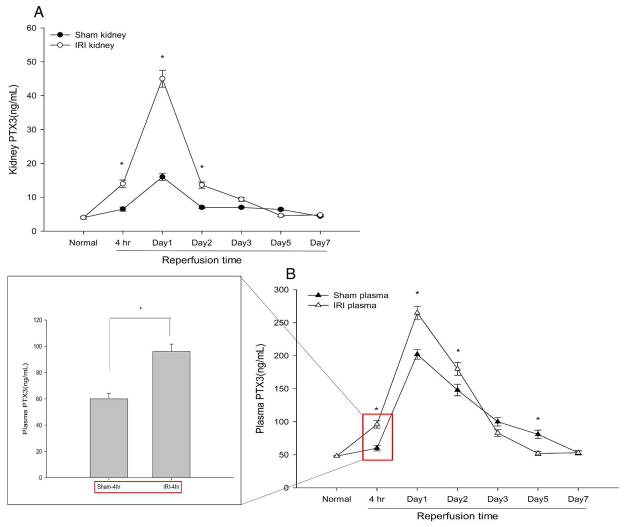
We found that IRI increased PTX3 protein in WT kidneys by ELISA. At 4hr reperfusion, PTX3 increased by 2.1-fold compared to sham controls (14.0±1.1ng/mL vs. 6.5±0.6ng/mL). It reached a peak value of 45±2.5ng/mL on day 1 (vs. sham 16.0±1.1ng/mL) and then gradually dropped down to baseline by day 7 (Figure 2A). In addition, we found a significant elevation of plasma PTX3 at 4hr reperfusion (96± 5.6ng/mL vs. 60±4.2ng/mL), which peaked at 24 hr reperfusion (265±9.8ng/mL vs. 202±7.3ng/mL) (Figure 2B). This increase is similar to increases seen after ischemic injury of other organs – brain after stroke, and myocardial ischemia (7, 8). Because PTX3 is produced at the site of injury (6), the plasma PTX3 is thought to enter the blood from the injured brain, heart, or, in our experiment, kidney. The increased plasma PTX3 in the sham mice may represent PTX3 produced by skin, muscle, and connective tissues that are injured by the surgery necessary to expose and then place the vascular clamp across (IRI) or beneath (sham) the renal arteries.
Figure 2.
PTX3 protein increases in kidney and plasma during AKI. Renal pedicles of WT mice were clamped for 16 min. Kidney samples were collected from normal kidneys and ischemic kidneys harvested at 4hr, day1, day3, day5, day7 reperfusion. Tissues were disrupted using homogenizer in RIPA buffer supplemented with protease inhibitors. Kidney protein concentration was determined by the Bradford assay in triplicate, and the same amount of protein from each kidney was assayed. Plasma and kidney PTX3 levels were measured using the Quantikine PTX3 immunoassay kit from R& D systems. (A) Time course study of kidney PTX3. Error bars represent mean ± SEM, n=4, *P< 0.001 compared to sham at each time point. (B) Time course study of plasma PTX3, Error bars represent mean ± SEM, n=4 per group, *P< 0.01 versus sham at the same time point. Plasma PTX3 at 4hr reperfusion was highlighted in red rectangle, and shown in the box.
The increase in plasma PTX3 at 4 hr reperfusion may be particularly important. See points enclosed in box in Figure 2B. This increase occurs in the IRI group well before the sham group, and well before the increase in serum creatinine (Scr) in our AKI model. This suggests that PTX3 may be a biomarker for the early detection of AKI. The search for such biomarkers, which appear before an increase in Scr, is a major ongoing effort in nephrology (12).
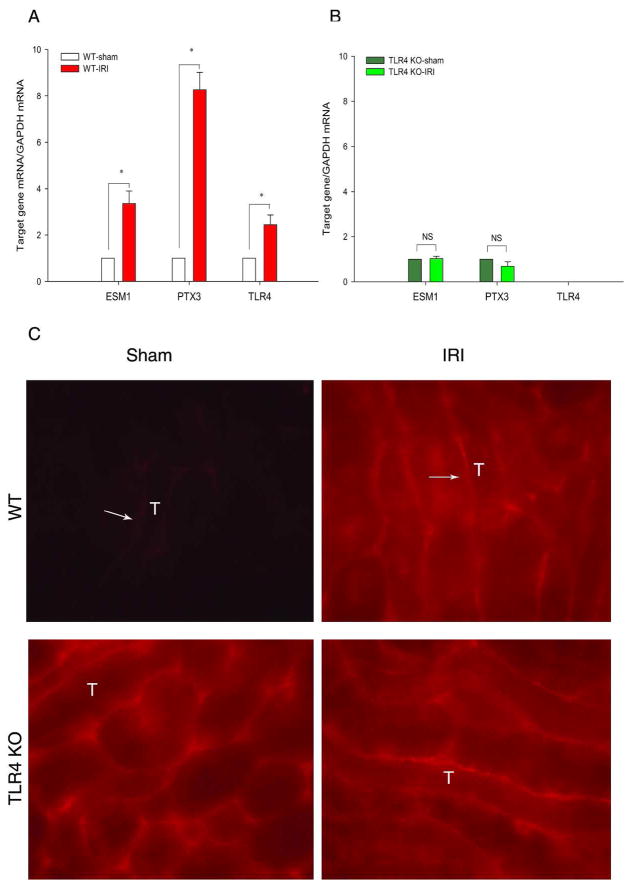
We previously showed the importance of endothelial TLR4 in the pathogenesis of ischemic AKI. DNA microarray data suggested that PTX3 increased on WT ischemic kidneys. Further qRT-PCR confirmed that IRI increased renal PTX3, and ESM1 (endothelial cell-specific molecule 1, or endocan) mRNA by 8.27±0.75-fold, and 3.35 ±0.55-fold, respectively, in WT kidneys. However, TLR4 KO prevented such increases (Figure 3A and B).
Figure 3.
TLR4 is required for increased PTX3 in AKI. Renal pedicles of WT B10 and TLR4 KO mice were clamped for 23min and kidneys harvested at 4hr reperfusion. The genes of interest (PTX3, Esm1, and TLR4) were determined by qRT-PCR and analyzed by the comparative Ct method. The calibrator gene is gene of interest taken from the sham kidney. (A) WT kidneys. (B) TLR4 KO kidneys. Error bars show mean±SEM, n= 6 in each group, *P < 0.01 IRI compared to sham; NS: not significant. (C) Immunohistology shows increased PTX3 in WT kidneys. at 4hr reperfusion. A rat anti-mouse PTX3 monoclonal antibody was used to stain frozen sections from PFA fixed tissues. Exactly the same staining conditions and exposures were used to compare PTX3 expression in sham and AKI kidneys. PTX3 is located on peritubular capillaries of the OM. PTX3 was increased on WT ischemic kidneys. No increased endothelial PTX3 was found on ischemic TLR4 KO kidneys.(X40). Arrows indicate some of many capillaries positive for PTX3; “T” indicates a few of many tubules.
We also compared PTX3 protein expression in sham versus ischemic WT kidneys by immunohistology. Consistent with the increase in PTX3 mRNA, Figure 3C shows increased peritubular PTX3 protein in the outer medulla (OM) at 4 hr reperfusion in WT kidneys. In the TLR4 KO mice, we did not see such increases. Supplemental Figure 1 shows that the anti-PTX3 antibody was specific because there was negligible staining of ischemic PTX3 knockout kidneys.
PTX3 is expressed predominantly on renal endothelia
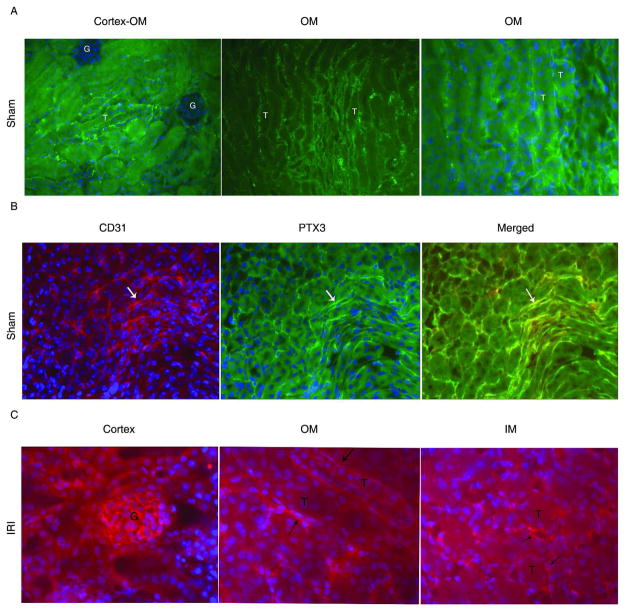
Since PTX3 was upregulated early during AKI, we performed immunostaining on frozen sections at 4hr reperfusion. In the sham kidney harvested 4hr reperfusion, we found PTX3 on peritubular structures of the OM (Figure 4A). In addition, there was scattered rare peritubular staining in the cortico-medullary junction, and we did not detect PTX3 on glomeruli (Figure 4A). No PTX3 was seen in the inner medulla (IM) (not shown). We localized PTX3 to peritubular endothelia by double staining with PTX3 and the endothelial marker CD31 (Figure 4B and Supplemental Figure 2). Previous studies in the kidney after IRI confirmed CD31 as an appropriate marker of endothelia (13, 14). At 4hr after IRI, PTX3 increased on the kidney. There was new expression of PTX3 on glomerular endothelia and on IM in addition to increased expression in the OM (Figure 4C).
Figure 4.
PTX3 co-localizes with CD31+ cells on kidney. Renal pedicles of WT mice were clamped for 23min. Kidney was in situ perfused with 4% PFA at 4hr reperfusion and then snap-frozen. (A) In sham kidney at 4hr post-op, PTX3 was detected on peritubular structure in the OM. Representative staining of PTX3 in the conjunction of cortex-OM area(x10), OM area(X20), and OM area(x40). (B) PTX3 co-localized with CD31 on the kidney section. A rabbit polyclonal anti-PTX3 was used in “Panels A and B”. In Panel B, a rat monoclonal antibody was also used to stain for CD31(x20), and species specific secondary antibodies allowed double staining for PTX3 and CD31. (C) PTX3 was detectable only by a rat monoclonal antibody after IRI. At 4hr reperfusion, PTX3 was expressed on glomeruli, peritubular capillaries in both OM and IM(x20). Arrows pointed to endothelia; “T” = tubules, “G” = glomeruli. Each stain was carried out on four individual mice per group and the images in the figures are representative of each group.
In addition to immunohistology, we developed techniques to isolate renal endothelia or leukocytes using beads coated with specific antibodies for endothelial cells or leukocytes. This technique, validated in our previous publication (5) confirmed that over 95% of PTX3 was on endothelia (Supplemental Figure 3A and Supplemental Text). Furthermore, our in vitro studies, discussed in the next section, also support the expression of PTX3 on endothelia.
Endothelial PTX3 is regulated by TLR4 and Reactive Oxygen Species (ROS)
We studied the regulation of PTX3 in the MS1 endothelial cell line, primary renal endothelial cultures (EC) from WT mice, and mice with conditional knockout of MyD88 on endothelia.
1) Regulation of PTX3 by TLR4 and ROS in MS1 endothelial cells in vitro
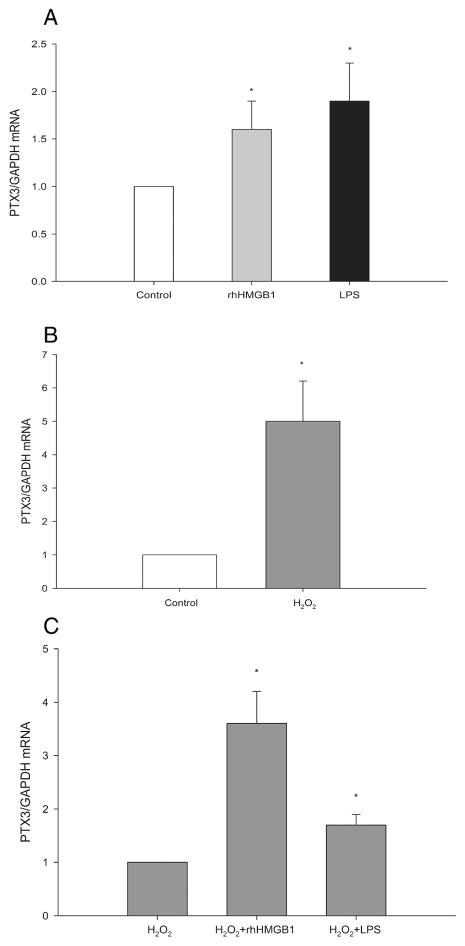
HMGB1 and ROS are potential signals for endothelial PTX3 production during ischemic AKI. Injured and dying renal cells release intracellular HMGB1 into the extracellular space where it has proinflammatory properties (15). We previously demonstrated that HMGB1 ligated endothelial TLR4 during ischemic AKI and induced expression of endothelial adhesion molecules necessary for maladaptive inflammation (5). In addition to HMGB1, ROS is produced during ischemic AKI and elicits maladaptive responses (16–18). Figure 5A shows that rhHMGB1 increases PTX3 expression by MS1 cells in vitro by 1.6±0.3 fold. As a positive control, we found that MS1 cells also responded by 1.9±0.4 fold to ultrapure lipopolysaccharide (LPS) that is the exogenous ligand for TLR4. To test the hypothesis that endothelial cells will produce more PTX3 under oxidative stress, we treated MS1 cells with H2O2 at 100μM for 30min and replenished cells with complete medium for 4hr in vitro. This is to mimic the in vivo IRI scenario. H2O2 induced PTX3 on MS1 by 5.0±1.2 fold (Figure 5B). Figure 5C shows that rhHMGB1 or LPS enhanced the PTX3 expression induced by H2O2.
Figure 5.
TLR4 ligands and ROS increase PTX3 expression in the MS1 cell line. MS1 cells were cultured in DMEM+ 10%FCS until reaching confluence. Cells were stimulated with (A)rhHMGB1 or LPS at 5μg/mL for 4hr; or (B) H2O2 at 100 μM in Earle’s Balanced Salt Solution for 30min and then replenished with complete medium for 4hr; Or (C) H2O2 at 100 μM for 30min and followed by rhHMGB1 or LPS(5μg/mL) in complete medium for 4hr. PTX3 mRNA was measured from harvested cells by qRT-PCR. Error bars shows mean ± SEM, n=5, *P< 0.05 compared to the control group.
2) PTX3 is regulated by TLR4 and ROS in primary renal endothelial cell cultures
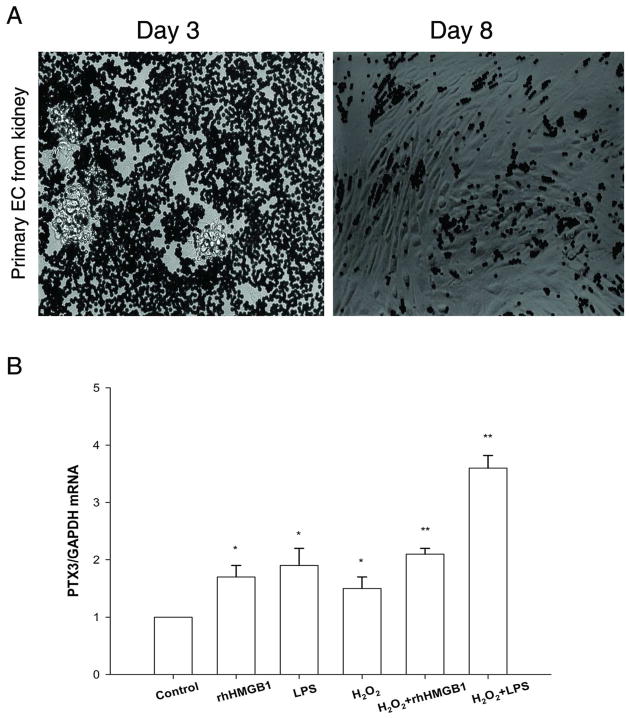
The MS1 endothelial cells above were originally derived from mouse pancreas (19, 20), and may not accurately reflect renal endothelial cells. We therefore developed techniques to isolate renal endothelial cells and study their PTX3 expression. We isolated CD31+ cells from renal digests and established a primary EC culture. When put into EC-culture medium, CD31+ cells started to grow into small cell clusters within 3 days. By day 8, cells would reach confluence and had the signature “cobblestone” appearance of endothelial cells (Figure 6A). We did not notice any growth difference between cells harvested from normal kidneys or ischemic kidneys. We used passage 1 for our experiment.
Figure 6.
Primary culture of renal EC. (A) Images of CD31+ Dynabead-isolated cells from kidney: Primary EC grown in vitro on Day 3, primary EC at confluence on Day 8. Cells showed typical cobblestone morphology. (B) PTX3 mRNA on primary EC was upregulated in vitro by H2O2(100 μM), and/or rhHMGB1(5μg/mL), and/or LPS(5μg/mL). Error bars show mean ± SEM, n=4, *P < 0.05, **P < 0.001 versus control.
Figure 6B showed that PTX3 was upregulated in the presence of H2O2 (1.5±0.2 fold), or rhHMGB1 (1.7±0.2 fold), or LPS (1.9±0.3 fold). Combined treatment of H2O2 and rhHMGB1 (2.1±0.1 fold), or H2O2 and LPS (3.6±0.2 fold), induced a much higher level of PTX3.
3) MyD88 conditional knockout on endothelium decreases PTX3 expression and other markers of endothelial activation
Our observations above suggest that PTX3 is regulated, in part, by TLR4. To better understand this regulation, we examined PTX3 expression with a conditional endothelial MyD88 knockout mouse. MyD88 is required for one of the two major pathways of TLR4 intracellular signaling, and contributes to the pathogenesis of ischemic AKI (21–23). To flox MyD88 on endothelial cells (“conditional MyD88 KO”), we used an established mouse with floxed MyD88 (24), and the Tie2Cre mouse (25, 26) that expresses cre recombinase uniquely on endothelia during ischemic AKI (13, 14). The MyD88 knockout mouse is normal except in its responses to infection and injury (27). Baseline renal and plasma PTX3 levels were similar in the “conditional MyD88 KO” and MyD88 f/f; Tie2Cre(−) (“wildtype”) mice. Using this cre-lox strategy, we were able to knockout MyD88 from renal endothelial cells by 50%. Despite this substantial residual endothelial MyD88, we found dramatic phenotypes in regard to renal and plasma PTX3 protein after IRI, and in endothelial activation markers.
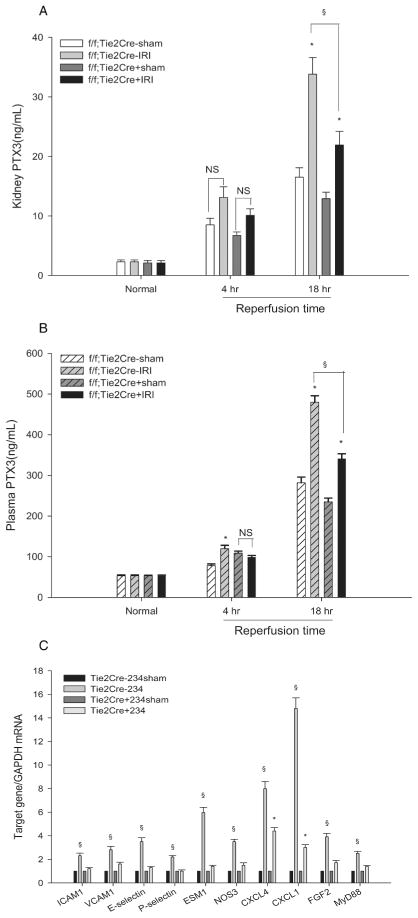
The main point of these studies was that at 18 hr reperfusion there was much less renal PTX3 protein in the “conditional MyD88 KO” kidneys (21.9±2.3ng/mL) than in “wildtype” kidneys (33.8±2.8ng/mL). See Figure 7A. This decreased PTX3 in ischemic “conditional MyD88 KO” kidneys was associated with decreased plasma PTX3 at 18 hr reperfusion (341.0±12.2ng/mL) compared to “wildtype” plasma (480.0±15.9ng/mL). See Figure 7B. The decreased plasma PTX3 confirms the decreased production in the “conditional KO” kidneys. These studies suggest that endothelial PTX3 is regulated by the endothelial MyD88-dependent pathway of TLR4 signaling during ischemic AKI.
Figure 7.
Conditional endothelial knockout of MyD88 decreases PTX3 production and endothelial activation. Renal pedicles of “Wildtype” [MyD88f/f; Tie2Cre(−)] and “conditional MyD88 KO” [MyD88f/f; Tie2Cre(+)] mice were clamped for 23min. (A) Kidney PTX3 was measured by ELISA at baseline, 4hr, and 18hr reperfusion. The right kidney was harvested as control. (B) Plasma PTX3 was measured by ELISA at the same time points. In both “A” and “B”, the error bars stand for mean±SEM, n=3 per group. The 4 groups at each time point were analyzed by one-way ANOVA, and then pairwise comparisons made by the Holm-Sidak method. *P < 0.05 IRI versus sham, §P < 0.05 IRI MyD88f/f; Tie2Cre(−) versus MyD88f/f, Tie2Cre(+) at 18hr reperfusion; NS: not significant. (C) Endothelial markers were detected by qRT-PCR on CD31+ cells isolated from kidneys at 4hr reperfusion. Data were analyzed by the comparative Ct method. The calibrator gene is gene of interest taken from the sham kidney. Error bars stand for mean±SEM, n=3 per group, §P < 0.05 MyD88f/f; Tie2Cre(−) IRI versus sham,*P < 0.05 MyD88f/f; Tie2Cre(+) IRI versus sham.
There was a much smaller increase in renal and plasma PTX3 after sham surgery in both “conditional MyD88 KO” and “wildtype” mice. Because this increase was the same in both types of mice, we believe that it was independent of endothelial Myd88 and due to cytokine released from the surgical trauma to skin and muscle that was common to both groups of mice and necessary to expose the renal pedicle. Cytokines such as TNF-α are known to regulate PTX3 production (6). These results are consistent with the idea that the uninjured kidney responds to cytokines produced by injured distant tissues (28).
Another major point of Figure 7 is the inhibitory effect of “conditional MyD88 KO” on endothelial adhesion molecules. In wildtype kidneys, such adhesion molecules are required for the inflammatory response to IRI that exacerbates injury (5). Figure 7C shows that after IRI endothelial cells isolated from “wildtype” endothelia increased their expression of ICAM1 (2.3±0.2 fold), VCAM1 (2.3± 0.8 fold), E-selectin (3.5± 0.3 fold), and P-selectin (2.2± 0.1 fold). In contrast, endothelia isolated from “conditional MyD88 KO” mice did not increase these adhesion molecules. Furthermore, “wildtype” but not “conditional MyD88 KO”, endothelia increased their expression of ESM1 (5.9± 0.4 fold) which is uniquely found on endothelia (29), NOS3 (3.5±0.2 fold), and FGF2 (3.9 ± 0.3 fold). Note that ESM1 expression after IRI was also decreased in TLR4 deficient kidneys (Figure 3). All three of these molecules are thought to ameliorate ischemic AKI (29–33). Finally, to our knowledge, these are the first studies to demonstrate increased expression of ESM1, NOS3, FGF2, CXCL4, and CXCL1 on renal endothelia isolated after IRI.
PTX3 KO differentially changes endothelial function at 4 hr versus 24 hr after renal IRI
The data below show that PTX3 KO profoundly alters the biology of renal endothelial cells during ischemic AKI. This may explain how PTX3 KO ameliorates this disease.
Using anti-CD31-conjugated Dynabeads, we isolated and compared renal endothelial from WT versus PTX KO mice at 4 and 24 hr after reperfusion. We found that PTX3 KO had opposite effects at these two time points.
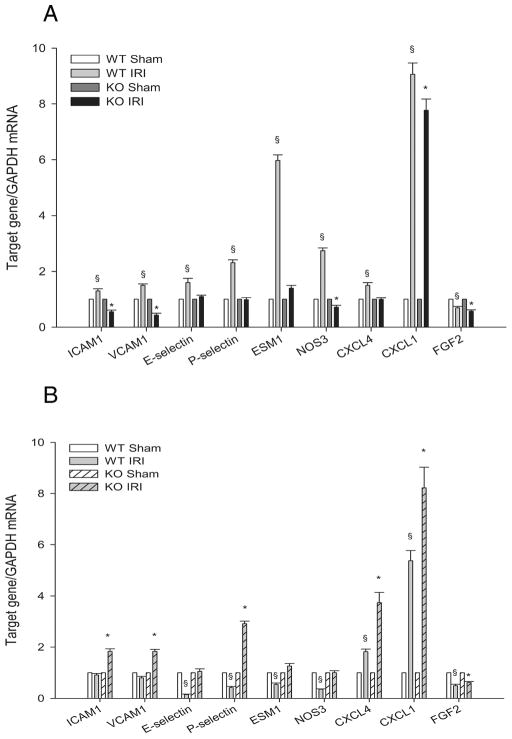
At 4hr reperfusion, inflammation exacerbates ischemic injury (34, 35). We previously showed that at this time WT endothelia increased their expression of adhesion molecules (ICAM1, VCAM1, E-selectin), and that TLR4 KO prevented maladaptive inflammation by preventing such adhesion molecule expression (5). Figure 8A shows that PTX3 KO also prevented expression of these adhesion molecules. We also examined other endothelial activation markers. Similar to the conditional MyD88 KO (Figure 7C) and unlike the WT endothelial cells, PTX3 KO did not increase expression of ESM1 or NOS3. This data suggest that PTX3 is downstream of TLR4, and that expression of these adhesion molecules is downstream of PTX3.
Figure 8.
CD31+ cells from PTX3 KO ischemic kidneys show delayed endothelial activation. Renal pedicles of WT and PTX3 KO mice were clamped for 23min. CD31 bound Dynabeads were used to isolate endothelial cells from kidney digest at 4hr and 24hr reperfusion. Endothelial markers were detected by qRT-PCR and data were analyzed by the comparative Ct method. The calibrator gene is gene of interest taken from the sham kidney. (A) 4hr reperfusion. (B) 24hr reperfusion. Error bars represent mean±SEM, n=3 per group, §P < 0.05 WT IRI versus WT sham, *P < 0.05 PTX3 KO IRI versus KO sham.
At 24 hr reperfusion, inflammation may have a different function. Instead of exacerbating injury, late inflammation may inhibit aggressive leukocyte activity, and reparative macrophages may facilitate repair (36–40). In contrast to the 4 hr reperfusion time point when PTX3 KO endothelial cells decreased adhesion molecule expression, Figure 8B shows the PTX3 KO increased expression of the adhesion molecules ICAM1, VCAM1, and P-selection. This suggests that PTX3 KO endothelia may facilitate immigration of regulatory and reparative leukocytes into the injured kidney.
PTX3 KO alters renal endothelial function in primary cultures
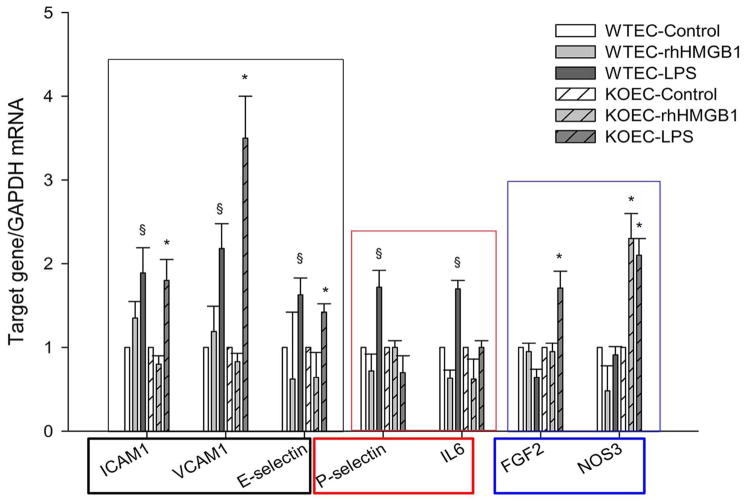
To further explore the effect of PTX3 KO on renal endothelium, we developed techniques to isolate and then culture primary endothelial cells from WT versus PTX3 KO kidneys. These primary endothelial cultures were then stimulated with the TLR4 ligands HMGB1 or LPS. As opposed to the endothelia directly isolated sham and ischemic kidneys in Figure 8 above, these primary endothelial cells have not been injured by IRI in vivo, and have been in the presence of growth factors (VEGF, FGF, IGF1, HGF, hydrocortisone, and heparin) which facilitate the survival of these cells in vitro and attempt to mimic the in vivo microenvironment. The main point learned from these primary cultures is that renal endothelia from PTX KO versus WT mice are fundamentally different.
Three different groups of endothelial responses were seen(Figure 9). The first group (ICAM-1, VCAM 1, E- selectin) of responses were the same in WT and PTX3 KO endothelial cells. These are proinflammatory adhesion molecules (34, 35). The second group (P-selectin, and IL6) were responses present in WT endothelia, but not PTX3 KO endothelia. These are also pro-inflammatory genes that have demonstrated maladaptive effects during ischemic AKI (41). The lesser expression of these genes by primary renal endothelia stimulated by TLR4 ligands in PTX3 KO mice may contribute to the lesser IRI seen in these mice. The third group (FGF2 and NOS3) were responses that were present in PTX3 KO but not WT mice. FGF2 ameliorates ischemic AKI (30, 31), and NOS3 should help preserve renal blood flow (32, 33).
Figure 9.
Fundamental differences in primary cultures of endothelial cells from WT and PTX3 KO kidneys. Primary renal EC was maintained in Clonetics EGM-2 endothelial cell growth medium-2. Cells were treated with rhHMGB1 or LPS at 5μg/mL for 4hr in culture. RNA was extracted and qRT-PCR performed. Error bars show mean mean±SEM, n=3, §P < 0.05 WT treated versus control, *P < 0.05 PTX3 KO treated versus control.
DISCUSSION
We found that knockout of PTX3 ameliorated IRI as assessed by both renal function and morphology. This is consistent with the detrimental effect of PTX3 on murine gastrointestinal IRI (42, 43).
This is, however, in contrast to the beneficial effect of PTX3 on murine cardiac IRI. Cardiac IRI exposes a non-muscle myosin heavy chain II. This is bound by a natural IgM autoantibody that activates complement the C1q-dependent classical pathway (44); soluble PTX3 binds soluble C1q and inhibits such classical pathway activation (45). In contrast, PTX3 has no effect on complement activation during renal IRI. Renal IRI does activate complement but not by natural autoantibodies and the classical pathway (46, 47). Instead tubular injury decreases Crry, the murine homologue of human MCP (membrane cofactor protein) and DAF (decay accelerating factor); Crry normally prevents amplification of the alternative complement pathway after “C3 tickover” (48). In the absence of tubular Crry, alternative complement activation continues unrestrained and renal tubular injury results (49, 50). As shown by others (49), we saw more C3 activation on injured and dead tubules in WT kidneys after IRI than in PTX3 KO kidneys (Supplemental Figure 4). The C3 was not associated with endothelia (the location of PTX3). There was decreased C3 deposition on PTX3 KO tubules; this was secondary to the decreased tubular injury, not a direct effect of PTX3 KO.
Although PTX3 is expressed by endothelia, leukocytes, and other tissues (6), we found PTX3 almost exclusively on endothelia in the kidney by immunohistology (Figure 4) and study of isolated endothelia and leukocytes (see Supplemental Figure 3A).
Although we cannot exclude the possibility that some PTX3 is deposited in the kidney from the blood during ischemic AKI, the following data show that most of the increased endothelial PTX3 is produced by endothelial cells: a) In Figure 3, the increased PTX3 mRNA in wildtype TLR4-sufficient ischemic kidneys is correlated with an increased PTX3 protein found on peritubular endothelia. This correlation is consistent with renal endothelial production of PTX3. b) In vitro stimulation of endothelia by HMGB1 and ROS, both present in the ischemic kidney, activates PTX3 gene expression (Figures 5 & 6). c) If PTX3 were extravasated from blood into the kidney, one would expect diffuse PTX3 staining because it binds to apoptotic cells (51), DNA released by dying cells (52) and extracellular complexes of tumor necrosis factor-stimulated gene 6/inter-alpha-trypsin inhibitor/hyaluronan (53, 54). The latter are found in the extracellular matrices of injured kidneys (55, 56). Instead, our immunostaining localized PTX3 protein to endothelia; this is consistent with its production by these cells. d) Finally, our idea that PTX3 is produced locally in the kidney is consistent with the literature. The literature indicates that PTX3 is produced locally at sites of inflammation and then escapes into blood rather than vice versa; this is unlike the other major member of the pentraxin family, CRP which is produced in the liver, enters the blood, and then extravasates into peripheral tissues (57).
We explored both upstream events which regulate renal endothelial PTX3, as well as downstream events by which PTX3 regulates renal IRI.
Upstream, TLR4 was one important positive regulator of PTX3 because PTX3 expression in vivo was increased after IRI in WT, but not in TLR4 deficient, kidneys. HMGB1, released by injured cells during renal IRI, is the major ligand for TLR4. Inhibition of HMGB1 with specific antibodies ameliorates ischemic AKI (58, 59). Although HMGB1 binds to a number of different receptors (15), TLR4 is the major maladaptive receptor during ischemic AKI. Thus, direct binding of HMGB1 to TLR4 has been demonstrated by biophysical techniques (60); knockout of RAGE, the other major receptor for HMGB1 has no effect on ischemic AKI (61); and, in contrast, knockout or inactivating natural mutations of TLR4 in mice ameliorate ischemic AKI (21, 23, 62, 63). In addition, mutations that decrease TLR4 signaling in humans decrease ischemic AKI after transplantation (64).
We found that recombinant HMGB1 increased PTX3 expression both in the MS1 endothelial cell line and WT renal endothelial primary cultures. Furthermore, we found that conditional MyD88 KO on endothelia decreased PTX3 protein from 33.8±2.8ng/mL to 21.9± 2.3ng/mL at 18 hr reperfusion. The residual PTX3 expression might be due to incomplete knockout of endothelial MyD88, or the MyD88-independent pathways of TLR4 signaling, or ROS released during IRI. Altogether our data suggest that PTX3 in increased on endothelia mainly by HMGB1 acting on TLR4 via the MyD88 signaling pathway.
We also made the important observation that partial conditional knockout of endothelial MyD88 markedly decreased expression of endothelial adhesion molecules after renal IRI; this confirms our previous observation that endothelial TLR4 is required for endothelial activation (5), and shows that this is due in large part to the MyD88 pathway.
In addition, we found that ROS produced during IRI (16–18) also contributes to the increased PTX3 expression. ROS synergize with the TLR4 ligands, HMGB1 or LPS, to increase PTX3 expression. To our knowledge, ROS has not previously been reported to regulate PTX3.
Downstream, we used two different experimental systems to study the effects of PTX3 on endothelial activation during ischemic AKI.
We isolated endothelia from ischemic WT and PTX KO kidneys. Knockout of PTX3 prevents the early expression of endothelial adhesion molecules and chemokines known to facilitate early maladaptive inflammation (65–69). Altogether this data suggests that increased PTX3 is one mediator of early maladaptive endothelial activation triggered by TLR4 that we previously reported (5). In contrast to the decreased endothelial adhesion molecule expression at 4 hr caused by PTX3 KO, we found increased expression of these adhesion molecules compared to WT at 24 hr after IRI. This should facilitate the late influx of leukocytes that participate in tissue repair and turn off the inflammatory response (36–40). Thus, our results show that PTX3 KO had different effects at 4 hr versus 24 hr reperfusion.
In addition, we also studied primary cultures of renal endothelia from unmanipulated WT and PTX KO mice. After stimulation by TLR4 ligands, the responses of the WT and PTX3 KO primary endothelia could be divided into three groups. Some activation functions were the same. Expression of other proinflammatory adhesion molecules required PTX3. Expression of some endothelial functions, such as FGF2 and NOS3, which should protect or repair the ischemic kidney, was increased in the PTX3 KO endothelia.
Each of our two experimental systems has advantages and disadvantages. The endothelial cells isolated from ischemic kidneys receive the entire spectrum of signals from the ischemic environment, but subpopulations may be selected and functions may be altered by the enzymatic and mechanical disruption during isolation. The endothelia grown in primary culture avoid the above problems but the artificial extracellular matrix and growth factors (VEGF, FGF, IGF1, HGF, hydrocortisone, and heparin) may not fully replicate the microenvironment in vivo.
The main point is both systems show that PTX3 knockout inhibits maladaptive endothelial responses to TLR4 ligands. The “minor” differences between the two systems may result from the following: In vivo, no intracellular signaling pathway, including that for TLR4, operates in isolation, and any change in cell functions integrates the “crosstalk” between multiple simultaneous signaling pathways (70–72). In other words, renal endothelial cells in vivo receive signals from a variety of growth factors and cytokines during AKI in addition to TLR4 ligands. Given this complexity, the profound anti-inflammatory effects of knockout of single pathway (PTX3) is remarkable both in vivo (Figure 8) and after stimulation in vitro (Figure 9). The differences in a few details are likely the result of “crosstalk” between TLR4 and other signaling pathways which are different after stimulation in vivo and in vitro, and will be the focus of future studies. These differences include the increase in adaptive FGF2 and NOS3 seen after in vitro stimulation (Figure 9), but not in endothelia isolated from ischemic kidneys (Figure 8).
In addition, although HMGB1 is a major and the best studied TLR4 ligand released during ischemic AKI (21, 23, 58–60, 62, 63), other TLR4 ligands such as stress fibronectin (73) are expected to be produced and may activate endothelial TLR4 during ischemic AKI in vivo. Each TLR4 ligand may elicit a slightly different response (74, 75), and contribute to the “crosstalk” discussed above. This is illustrated by the different responses to HMGB1 and endotoxin in Figure 9.
We suggest that PTX3 may exacerbate the above endothelial stress responses during renal IRI by binding and inhibiting growth factors which would otherwise ameliorate endothelial injury and promote repair. The N-terminus of PTX3 specifically binds and inhibits FGF8 (76) and FGF2 (77, 78). Such inhibition would have detrimental effects on renal endothelia because FGF2 ameliorates rodent ischemic AKI(30, 31).
Another possibility is that PTX3 increases the exposure of renal endothelial to proinflammatory cytokines by inhibiting “efferocytosis”, which is defined as the phagocytosis of apoptotic cells and which does not result in the release of proinflammatory cytokines. Apoptosis is a major type of cell death during ischemic AKI [see review (79)]. PTX3 inhibits efferocytosis (80–83); such inhibition of apoptotic renal cells would allow these apoptotic cells to degenerate (die a post-apoptotic death) and release proinflammatory damage associated molecular pattern molecules (DAMPS) (84–86). Consistent with this formulation is the increased necrosis and inflammation we saw in the WT compared to the PTX3 KO ischemic kidneys. The idea that efferocytosis inhibits the maladaptive inflammatory response to renal IRI is supported by the beneficial effects of increasing efferocytosis by injections of MFG-E8 (milk fat globule-EGF factor 8/lactadherin) (87, 88); MFG-E8 is a “bridging molecule” that links the apoptotic cells to phagocytes, and thus increases efferocytosis (89).
In summary, PTX3 may have fundamental importance for biology and disease because it has been so conserved during evolution (6). We have now demonstrated important links between PTX3 and renal endothelium, TLR4, MyD88, and ROS in the pathogenesis of ischemic IRI. We showed that PTX3 knockout ameliorates ischemic AKI. PTX3 expression is increased via ROS, and a MyD88-dependent TLR4-mediated mechanism on renal endothelia. PTX3 is required for the early expression of endothelial adhesion molecules and chemokines that facilitate the maladaptive inflammatory response to IRI. PTX3 inhibits the late endothelial expression of adhesion molecules that may facilitate immigration of regulatory and reparative leukocytes.
MATERIALS AND METHODS
Mice
6–8 weeks old, male C57BL/10, TLR4 null mice C57BL/10ScNJ, MyD88 floxed mice B6.129P2(SJL)-Myd88tm1Defr/J, and Tie2 Cre “driver” B6.Cg-Tg(Tek-cre)1Ywa/J mice were from The Jackson Laboratory (Bar harbor, ME). To avoid non cell-specific deletion of floxed alles arising from germ line Cre-recombinase activity(90), female MyD88f/f mice were interbred with male MyD88f/f; Tie2Cre(+) mice to generate conditional endothelial cell-specific knockout MyD88 mice(MyD88f/f; Tie2Cre+) and littermates control mice(MyD88f/f; Tie2Cre−). PTX3 KO founders were obtained from Dr. Martin M. Matzuk (Baylor College of Medicine, Houston, Texas). Due to the subfertility of female PTX3 KO mice (91), we bred heterozygous males and heterozygous females. Littermates of PTX3+/+ were used as controls. Genomic DNA from offspring was extracted using Extract-N-Amp Tissue PCR kit(Sigma, Saint Louis, MO). Primers for genotyping were described in detail in Supplemental Table 1.
Renal ischemia reperfusion injury
After right nephrectomy, the left renal pedicle was occluded for either 16 min or 23 min; sham-operated mice were used as controls(5). All mouse work was approved by the UT Southwestern Institutional Animal Care and Use Committee (IACUC).
DNA microarray
Detailed methods can be found in the Supplement.
Renal function
Serum creatinine was measured using P/ACE MDQ Capillary Electrophoresis System with a PDA Detector (Beckman Coulter, Indianapolis, IN) (9). BUN was measured using the VITROS BUN slides on VITROS 250 Chemistry Analyzer(Ortho-clinical Diagnostics, Raritan, NJ).
Histology and Immunohistology
For histology, kidneys were harvested at 24 hr reperfusion and fixed in 10% neutral buffered formalin(Sigma). Paraffin-embedded sections were stained with hematoxylin and eosin(H&E), and anti-myeloperoxidase (rabbit anti-MPO polyclonal antibody, Thermo Scientific, Rockford, IL). Tissue damage and inflammation was evaluated as previously reported(53). For immunofluorescence staining, mice were in situ perfused with cold PBS followed by cold 4% PFA. Detailed sample processing can be found as previously reported(5). Sections were blocked with 10% goat serum and stained with Rat anti-mouse Pentraxin 3(clone 265629, R& D Systems, Minneapolis, MN) or rat anti-mouse Complement component 3 (clone RmC11H9, Cedarlane Laboratories, Burlington, NC). Rat IgG2b or IgG2a was used as isotype control. For double-staining of CD31 and PTX3, rat anti-mouse CD31 mAb (clone MEC13.3, BD Pharmingen, San Diego, CA) was applied to the section followed by Texas Red-conjugated goat anti-rat IgG(Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-human PTX3(which cross reacts with mouse and rat, Santa Cruz), and then fluorescein goat anti-rabbit IgG(Santa Cruz). Sections were mounted with VECTASHIELD Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA), and visualized using a Carl Zeiss Axioplan2 Imaging microscope (Carl Zeiss MicroImaging, Thornwood, NY). Each stain was carried out on four individual mice per group and the images in the figures are representative of each group.
Isolation of endothelial cells and leukocytes
Detailed procedure can be found in our previous reports(5, 53).
Primary renal endothelial cell culture
Bead-bound CD31+ cells isolated from kidneys were resuspended in Clonetics EGM-2 endothelial cell growth medium-2 BulletKit (Lonza, Allendale, NJ) and plated into BD BioCoat Fibronectin 24-well Multiwell plate(BD Pharmingen). Medium was changed every 3 days. Primary cells passage 1 were used for experiment.
Hydrogen peroxide(H2O2), lipopolysaccharide(LPS), recombinant human High-mobility group protein B1(rhHMGB1) treatment
Confluent MS1 monolayers or primary renal endothelial cells, were washed with Dulbecco’s Phosphate Buffered Saline three times. Cells were exposed to H2O2 (Sigma) at 100μM for 30 min in Earle’s Balanced Salt Solution at 37°C, then replenished with complete medium containing 10% FCS. Some cells were treated with rhHMGB1(R&D Systems) at 5 ug/mL or with an ultrapure form of LPS that activates only TLR4 (Escherichia coli 0111:B4, InvivoGen, San Diego, CA) for an additional 4 hr followed by RNA extraction using RNeasy Mini kit(Qiagen).
Real-Time RT-PCR
Detailed procedure can be found in our previous reports(5, 53). PCR primers are in Supplemental Table 2.
Enzyme-linked immunosorbent assay (ELISA)
Blood and kidney samples were collected at 4 hr, 18 hr, 24 hr, day 2, day 3, day 5, and day 7 reperfusion. Plasma was obtainted by centrifuging blood samples at 2000 × g for 15 min at 4°C. Kidneys were homogenized in RIPA buffer supplemented with Protease inhibitor cocktail(Sigma) on ice. Samples were centrifuged at 12000 rpm for 15 min at 4°C. The protein concentration was measured by Coomassie Plus(Bradford) Assay Reagent(Thermo Scientific) in triplicate. All samples were stored at −80°C until analysis. PTX3 protein of each sample was assayed in duplicate using Mouse Pentraxin 3 Quantikine ELISA Kits(R&D Systems) according to the manufacturer’s instructions. Absorbance was read on Pelkin Elmer 1420 Multilabel Counter Victor 3 plate reader at 450 nm, with correction wavelength set at 540 nm.
Western blot analysis
Protein extracts were separated by 10% precast SDS/PPAGE, transferred to a PDVF membrane (Millipore), probed with goat anti-mouse C3 antibody (ICL, Portland, OR) or rat anti-mouse C3(RMC11H9, Cedarlane Labs), exposed to an HRP conjugated secondary antibody (Sigma) and visualized using Pierce ECL Western Blotting Substrate(Thermo Scientific) according to the supplier’s instructions. The beta Actin Antibody[HRP](GenScript, Piscataway, NJ) was used as loading control.
Statistics
The data were presented as mean±SEM. One-way analysis of variance (ANOVA) Holm-Sidak method and 2-tailed Student’s t test were carried out using Sigma Plot 11.0. Differences with a P value of <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by NIH RO-1 DK069633 to CYL, a grant from the Beecherl Foundation to CYL, and by NIH DK079328 UT Soutwestern O’Brien Kidney Research Core Center. The pentraxin 3 knockout mice were created with the support of NIH grant HD033438 (to MMM) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors appreciate the excellent technical assistance of Ms. Jessica Lucas for measurement of serum creatinine by capillary electrophoresis.
Footnotes
See NCBI Gene Expression Omnibus(Edgar), GEO Series accession number GSE34351 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE34351).
DISCLOSURE
All the authors declared no competing interests.
References
- 1.Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. 2007 Jul;72(2):208–12. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2011 Nov 23; doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010 Mar 3;298:F1078. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol. 2011 Mar;22(3):416–25. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, John R, Richardson JA, Shelton JM, Zhou XJ, Wang Y, et al. Toll-like receptor 4 regulates early endothelial activation during ischemic acute kidney injury. Kidney Int. 2011 Oct 6;79:288–99. doi: 10.1038/ki.2010.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inforzato A, Jaillon S, Moalli F, Barbati E, Bonavita E, Bottazzi B, et al. The long pentraxin PTX3 at the crossroads between innate immunity and tissue remodelling. Tissue Antigens. 2011 Apr;77(4):271–82. doi: 10.1111/j.1399-0039.2011.01645.x. [DOI] [PubMed] [Google Scholar]
- 7.Kume N, Mitsuoka H, Hayashida K, Tanaka M. Pentraxin 3 as a biomarker for acute coronary syndrome: Comparison with biomarkers for cardiac damage. J Cardiol. 2011 May 25; doi: 10.1016/j.jjcc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Ryu WS, Kim CK, Kim BJ, Kim C, Lee SH, Yoon BW. Pentraxin 3: A novel and independent prognostic marker in ischemic stroke. Atherosclerosis. 2012 Feb;220(2):581–6. doi: 10.1016/j.atherosclerosis.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 9.Zinellu A, Caria MA, Tavera C, Sotgia S, Chessa R, Deiana L, et al. Plasma creatinine and creatine quantification by capillary electrophoresis diode array detector. Anal Biochem. 2005 Jul 15;342(2):186–93. doi: 10.1016/j.ab.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 10.Yuen PS, Dunn SR, Miyaji T, Yasuda H, Sharma K, Star RA. A simplified method for HPLC determination of creatinine in mouse serum. AJP - Renal Physiology. 2004;286(6):F1116–F9. doi: 10.1152/ajprenal.00366.2003. [DOI] [PubMed] [Google Scholar]
- 11.Dunn SR, Qi Z, Bottinger EP, Breyer MD, Sharma K. Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int. 2004;65(5):1959–67. doi: 10.1111/j.1523-1755.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 12.Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect Gold Standards for Kidney Injury Biomarker Evaluation. J Am Soc Nephrol. 2012 Oct 21;23:13–21. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horbelt M, Lee SY, Mang HE, Knipe NL, Sado Y, Kribben A, et al. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol. 2007 Sep;293(3):F688–95. doi: 10.1152/ajprenal.00452.2006. [DOI] [PubMed] [Google Scholar]
- 14.Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol. 2003 Aug;285(2):F191–8. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- 15.Andersson U, Tracey KJ. HMGB1 Is a Therapeutic Target for Sterile Inflammation and Infection. Annu Rev Immunol. 2011 Apr;5:29. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, et al. Mitochodria-Targeted Peptide Accelerates ATP Recovery and Reduces Ischemic Kidney Injury. J Am Soc Nephrol. 2011 May;5:22. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunyn-Schmiedebergs Arch Pharmacol [Review] 2007 Oct;376(1–2):1–43. doi: 10.1007/s00210-007-0183-5. [DOI] [PubMed] [Google Scholar]
- 18.Hall AM. Pores for Thought: New Strategies to Re-energize Stressed Mitochondria in Acute Kidney Injury. J Am Soc Nephrol. 2011 May 12;22:986–9. doi: 10.1681/ASN.2011030309. [DOI] [PubMed] [Google Scholar]
- 19.Arbiser JL, Moses MA, Fernandez CA, Ghiso N, Cao Y, Klauber N, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc NatlAcadSci USA. 1997;94(3):861–6. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arbiser JL, Larsson H, Claesson-Welsh L, Bai X, LaMontagne K, Weiss SW, et al. Overexpression of VEGF 121 in immortalized endothelial cells causes conversion to slowly growing angiosarcoma and high level expression of the VEGF receptors VEGFR-1 and VEGFR-2 in vivo. American Journal of Pathology. 2000;156(4):1469–76. doi: 10.1016/S0002-9440(10)65015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007 Oct;117(10):2847–59. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, et al. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol. 2007 May 15;178(10):6252–8. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- 23.Pulskens WP, Teske GJ, Butter LM, Roelofs JJ, van der Poll T, Florquin S, et al. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS ONE. 2008;3(10):e3596. doi: 10.1371/journal.pone.0003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008 Aug 15;29(2):272–82. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, et al. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):3058–63. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001 Feb 15;230(2):230–42. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 27.O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev. 2008 Dec;226:10–8. doi: 10.1111/j.1600-065X.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 28.Hauser P, Kainz A, Perco P, Bergmeister H, Mitterbauer C, Schwarz C, et al. Transcriptional response in the unaffected kidney after contralateral hydronephrosis or nephrectomy. Kidney Int. 2005 Dec;68(6):2497–507. doi: 10.1111/j.1523-1755.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 29.Sarrazin S, Adam E, Lyon M, Depontieu F, Motte V, Landolfi C, et al. Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim Biophys Acta. 2006 Jan;1765(1):25–37. doi: 10.1016/j.bbcan.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Villanueva S, Cespedes C, Gonzalez A, Vio CP. bFGF induces an earlier expression of nephrogenic proteins after ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2006 Dec;291(6):R1677–87. doi: 10.1152/ajpregu.00023.2006. [DOI] [PubMed] [Google Scholar]
- 31.Villanueva S, Cespedes C, Gonzalez AA, Roessler E, Vio CP. Inhibition of bFGF-receptor type 2 increases kidney damage and suppresses nephrogenic protein expression after ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2008 Mar;294(3):R819–28. doi: 10.1152/ajpregu.00273.2007. [DOI] [PubMed] [Google Scholar]
- 32.Goligorsky MS, Brodsky SV, Noiri E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002;61(3):855–61. doi: 10.1046/j.1523-1755.2002.00233.x. [DOI] [PubMed] [Google Scholar]
- 33.Legrand M, Mik EG, Johannes T, Payen D, Ince C. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol Med. 2008 Jul-Aug;14(7–8):502–16. doi: 10.2119/2008-00006.Legrand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66(2):480–5. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 35.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol. 2009 Oct 13;130:41–50. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010 Mar 2;107(9):4194–9. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011 Feb;22(2):317–26. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Soloski MJ, et al. Mycophenolate mofetil modifies kidney tubular injury and Foxp3+ regulatory T cell trafficking during recovery from experimental ischemia-reperfusion. Transpl Immunol. 2010 May;23(1–2):45–52. doi: 10.1016/j.trim.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. 2010 Feb 17; doi: 10.1038/ki.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai LW, Yong KC, Lien YH. Pharmacologic recruitment of regulatory T cells as a therapy for ischemic acute kidney injury. Kidney Int. 2011 Dec 21; doi: 10.1038/ki.2011.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L, et al. Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol. 2005 Nov;16(11):3315–25. doi: 10.1681/ASN.2003090757. [DOI] [PubMed] [Google Scholar]
- 42.Souza DG, Soares AC, Pinho V, Torloni H, Reis LF, Teixeira MM, et al. Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am J Pathol. 2002 May;160(bett5):1755–65. doi: 10.1016/s0002-9440(10)61122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Souza DG, Amaral FA, Fagundes CT, Coelho FM, Arantes RM, Sousa LP, et al. The Long Pentraxin PTX3 Is Crucial for Tissue Inflammation after Intestinal Ischemia and Reperfusion in Mice. Am J Pathol. 2009 Mar 12;174:1309–18. doi: 10.2353/ajpath.2009.080240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haas MS, Alicot EM, Schuerpf F, Chiu I, Li J, Moore FD, et al. Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infarction. Cardiovasc Res. 2010 Sep 1;87(4):618–27. doi: 10.1093/cvr/cvq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salio M, Chimenti S, De Angelis N, Molla F, Maina V, Nebuloni M, et al. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008 Feb 26;117(8):1055–64. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 46.Renner B, Strassheim D, Amura CR, Kulik L, Ljubanovic D, Glogowska MJ, et al. B cell subsets contribute to renal injury and renal protection after ischemia/reperfusion. J Immunol. 2010 Oct 1;185(7):4393–400. doi: 10.4049/jimmunol.0903239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, et al. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105(10):1363–71. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010 Sep;11(9):785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thurman JM, Renner B. Dynamic control of the complement system by modulated expression of regulatory proteins. Lab Invest. 2011 Jan;91(1):4–11. doi: 10.1038/labinvest.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bao L, Wang Y, Chang A, Minto AW, Zhou J, Kang H, et al. Unrestricted C3 activation occurs in Crry-deficient kidneys and rapidly leads to chronic renal failure. J Am Soc Nephrol. 2007 Mar;18(3):811–22. doi: 10.1681/ASN.2006101176. [DOI] [PubMed] [Google Scholar]
- 51.Deban L, Jarva H, Lehtinen MJ, Bottazzi B, Bastone A, Doni A, et al. Binding of the long pentraxin PTX3 to factor H: interacting domains and Function in the regulation of complement activation. J Immunol. 2008 Dec 15;181(12):8433–40. doi: 10.4049/jimmunol.181.12.8433. [DOI] [PubMed] [Google Scholar]
- 52.Jaillon S, Peri G, Delneste Y, Fremaux I, Doni A, Moalli F, et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med. 2007 Apr 16;204(4):793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004 Apr;131(7):1577–86. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- 54.Scarchilli L, Camaioni A, Bottazzi B, Negri V, Doni A, Deban L, et al. PTX3 interacts with inter-alpha-trypsin inhibitor: implications for hyaluronan organization and cumulus oophorus expansion. J Biol Chem. 2007 Oct 12;282(41):30161–70. doi: 10.1074/jbc.M703738200. [DOI] [PubMed] [Google Scholar]
- 55.Janssen U, Thomas G, Glant T, Phillips A. Expression of inter-alpha-trypsin inhibitor and tumor necrosis factor-stimulated gene 6 in renal proximal tubular epithelial cells. Kidney Int. 2001 Jul;60(1):126–36. doi: 10.1046/j.1523-1755.2001.00779.x. [DOI] [PubMed] [Google Scholar]
- 56.Bommaya G, Meran S, Krupa A, Phillips AO, Steadman R. Tumour necrosis factor-stimulated gene (TSG)-6 controls epithelial-mesenchymal transition of proximal tubular epithelial cells. Int J Biochem Cell Biol. 2011 Dec;43(12):1739–46. doi: 10.1016/j.biocel.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Bottazzi B, Garlanda C, Cotena A, Moalli F, Jaillon S, Deban L, et al. The long pentraxin PTX3 as a prototypic humoral pattern recognition receptor: interplay with cellular innate immunity. Immunol Rev. 2009 Jan;227(1):9–18. doi: 10.1111/j.1600-065X.2008.00719.x. [DOI] [PubMed] [Google Scholar]
- 58.Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, et al. HMGB1 Contributes to Kidney Ischemia Reperfusion Injury. J Am Soc Nephrol. 2010 Sep 16;21:1878–90. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Gong Q, Zhong S, Wang L, Guo H, Xiang Y, et al. Neutralization of the extracellular HMGB1 released by ischaemic damaged renal cells protects against renal ischaemia-reperfusion injury. Nephrol Dial Transplant. 2010 Aug 2;26:469–78. doi: 10.1093/ndt/gfq466. [DOI] [PubMed] [Google Scholar]
- 60.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11942–7. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dessing MC, Pulskens WP, Teske GJ, Butter LM, van der Poll T, Yang H, et al. RAGE Does Not Contribute to Renal Injury and Damage upon Ischemia/Reperfusion-Induced Injury. J Innate Immun. 2011 Nov 4; doi: 10.1159/000334251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rusai K, Sollinger D, Baumann M, Wagner B, Strobl M, Schmaderer C, et al. Toll-like receptors 2 and 4 in renal ischemia/reperfusion injury. Pediatr Nephrol. 2010 May;25(5):853–60. doi: 10.1007/s00467-009-1422-4. [DOI] [PubMed] [Google Scholar]
- 63.Chen J, Hartono J, John R, Bennett M, Zhou X, Wang Y, et al. Interleukin 6 production by leukocytes during ischemic acute kidney injury is regulated by TLR4. Kidney Int. 2011;80(5):504–15. doi: 10.1038/ki.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kruger B, Krick S, Dhillon N, Lerner SM, Ames S, Bromberg JS, et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009 Mar 3;106(9):3390–5. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-ramos JC, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97(4):1056–63. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelly KJ, Williams WW, Jr, Colvin RB, Bonventre JV. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc Natl Acad Sci U S A. 1995;32676;91:812–6. doi: 10.1073/pnas.91.2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rabb H, Mendiola CC, Dietz J, Saba SR, Issekutz TB, Abanilla F, et al. Role of CD11a and CD11b in ischemic acute renal failure in rats. American Journal of Physiology. 1994;32676;267(6 2):F1052–F8. doi: 10.1152/ajprenal.1994.267.6.F1052. [DOI] [PubMed] [Google Scholar]
- 68.Kiew LV, Munavvar AS, Law CH, Azizan AN, Nazarina AR, Sidik K, et al. Effect of antisense oligodeoxynucleotides for ICAM-1 on renal ischaemia-reperfusion injury in the anaesthetised rat. J Physiol. 2004 Jun 15;557(Pt 3):981–9. doi: 10.1113/jphysiol.2004.061788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haller H, Dragun D, Miethke A, Park JK, Weis A, Lippoldt A, et al. Antisense oligonucleotides for ICAM 1 attenuate reperfusion injury and renal failure in the rat. Kidney Int. 1996;32676;50(2):473–80. doi: 10.1038/ki.1996.338. [DOI] [PubMed] [Google Scholar]
- 70.O’Neill LA. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008 Jul;29(1):12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Bezbradica JS, Medzhitov R. Integration of cytokine and heterologous receptor signaling pathways. Nat Immunol. 2009 Apr;10(4):333–9. doi: 10.1038/ni.1713. [DOI] [PubMed] [Google Scholar]
- 72.Ostuni R, Zanoni I, Granucci F. Deciphering the complexity of Toll-like receptor signaling. Cell Mol Life Sci. 2010 Jul 31; doi: 10.1007/s00018-010-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.You R, Zheng M, McKeown-Longo PJ. The first type III repeat in fibronectin activates an inflammatory pathway in dermal fibroblasts. J Biol Chem. 2010 Nov 19;285(47):36255–9. doi: 10.1074/jbc.C110.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mittal D, Saccheri F, Venereau E, Pusterla T, Bianchi ME, Rescigno M. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J. 2010 Jun 4; doi: 10.1038/emboj.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piccinini AM, Midwood KS. DAMPening Inflammation by Modulating TLR Signalling. Mediators Inflamm. 2010 doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leali D, Alessi P, Coltrini D, Ronca R, Corsini M, Nardo G, et al. Long pentraxin-3 inhibits FGF8b-dependent angiogenesis and growth of steroid hormone-regulated tumors. Mol Cancer Ther. 2011 Sep;10(9):1600–10. doi: 10.1158/1535-7163.MCT-11-0286. [DOI] [PubMed] [Google Scholar]
- 77.Leali D, Bianchi R, Bugatti A, Nicoli S, Mitola S, Ragona L, et al. Fibroblast growth factor 2-antagonist activity of a long-pentraxin 3-derived anti-angiogenic pentapeptide. J Cell Mol Med. 2010 Aug;14(8):2109–21. doi: 10.1111/j.1582-4934.2009.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rusnati M, Camozzi M, Moroni E, Bottazzi B, Peri G, Indraccolo S, et al. Selective recognition of fibroblast growth factor-2 by the long pentraxin PTX3 inhibits angiogenesis. Blood. 2004 Jul 1;104(1):92–9. doi: 10.1182/blood-2003-10-3433. [DOI] [PubMed] [Google Scholar]
- 79.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011 May 11; doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rovere P, Peri G, Fazzini F, Bottazzi B, Doni A, Bondanza A, et al. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood. 2000 Dec 15;96(13):4300–6. [PubMed] [Google Scholar]
- 81.Baruah P, Dumitriu IE, Peri G, Russo V, Mantovani A, Manfredi AA, et al. The tissue pentraxin PTX3 limits C1q-mediated complement activation and phagocytosis of apoptotic cells by dendritic cells. J Leukoc Biol. 2006 Jul;80(1):87–95. doi: 10.1189/jlb.0805445. [DOI] [PubMed] [Google Scholar]
- 82.van Rossum AP, Fazzini F, Limburg PC, Manfredi AA, Rovere-Querini P, Mantovani A, et al. The prototypic tissue pentraxin PTX3, in contrast to the short pentraxin serum amyloid P, inhibits phagocytosis of late apoptotic neutrophils by macrophages. Arthritis Rheum. 2004 Aug;50(8):2667–74. doi: 10.1002/art.20370. [DOI] [PubMed] [Google Scholar]
- 83.van Rossum AP, Pas HH, Fazzini F, Huitema MG, Limburg PC, Jonkman MF, et al. Abundance of the long pentraxin PTX3 at sites of leukocytoclastic lesions in patients with small-vessel vasculitis. Arthritis Rheum. 2006 Mar;54(3):986–91. doi: 10.1002/art.21669. [DOI] [PubMed] [Google Scholar]
- 84.Poon IK, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ. 2010 Mar;17(3):381–97. doi: 10.1038/cdd.2009.195. [DOI] [PubMed] [Google Scholar]
- 85.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010 Jan;10(1):36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peter C, Wesselborg S, Herrmann M, Lauber K. Dangerous attraction: phagocyte recruitment and danger signals of apoptotic and necrotic cells. Apoptosis. 2010 Sep;15(9):1007–28. doi: 10.1007/s10495-010-0472-1. [DOI] [PubMed] [Google Scholar]
- 87.Matsuda A, Wu R, Jacob A, Komura H, Zhou M, Wang Z, et al. Protective effect of milk fat globule-epidermal growth factor-factor VIII after renal ischemia-reperfusion injury in mice. Crit Care Med. 2011 Sep;39(9):2039–47. doi: 10.1097/CCM.0b013e3182227a3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harrois A, Duranteau J. Acute kidney injury: clear the kidney of apoptotic debris! Crit Care Med. 2011 Sep;39(9):2180–1. doi: 10.1097/CCM.0b013e318226619c. [DOI] [PubMed] [Google Scholar]
- 89.Matsuda A, Jacob A, Wu R, Zhou M, Nicastro JM, Coppa GF, et al. Milk fat globule-EGF factor VIII in sepsis and ischemia-reperfusion injury. Mol Med. 2011 Jan-Feb;17(1–2):126–33. doi: 10.2119/molmed.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Lange WJ, Halabi CM, Beyer AM, Sigmund CD. Germ line activation of the Tie2 and SMMHC promoters causes noncell-specific deletion of floxed alleles. Physiol Genomics. 2008 Sep 17;35(1):1–4. doi: 10.1152/physiolgenomics.90284.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, et al. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002 Jun;16(6):1154–67. doi: 10.1210/mend.16.6.0859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.