Abstract
Caveolin-1 has emerged as a critical regulator of signaling pathways involved in lung fibrosis and inflammation. Therefore, we investigated whether caveolin-1 is deficient in asthmatic patients and in a murine model of asthma. Immunohistochemical analyses of endobronchial biopsies showed a remarkable loss of caveolin-1 in the lungs of asthmatic patients compared to controls. This loss was most evident in bronchial epithelial cells, and associated with an increase in the expression of extracellular matrix proteins collagen I, tenascin, and periostin. Cultured primary bronchial epithelial cells of asthmatics had lower caveolin-1 expression compared to control cells. In addition, caveolin-1 expression was significantly decreased in peripheral blood monocytes from asthma patients. The loss of caveolin-1 was also observed in a mouse model for asthma (mice sensitized and challenged with aspergillus fumigatus). To our knowledge, this is the first demonstration that the regulatory protein caveolin-1 is reduced in patients with asthma.
Keywords: Asthma, allergen, epithelium
Introduction
Caveolin-1 (cav-1) is the major structural protein of plasma membrane caveolae and is also associated with the membranes of other organelles. Cav-1 is a master regulatory protein in that it binds to and thereby inhibits the function of kinases in several major families including PKC, MAPK, Src, and G protein1-2 and regulates signaling and cell functions induced by the major pro-fibrotic cytokine, TGFβ.3-4 Cav-1 plays a central role in interstitial lung diseases such as scleroderma (SSc) and idiopathic pulmonary fibrosis (IPF).2-3 Cav-1 is underexpressed in SSc and IPF fibroblasts resulting in overexpression of collagen, and it is underexperessed in SSc monocytes resulting in hypermigration3, 5. It is also underexpressed in various cell types in mice in which fibrosis has been induced using bleomycin. Moreover, the pathology of bleomycin treatment can be reversed by treating the mice with the caveolin-1 scaffolding domain peptide which restores caveolin-1 function to cell deficient in caveolin-16. In a murine model of asthma, cav-1 levels were decreased in the lungs of mice sensitized and challenged with ovalbumin4, 7.
In the current study, our goals were to: 1) Determine whether cav-1 is reduced in patients with asthma, 2) Identify the cell type(s) in which loss of cav-1 occurs, 3) Identify ECM proteins whose expression is enhanced concomitant with the loss of cav-1, and 4) Determine whether cav-1 is also lost in a murine model of asthma in which mice are sensitized and challenged with Aspergillus fumigatus (Af).
Methods
Patients
Six patients with mild to severe asthma and six controls were recruited under an IRB-approved protocol. Patients and controls were well-matched in terms of age, gender distribution, and race with ~50% of subjects African American. All patients had a positive skin test to at least one perennial aeroallergen. Endobronchial biopsy sections from five patients with mild to moderate persistent asthma and from five control subjects were obtained from the National Jewish Medical Center (Denver, CO).
Mice
In accord with an IACUC-approved protocol, six Balb/c female mice were sensitized to Af with an intraperitoneal injection of 200 μg Af (mixture of mycelia extract and culture filtrate, Greer, NC), then challenged with intranasal Af (200 μg /10 μl PBS), three days/week for 4 weeks.8 Six control mice were sensitized and challenged with saline.
Primary antibodies used
Rabbit anti-cav-1 (Santa Cruz sc-894), rabbit anti-collagen (PhosphoSolutions 322-COLT), rabbit anti-periostin (PhosphoSolutions 1621-PERI) and rabbit anti-tenascin (Millipore AB19011).
Results
Caveolin-1 levels are reduced and ECM protein levels are increased in asthmatic lungs
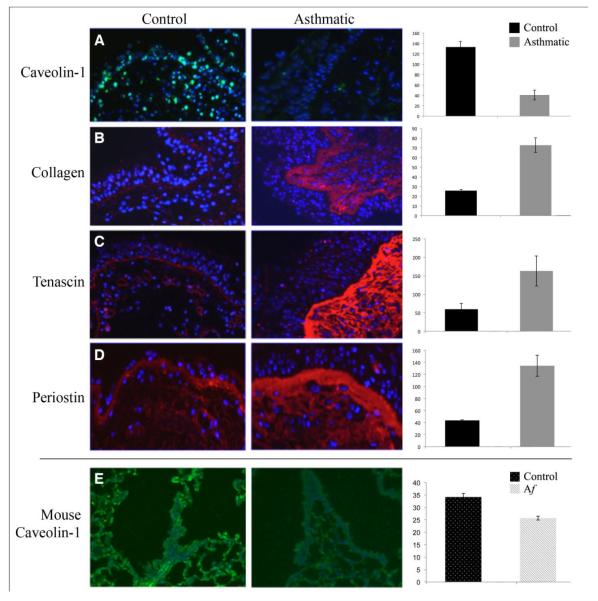
Caveolin-1 staining intensity in endobronchial biopsy sections was decreased in four out of five asthmatics examined (Fig. 1A). When compared to three control subjects, this decrease was highly significant (p < 0.002). This loss was most evident in the bronchial epithelial cells. The loss of caveolin-1 was associated with basement membrane thickening and a major increase in collagen, tenascin, and periostin deposition (Figs. 1B, C and D). Again, these data were statistically significant or approached significance (p < 0.005, 0.05, and 0.08 respectively). To determine whether this loss of caveolin-1 was also replicated in a mouse model system for asthma, caveolin-1 staining intensity was compared in the lungs of Af-sensitized and challenged mice, and in non-sensitized controls (Fig. 1E). A highly significant decrease in caveolin-1 expression (p < 0.001, n = 6) occurred that was associated with hypercellularity and peribronchial fibrosis (data not shown).
Figure 1. Caveolin-1 and ECM protein expression in human asthma lung tissue and in a murine model of allergic asthma.
A-D: Representative images of GMA-embedded human endobronchial lung biopsy sections from Control and Asthmatic subjects were stained with anti-cav-1 (A), anti-collagen (B), anti-tenascin (C) and anti-periostin (D), followed by appropriate secondary antibodies (green for A; red for B-D) and counterstained with the nuclear stain DAPI (blue). E: Representative images of anti-cav-1 stained formalin-fixed, paraffin-embedded sections from saline sensitized and challenged mice (left) and Af sensitized and challenged mice (right). In A through E, staining intensity was quantified by densitometry using Image J1.32 NIH software (five images per human subject, four subjects in each category). Average staining intensity ± s.e.m. is expressed in arbitrary units and shown to the right of each set of micrographs. The statistical significance of these data was determined using Student’s t-test. Key: Af: Aspergillus fumigatus.
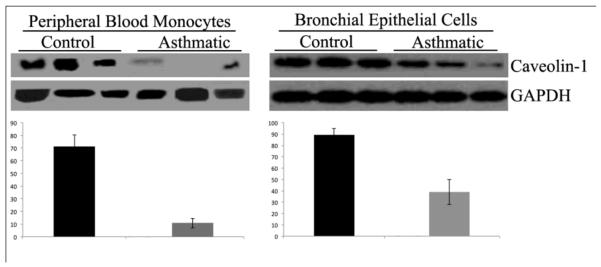
To determine whether the reduced level of caveolin-1 in asthmatic bronchial epithelial cells is maintained in vitro, cells derived from asthmatics and control subjects were cultured and caveolin-1 levels determined by Western blotting. Indeed, the level of caveolin-1 was significantly lower (p < 0.03) in asthmatic cells (Fig. 2 right) indicating that these cell stably underexpress caveolin-1.
Figure 2. Caveolin-1 expression is reduced in asthmatic peripheral blood monocytes and bronchial epithelial cells.
Peripheral blood monocytes were isolated by adhesion9from six subjects in each category., Phenotypic analysis showed the populations to be >75% monocytes. Asthmatic and Control primary human bronchial epithelial cells were purchased from Lonza, cultured following the manufacturer’s instructions, and used in experiments between passages 3 and 5. Extracts of asthmatic peripheral blood monocytes, asthmatic bronchial epithelial cells, and control cells were prepared as previously described9,12 and Western blotted with anti-cav-1 and anti-GAPDH which served as a loading control. Cav-1 expression is reduced in asthmatic monocytes (left) and bronchial epithelial cells (right). Representative blots are shown containing three independent Control and three independent Asthmatic monocyte extracts and triplicate wells of bronchial epithelial cells. Intensity of signals in images from six subjects or wells in each category was quantified by densitometry using Image J1.32 NIH software and is expressed in arbitrary units and shown below each Western blot. The statistical significance of these data was determined using Student’s t-test.
Caveolin-1 levels are reduced in asthmatic monocytes
Given that caveolin-1 levels are reduced in both the affected lung tissue and in circulating monocytes in scleroderma patients5, we determined whether asthmatic monocytes also exhibit reduced caveolin-1 expression. Indeed, as in scleroderma, the level of caveolin-1 was significantly reduced (p < 0.001, n=6) in asthmatic monocytes compared to control monocytes (Fig. 2 left).
Discussion
To our knowledge this is the first report of loss of caveolin-1 in human subjects with asthma. The loss of caveolin-1 affected both bronchial epithelial cells and peripheral blood monocytes and was associated with enhanced deposition of ECM proteins. We also demonstrated loss of caveolin-1 following Af challenge in an Af-sensitized chronic murine model of asthma. Previous studies suggest that the loss of caveolin-1 may lead to the overexpression of ECM proteins in that the reduced levels of caveolin-1 in scleroderma lung fibroblasts results in the hyperactivation of MAP kinases and increased collagen production.6 Based on previous studies in which the loss of caveolin-1 from scleroderma monocytes was shown to enhance their expression of CXCR4 and migration towards CXCL12 via a MAP kinase-mediated pathway5, we propose that the loss of caveolin-1 from monocytes may play a role in the pathology of asthma by promoting their migration into damaged lung tissue.
These observations also raise the possibility that asthma may be treated with a novel drug based on the caveolin-1 scaffolding domain peptide (CSD) which can restore caveolin-1 function to cells deficient in caveolin-1. This approach has already been shown to be effective in vitro (reversing the overexpression of collagen by scleroderma lung fibroblasts and the hypermigration of scleroderma monocytes) and in vivo (reversing inflammation and fibrosis in mice treated with bleomycin)6, 9. It is also noteworthy that asthmatic airway epithelial cells overproduce pro-inflammatory cytokines (IL-6, IL-8 and GM-CSF), TH2 amplifying cytokines (TSLP, IL-33 and IL-25), profibrogenic growth factors (TGF-β and FGF-2) 10-12, and the ECM protein periostin13. Given that the production of most, and perhaps all, of these effectors is regulated by MAP kinases,14-15 that MAP kinase activity is regulated by caveolin-1, and that we show here that asthmatic airway epithelial cells are deficient in cav-1, we predict that the CSD peptide will reverse the overproduction of these pro-asthmatic effectors as part of its beneficial effect on asthma patients.
Acknowledgments
The authors thank Richard P. Visconti PhD, Michael Bonner, PhD and Allen P. Kaplan MD for advice and helpful discussions related to various experiments.
Funding source: Sonia Bains was supported by NIH/NCRR Grant number UL1RR029882. Elena Tourkina was supported by NIH NIAMS grants R03 AR056767 and K01 AR054143 and a grant from the Scleroderma Foundation; Stanley Hoffman was supported by NIH NCCAM grant AT004450, a South Carolina Clinical and Translational Research Institute Pilot Project grant, and grant W81XWH-11-1-0508 from the DOD. The Medical University of South Carolina received NCRR Construction grant C06 RR015455. Carl Atkinson was supported by the FAMRI CIA092079.
Footnotes
Authors’ contribution:
Dr. Bains was the primary hands-on person responsible for performing and designing the experiments. She also recruited patients from her clinic and obtained peripheral blood monocytes. She was responsible for writing the manuscript. Drs. Hoffman and Tourkina were involved in the conception and design of the experiments, as well as in data interpretation and statistical analysis. Drs. Joseph and Tholanikunnel helped perform the cell culture work, including growing primary bronchial epithelial cell cultures derived from asthmatic and control subjects. Dr. Atkinson was responsible for the aspergillus sensitization and challenge murine model of asthma. Drs. Chu and Martin prepared endobronchial lung biopsy sections from subjects with asthma and controls for use in this study. Dr. Riemer is a lung pathologist who evaluated the slides used in this study in a blinded fashion. All authors offered their criticisms of the manuscript and approve this version for publication.
Conflicts of Interest: While none of the authors have received any financial benefit from this work, Drs. Hoffman and Tourkina are the Inventors on a use patent (# 8,058,227) issued to the Medical University of South Carolina on the caveolin-1 scaffolding domain peptide as a treatment for fibrotic diseases. Drs. Hoffman and Tourkina are also the founders of a company, FibroTherapeutics, Inc., which has an option to license this patent from MUSC for the purpose of developing a drug based on the caveolin-1 scaffolding domain peptide.
Literature Cited
- 1.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–33. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 2.Tourkina E, Gooz P, Pannu J, et al. Opposing effects of protein kinase Calpha and protein kinase Cepsilon on collagen expression by human lung fibroblasts are mediated via MEK/ERK and caveolin-1 signaling. J Biol Chem. 2005;280:13879–87. doi: 10.1074/jbc.M412551200. [DOI] [PubMed] [Google Scholar]
- 3.Wang XM, Zhang Y, Kim HP, et al. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203:2895–906. doi: 10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Saux CJ, Teeters K, Miyasato SK, et al. Down-regulation of caveolin-1, an inhibitor of transforming growth factor-beta signaling, in acute allergen-induced airway remodeling. J Biol Chem. 2008;283:5760–8. doi: 10.1074/jbc.M701572200. [DOI] [PubMed] [Google Scholar]
- 5.Tourkina E, Richard M, Oates J, et al. Caveolin-1 regulates leucocyte behaviour in fibrotic lung disease. Ann Rheum Dis. 2010;69:1220–6. doi: 10.1136/ard.2009.117580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tourkina E, Richard M, Gooz P, et al. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2008;294:L843–61. doi: 10.1152/ajplung.00295.2007. [DOI] [PubMed] [Google Scholar]
- 7.Chen CM, Wu MY, Chou HC, Lang YD, Wang LF. Downregulation of caveolin-1 in a murine model of acute allergic airway disease. Pediatr Neonatol. 2011;52:5–10. doi: 10.1016/j.pedneo.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Seymour BW, Schelegle ES, Pinkerton KE, et al. Second-hand smoke increases bronchial hyperreactivity and eosinophilia in a murine model of allergic aspergillosis. Clin Dev Immunol. 2003;10:35–42. doi: 10.1080/10446670310001598483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tourkina E, Bonner M, Oates J, et al. Altered monocyte and fibrocyte phenotype and function in scleroderma interstitial lung disease: reversal by caveolin-1 scaffolding domain peptide. Fibrogenesis Tissue Repair. 2011;4:15. doi: 10.1186/1755-1536-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol. 2010;88:257–68. doi: 10.1038/icb.2009.113. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Smartt H, Holgate ST, Roche WR. Growth factors secreted by bronchial epithelial cells control myofibroblast proliferation: an in vitro co-culture model of airway remodeling in asthma. Lab Invest. 1999;79:395–405. [PubMed] [Google Scholar]
- 12.Mulligan RM, Atkinson C, Vertegel AA, Reukov V, Schlosser RJ. Cigarette smoke extract stimulates interleukin-8 production in human airway epithelium and is attenuated by superoxide dismutase in vitro. Am J Rhinol Allergy. 2009;23:e1–4. doi: 10.2500/ajra.2009.23.3400. [DOI] [PubMed] [Google Scholar]
- 13.Sidhu SS, Yuan S, Innes AL, et al. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci U S A. 2010;107:14170–5. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puddicombe SM, Davies DE. The role of MAP kinases in intracellular signal transduction in bronchial epithelium. Clin Exp Allergy. 2000;30:7–11. doi: 10.1046/j.1365-2222.2000.00709.x. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Fan D, Wang C, et al. Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-beta1 pathways in cardiac fibroblasts. Cardiovasc Res. 2011;91:80–9. doi: 10.1093/cvr/cvr067. [DOI] [PubMed] [Google Scholar]