Abstract
Objectives
We explored the age-stratified correlates and correlations between HR-HPV infection and cervical abnormalities in perimenopausal women.
Materials and methods
HPV testing and Pap smear screening were performed at baseline on 841 routinely screened women age 35–60 years in the HPV in Perimenopause (HIP) cohort. Demographic, behavioral and medical information was collected through telephone administered questionnaires. Descriptive analyses were used to examine the correlation between HR-HPV infection and cervical abnormalities by age. Logistic regression was used to determine correlates of HPV and abnormalities in women under and over 45 years of age.
Results
The prevalence of HPV, HR-HPV and cervical abnormalities decreased significantly with increasing age, as did the correlation between HR-HPV and cervical abnormalities. The prevalence of HR-HPV was 50% among younger women with abnormalities but this decreased steadily to 20% HR-HPV detection among 50–54 year old, and no abnormalities were detected in 55–60 year old women. Different correlates of HR-HPV infection and abnormalities were observed in women ≥45 years, a pattern not seen in the younger women.
Conclusions
Although the relative proportion of low and high-grade abnormalities did not change with age, we saw a loss of concordance between HR-HPV detection and cytological abnormalities with increasing age. Current guidelines for cervical cancer screening group together all women age 30 and above. Our data raise important questions about the interpretation of HPV and Pap test results in this age group and suggest that ongoing surveillance of HPV and cytology in cervical cancer screening programs consider a third age stratification among older women.
Keywords: perimenopausal women, menopause, human papillomavirus, HPV, cervical lesions, cytology, cervical cancer, screening, guidelines
INTRODUCTION
Annual screening with cervical Pap smears has dramatically reduced morbidity and mortality due to cervical cancer in the United States in the past 40 years (1). Current consensus guidelines from the American Society for Colposcopy and Cervical Pathology (ASCCP) and the American College of Obstetricians and Gynecologists (ACOG) recommend that HR-HPV testing can be performed in addition to routine cytology screening in women 30 years and older (2, 3). Current guidelines stratify their recommendations at age 30 years because women under 30 are at very low risk of invasive cervical cancer but have a very high probability of prevalent, but transient, HPV infection (4–7). On the other hand, the prevalence of HPV among women 30 years and older is lower than among younger women, resulting in a substantial improvement in the specificity of HPV DNA testing when screening older women.
Although there is an obvious justification for age stratification at 30 years when making recommendations for use of HPV DNA testing in screening, it is unclear whether the guidelines for women over 30 are equally applicable throughout a woman’s screening lifetime. Because the rate of pre-neoplastic disease is low (8), few studies of women over age 30 have the power to further stratify by age in order to better inform guidelines. Recently, the ATHENA trial, which included data on over 35,000 women age 30 years and older, reported a decrease in HR-HPV detection with increasing age among women with all grades of pathology-confirmed cervical intraepithelial neoplasia (CIN), with the most striking decrease observed for HPV 16 infection (9). In the HPV in Perimenopause (HIP) cohort of routinely screened women age 35–60 years, we observed a similar trend of decreasing correlation between HR-HPV detection and cervical abnormalities with increasing age While the HIP cohort is small in size relative to the large studies of screening populations (9, 10), it is unique in that we are able to use the extensive personal, behavioral and medical information collected in the HIP study to explore age-stratified patterns and correlates of HPV infection and abnormal cervical cytology in perimenopausal women. Therefore, in this secondary analysis of the HIP study cohort we aimed to further explore the association between any-HPV and HR-HPV detection and abnormal cytology by age.
METHODS
Study population and data collection
Women attending outpatient OB/GYN clinics for routine examination in and around Baltimore, MD from March, 2008 to March, 2011 were recruited to participate in an ongoing prospective cohort study on the natural history of HPV infection through the perimenopausal transition. Women were eligible to participate if they were aged 35–60 years, had an intact cervix, and were willing to provide informed consent. Women were not eligible for enrollment if they were pregnant, had plans to become pregnant, had a history of organ transplantation or were known to be HIV-positive.
After providing informed consent, women completed a baseline questionnaire and gynecological examination. Information on sociodemographic characteristics, reproductive and menstrual history, hormonal and non-hormonal medication use, lifetime sexual history and current sexual behavior were collected using a telephone-administered questionnaire. Data on women’s cervical screening and treatment history were also collected, including whether they had obtained previous Pap or HPV tests, had a previous Pap abnormality, and had ever had a colposcopy or treatment for cervical abnormalities. A trained study physician conducted a speculum exam to collect a cervical brush for HPV DNA testing (Digene HPV sampler, Digene, Gaithersburg, MD, USA) as part of the standardized study protocol. Cervical brushes were placed in standard transport medium at 4° for less than 24 hours before being vortexed and stored at −80 °C. All study procedures were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Detection of cervical abnormalities and HPV infection
Women provided signed consent to allow retrieval of their Pap results from the clinical cytopathology labs. The result of the Pap test that was associated with study enrollment was abstracted from the cytopathology report onto a standardized case report form. If the enrollment visit coincided with an annual exam where a Pap test was indicated by clinic protocol, the clinician collected a liquid-based cytology specimen prior to collecting the study samples. Pap results were managed according to standard clinical practice. Because some physicians do not screen annually by cytology, 12% of women did not receive a Pap test within 3 months of the enrollment exam and the HPV DNA test. Pap results were classified according to the most severe diagnosis as negative for intraepithelial lesion or malignancy (NILM), atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithial lesion (LSIL), atypical squamous cells, cannot exclude high-grade squamous intraepithial lesion (ASC-H), high-grade squamous intraepithial lesion (HSIL), or cancer. For study purposes abnormal cytology was considered ASCUS or greater.
Detection and genotyping of HPV DNA from cervical specimens of all women at baseline was performed at Johns Hopkins University in Baltimore, MD. DNA was extracted using the QIAamp DNA Blood Kit (Qiagen,Valencia, CA, USA) according to manufacturer’s instructions with modification (11). An 8µl aliquot of extracted DNA was tested using the Roche HPV Linear Array PCR-based assay (Roche Diagnostics, Indianapolis, IN, USA). Detection of the presence of human DNA by beta-globin-specific polymerase chain reaction (PCR) is a component of the LA assay, and was used to determine sample adequacy. The HPV Linear Array is based on the PGMY09/11 PCR primer system that allows for high efficiency amplification of 37 distinct HPV genotypes (12, 13). For this analysis, HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 were classified as high-risk (carcinogenic) HPV types.
Statistical analysis
Descriptive analyses were conducted to characterize the pattern of HPV infection and cervical abnormalities among women in five-year age categories: 35–39, 40–44, 45–49, 50–54 and 55–60 years. The correlation between HPV-positivity and abnormal cervical cytology was examined by calculating the prevalence of HR-HPV detection among women with current abnormalities and the prevalence of abnormalities among women with detectable HR-HPV. To determine if the association between HPV and abnormalities changed with increasing age, a two-sided test for trend across ordered groups was performed with the significance level set at p<0.05. Exact logistic regression was used to estimate unadjusted odds ratios and corresponding 95% confidence intervals for the association between potential risk factors for HPV positivity and cervical abnormalities. Regression analyses were conducted separately for women under the median age of 45 years and for women 45 years and older. To examine correlates of abnormal cytology independent of HR-HPV infection, additional regression models were adjusted for the presence of HR-HPV. All analyses were carried out using SAS version 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
Description of routinely screened perimenopausal women
Of the 949 women enrolled in this study, 101 women were excluded from this analysis because they did not have a Pap smear within 3 months of study enrollment, 64 women were excluded because of incomplete baseline questionnaire data, and 3 because their cervical samples for HPV DNA detection were beta-globin negative. In the remaining 781 women, the median age was 47 years (IQR: 41–52) and 64% (n=497) were currently married (Table 1). The majority of women were white (n=594, 74%) and had completed at least some education beyond high school (83%; n=651). All women reported having previous Pap smear screening, with over 90% (n=700) of women reporting a Pap smear in the 3 years prior to enrollment. Almost half of the women (46%; n=416) reported having received an abnormal Pap result prior to enrollment and 20% reported ever having a colposcopy (n=165) or treatment for an abnormal Pap smear (n=148).
Table 1.
Characteristics of the mid-adult screening population (N=781)
| Total cohort N(%) |
|
|---|---|
| Age in years | |
| 35–39 | 137 (18) |
| 40–44 | 172 (22) |
| 45–49 | 195 (25) |
| 50–54 | 161 (21) |
| 55–60 | 116 (15) |
| Marital status1 | |
| Married | 497 (64) |
| Divorced/Separated/Widowed | 143 (18) |
| Single | 140 (18) |
| Race | |
| White/Caucasian | 579 (74) |
| Black/African-American | 146 (19) |
| Other | 56 (7) |
| Education completed | |
| High School | 130 (17) |
| Post high school | 182 (23) |
| College | 231 (30) |
| Post graduate | 238 (30) |
| Yearly Income (US dollars) | |
| <40,000 | 54 (7) |
| 40–80,000 | 186 (24) |
| 80–120,000 | 175 (22) |
| >120,000 | 234 (30) |
| unknown | 132 (17) |
| BMI | |
| <25 | 301 (39) |
| 25–29.9 | 236 (30) |
| >30 | 243 (31) |
| Smoking history | |
| Never | 538 (69) |
| Former | 159 (20) |
| Current smoker | 84 (11) |
| Exogenous Hormone Use2 | |
| Never/Former | 582 (75) |
| Current | 199 (25) |
| Menopausal status | |
| Premenopausal | 329 (43) |
| Perimenopausal | 231 (30) |
| Postmenopausal | 203 (27) |
| Live Births | |
| 0 | 220 (28) |
| 1 | 140 (18) |
| 2 | 280 (36) |
| 3 or more | 139 (18) |
| Lifetime number of sexual partners | |
| 0–1 | 105 (14) |
| 2 | 56 (7) |
| 3 | 79 (10) |
| 4 | 64 (8) |
| 5 | 95 (12) |
| 6–10 | 212 (27) |
| 11–20 | 109 (14) |
| >20 | 58 (7) |
| Recent sexual partners3 | |
| No recent sex | 162 (21) |
| Sex, no new partner | 591 (76) |
| Sex with new partner(s) | 23 (3) |
| Ever diagnosed with STI(s)4 | |
| No | 507 (65) |
| Yes | 270 (35) |
| Ever previous Pap screening | |
| Yes | 781 (100) |
| Ever previous Abnormal Pap | |
| No | 416 (54) |
| Yes | 356 (46) |
| Ever had colposcopy | |
| No | 616 (79) |
| Yes | 165 (21) |
| Ever treated for abnormal Pap5 | |
| No | 633 (81) |
| Yes | 148 (19) |
Widowed (n=13), separated (n=26), divorced (n=104), 1 missing marital status
Includes both hormonal contraceptives and hormone replacement therapy
Recent refers to the 6 months prior to study baseline
Includes chlamydia, gonorrhea, herpes, trichomonas, syphilis, chancroid, and genital warts
Treatment defined as laser, cryotherapy, LEEP, or conization
Missing data: marital status-1, bmi-1, menopausal status-18, live births-2, lifetime number of sexual partners-3, recent sex-5, ever diagnosed with STI-4, previous abnormal pap- 9
Abbreviations: N=number, %=percent, BMI=body mass index, STI=sexually transmitted infection
Correlation between HPV positivity and abnormal cervical cytology
At baseline, the prevalence of any-HPV detection was 18% (n=142), the prevalence of HR-HPV detection was 9% (n=68), and the prevalence of cytologic abnormalities was 5% (n=40), which included 25 cases of ASCUS, 13 cases of LSIL and 2 cases of ASC-H. There was a significant decrease in the prevalence of any-HPV (p=0.003), HR-HPV (p<0.001), and abnormal cervical cytology (p<0.001) with age (Table 2). The decrease in prevalence from youngest to oldest was almost 5-fold for high-risk HPV, from 17% (23/137) to 4% (4/116). A similar decrease was seen for abnormal cytology, and no abnormalities were detected in the oldest group of women. Similar patterns were observed for combinations of concurrent Pap smear and HR-HPV DNA results by age (Table 3). Corresponding with the decreasing prevalence, the percentage of women who were negative on both tests increased, while those who were positive on both decreased with age.
Table 2.
The prevalence of HPV and cervical abnormalities across age strata among women over 35 years old.
| Any-HPV DNA | HR-HPV DNA1 | Only LR-HPV DNA | Cervical cytology | |||||
|---|---|---|---|---|---|---|---|---|
| Negative N (%) |
Positive N (%) |
Negative N (%) |
Positive N (%) |
Negative N (%) |
Positive N (%) |
Normal N (%) |
Abnormal2 N (%) |
|
| Age 35–39 (n=137) | 98 (72) | 39 (28) | 114 (83) | 23 (17) | 121 (88) | 16 (12) | 123 (90) | 14 (10) |
| Age 40–44 (n=172) | 143 (83) | 29 (17) | 153 (89) | 19 (11) | 162 (94) | 10 (6) | 162 (94) | 10 (6) |
| Age 45–49 (n=195) | 162 (83) | 33 (17) | 182 (93) | 13 (7) | 175 (90) | 20 (10) | 183 (94) | 12 (6) |
| Age 50–54 (n=161) | 134 (83) | 27 (17) | 152 (94) | 9 (6) | 143 (89) | 18 (11) | 157 (98) | 4 (2) |
| Age 55–60 (n=116) | 102 (82) | 14 (12) | 112 (97) | 4 (3) | 106 (91) | 10 (9) | 116 (100) | 0 (0) |
| Trend p-value | 0.003 | <0.001 | 0.924 | <0.001 | ||||
HR-HPV with or without LR-HPV infection
Abnormal is defined as ≥ASCUS (ASCUS=25, LSIL=13, ASC-H=2, HSIL=0)
Abbreviations: HPV=high-risk human papillomavirus, HR=high-risk, LR=low-risk,N=number, %=percentage, ASCUS=atypical squamous cells, undetermined significance, LSIL=low-grade intraepithelial lesion, ASC-H=atypical squamous cells, can’t exclude high-grade squamous intraepithelial lesion, HSIL= high-grade squamous intraepithelial lesion
Table 3.
Correlation between HR-HPV and cytology results stratified by age1
| HPV− Pap- N (%) |
HPV+ Pap- N (%) |
HPV− Pap+ N (%) |
HPV+ Pap+ N (%) |
|
|---|---|---|---|---|
| 35–39 (n=137) | 107 (78) | 16 (12) | 7 (5) | 7 (5) |
| 40–44 (n=172) | 147 (85) | 15 (9) | 6 (3) | 4 (2) |
| 45–49 (n=195) | 173 (89) | 10 (5) | 9 (5) | 3 (2) |
| 50–54 (n=161) | 149 (93) | 8 (5) | 3 (2) | 1 (1) |
| 55–60 (n=116) | 112 (97) | 4 (3) | 0 (0) | 0 (0) |
| Trend p-value | <0.001 | 0.003 | 0.02 | 0.002 |
Categories are mutually exclusive combinations of HR-HPV DNA status and Pap Smear results: HR-HPV negative and normal Pap, HR-HPV positive and normal Pap, HR-HPV negative and ≥ASCUS, HR-HPV positive and ≥ASCUS
Abbreviations: HPV=high-risk human papillomavirus, HR=high-risk, N=number, %=percentage, ASCUS=atypical squamous cells, undetermined significance
There was a trend of decreased concordance between Pap and HR-HPV test results with age, but an increase in the concordance between Pap and any-HPV test results (Fig. 1). Specifically, among women with HR-HPV infection, there was a decrease in the proportion of cervical abnormalities with increasing age, from 30% (7/23) among women in the youngest age group to 0% (0/4) in the oldest. A similar decrease was seen in the proportion of abnormalities among women with any-HPV detected. Among women with abnormal cervical cytology, there was a decrease in the prevalence of HR-HPV with increasing age. In women aged 35–39 years, 50% (7/14) of women with cervical abnormalities had HR-HPV infection, whereas only 25% (1/4) of 50–54 year old women with cervical abnormalities had detectable HR-HPV. On the other hand, the prevalence of any-HPV among women with abnormal cytology was highest in the women 50–54 years old (75%; n=3).
Figure 1.
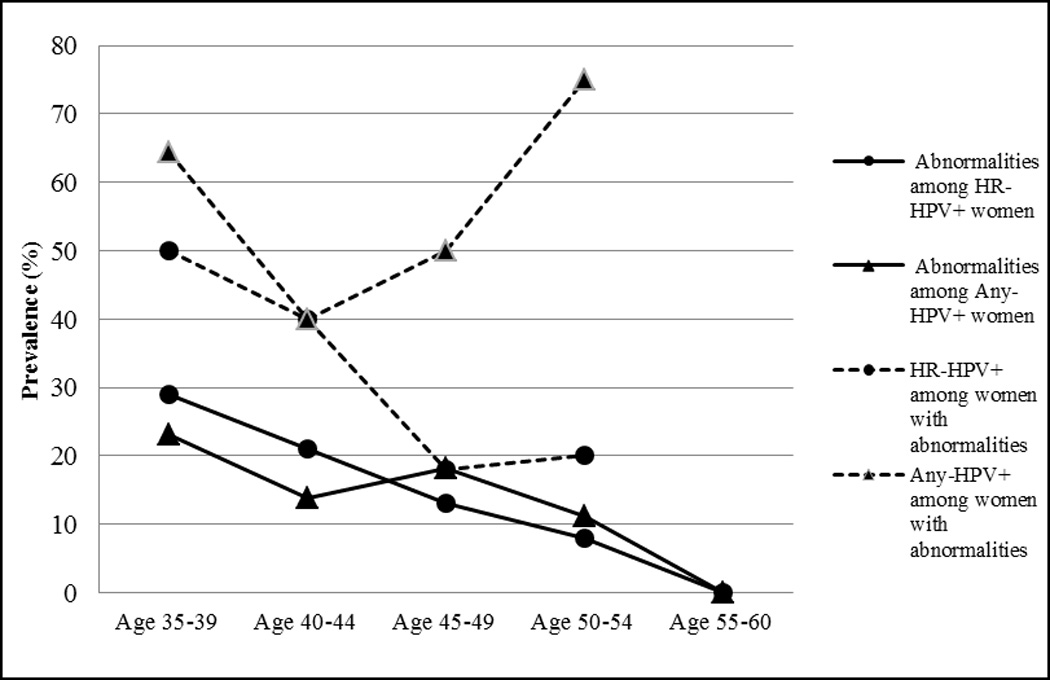
The correlation between abnormal cervical cytology and HR-HPV DNA detection stratified by age.
Abbreviations: HR-HPV+=positive for high-risk human papillomavirus types; Any-HPV+=positive for any human papillomavirus types
Risk factors for current HPV and cervical abnormalities
To better understand the discordance between HR-HPV and cervical abnormalities with age, we made individual comparisons of the correlates of current HPV infection with the correlates of cervical abnormalities among women under and over 45 years of age (Table 4). The odds of HR-HPV detection were higher among unmarried women compared with married women in both age groups. In addition, the odds of HR-HPV detection were higher among women who reported a new sex partner in the last 6 months, regardless of age, compared with women without sexual partners.
Table 4.
Correlates ofHR-HPV infection and cervical abnormalities stratified by age.
| Women <45 years | Women ≥ 45 years | |||||||
|---|---|---|---|---|---|---|---|---|
| HR-HPV [n(%)] |
uOR [95% CI] | Abnormal [n(%)] |
uOR [95% CI] | HR-HPV [n(%)] |
uOR [95% CI] | Abnormal [n(%)] |
uOR [95% CI] | |
| Marital status | ||||||||
| Married | 15 (8) | REF | 14 (8) | REF | 12 (4) | REF | 10 (3) | REF |
| Divorced/Separated/Widowed | 10 (19) | 2.6 (1.0, 6.6) | 5 (9) | 1.2 (0.3, 3.9) | 9 (10) | 2.8 (1.0, 7.5) | 3 (3) | 1.1 (0.2, 4.2) |
| Single | 17 (25) | 3.7 (1.6, 8.6) | 5 (7) | 1.0 (0.3, 3.0) | 5 (7) | 1.9 (0.5, 6.0) | 3 (4) | 1.3 (0.2, 5.3) |
| Race | ||||||||
| White/Caucasian | 27 (13) | REF | 16 (8) | REF | 19 (5) | REF | 14 (4) | REF |
| Black/African-American | 10 (14) | 1.1 (0.5, 2.6) | 6 (9) | 1.1 (0.3, 3.2) | 5 (7) | 1.2 (0.4, 3.8) | 1 (1) | 0.3 (0.0, 2.3) |
| Other | 5 (17) | 1.3 (0.4, 4.0) | 2 (7) | 0.9 (0.1, 4.0) | 2 (8) | 1.5 (0.2, 7.0) | 1 (4) | 1.0 (0.0, 7.2) |
| Education completed | ||||||||
| High School | 6 (11) | REF | 4 (7) | REF | 3 (4) | REF | 3 (4) | REF |
| Post high school | 15 (22) | 2.3 (0.8, 8.0) | 10 (15) | 2.2 (0.6, 10.3) | 10 (9) | 2.3 (0.6, 13.3) | 3 (3) | 0.6 (0.1, 4.9) |
| College | 13 (13) | 1.3 (0.4, 4.3) | 3 (3) | 0.4 (0.1, 2.5) | 6 (5) | 1.1 (0.2, 7.2) | 3 (2) | 0.6 (0.1, 4.2) |
| Post graduate | 8 (9) | 0.9 (0.2, 3.2) | 7 (8) | 1.2 (0.3 (5.6) | 7 (5) | 1.1 (0.3, 7.1) | 7 (5) | 1.1 (0.3, 7.0) |
| Yearly Income | ||||||||
| >120,000 | 6 (7) | REF | 3 (4) | REF | 2 (1) | REF | 6 (4) | REF |
| 80–120,000 | 10 (13) | 1.9 (0.6, 6.7) | 6 (8) | 2.2 (0.5, 14.2) | 7 (7) | 5.7 (1.1, 57.4) | 2 (2) | 0.5 (0.1, 2.9) |
| 40–80,000 | 16 (18) | 2.8 (1.0, 9.2) | 12 (14) | 4.1 (1.1, 23.7) | 7 (7) | 5.7 (1.1, 57.4) | 3 (3) | 0.8 (0.1, 3.7) |
| <40,000 | 2 (8) | 1.2 (0.1, 7.3) | 1 (4) | 1.2 (0.0, 15.5) | 1 (3) | 2.5 (0.0, 49.5) | 3 (10) | 2.6 (0.4, 13.1) |
| Unknown | 8 (19) | 2.9 (0.8, 11.0) | 2 (5) | 1.3 (0.1, 11.8) | 9 (10) | 8.3 (1.7, 80.9) | 2 (2) | 0.6 (0.1, 3.2) |
| Smoking history | ||||||||
| Never | 34 (15) | REF | 14 (6) | REF | 13 (4) | REF | 10 (3) | REF |
| Former | 6 (12) | 0.7 (0.2, 1.9) | 5 (10) | 1.6 (0.4, 5.0) | 6 (6) | 1.4 (0.4, 4.0) | 5 (5) | 1.5 (0.4, 4.9) |
| Current smoker | 2 (7) | 0.4 (0.0, 1.7) | 5 (16) | 2.9 (0.8, 9.4) | 7 (13) | 3.5 (1.1, 10.0) | 1 (2) | 0.6 (0.0, 4.2) |
| Live Births | ||||||||
| 0 | 10 (16) | REF | 5 (8) | REF | 3 (4) | REF | 1 (1) | REF |
| 1 | 8 (18) | 0.8 (0.2, 2.3) | 3 (9) | 0.6 (0.1, 2.7) | 6 (8) | 1.0 (0.2, 3.7) | 0 (0) | 1.4 (0.1, 19.4) |
| 2 | 7 (11) | 0.6 (0.3, 1.5) | 6 (10) | 0.6 (0.2, 1.9) | 8 (6) | 1.0 (0.3, 3.3) | 5 (4) | 2.0 (0.3, 21.2) |
| 3 or more | 17 (12) | 0.9 (0.3, 2.6) | 10 (7) | 1.0 (0.2, 3.5) | 9 (5) | 0.8 (0.2, 3.4) | 10 (5) | 5.5 (1.0, 55.3) |
| Exogenous Hormone Use | ||||||||
| Never/ Former | 21 (11) | REF | 13 (7) | REF | 21 (6) | REF | 15 (4) | REF |
| Current | 21 (19) | 2.1 (1.0, 4.2) | 11 (10) | 1.6 (0.6, 4.1) | 5 (6) | 1.0 (0.3, 2.8) | 1 (1) | 0.3 (0.0, 1.8) |
| Lifetime sex partners | ||||||||
| < 5 | 11 (11) | REF | 5 (5) | REF | 5 (3) | REF | 10 (5) | REF |
| ≥ 5 | 31 (15) | 1.4 (0.7, 3.3) | 19 (9) | 1.9 (0.7, 6.7) | 21 (8) | 3.4 (1.2, 11.8) | 6 (2) | 0.5 (0.1, 1.4) |
| Recent sex partners | ||||||||
| Sex, no new partner(s) | 28 (11) | REF | 17 (7) | REF | 16 (5) | REF | 12 (4) | REF |
| No recent sex | 4 (10) | 0.9 (0.2, 2.9) | 3 (8) | 1.2 (0.2, 4.3) | 7 (6) | 1.2 (0.4, 3.2) | 2 (2) | 0.5 (0.1, 2.1) |
| Sex with new partner(s) | 9 (69) | 17.7 (5.6, 84.0) | 3 (23) | 4.1 (0.7, 18.2) | 3 (30) | 8.5 (1.3, 41.9) | 2 (20) | 6.7 (0.6, 39.2) |
| Ever previous abnormality | ||||||||
| No | 18 (10) | REF | 7 (4) | REF | 8 (3) | REF | 8 (3) | REF |
| Yes | 24 (18) | 1.8 (0.9, 3.8) | 17 (13) | 3.4 (1.3, 9.9) | 18 (8) | 2.6 (1.1, 7.1) | 8 (4) | 1.1 (0.4, 3.5) |
| Time since last abnormal pap 1 | ||||||||
| No abnormal pap | 18 (10) | REF | 7 (4) | REF | 8 (3) | REF | 8 (3) | REF |
| 1–5 years | 6 (14) | 1.4 (0.4, 4.0) | 4 (9) | 2.4 (0.5, 10.1) | 6 (9) | 2.8 (0.8, 9.7) | 2 (3) | 0.9 (0.1, 4.6) |
| 6–10 years | 4 (15) | 1.6 (0.4, 5.4) | 5 (19) | 5.6 (1.3, 22.6) | 5 (14) | 4.9 (1.8, 18.1) | 0 (0) | 0.6 (0.0, 4.1) |
| >10 years | 9 (16) | 1.6 (0.6, 4.0) | 4 (7) | 1.8, (0.4, 7.2) | 4 (4) | 1.2 (0.3, 4.8) | 0 (0) | 0.2 (0.0, 1.4) |
| Ever previous colposcopy | ||||||||
| No | 24 (10) | REF | 11 (5) | REF | 17 (4) | REF | 11 (3) | REF |
| Yes | 18 (23) | 2.6 (1.3, 5.5) | 13 (17) | 4.1 (1.6, 10.5) | 9 (10) | 2.5 (0.9, 6.1) | 5 (6) | 2.0 (0.5, 6.6) |
N=744; 21 excluded because abnormal Pap within 1 year of enrollment and so could not be conclusively separated from baseline result, 16 excluded because missing abnormal Pap date
Abbreviations: N=number, %=percent, HR-HPV=high-risk human papillomavirus, uOR=unadjusted odds ratio, CI=confidence interval, REF=referent group
Among younger women (under 45 years of age), there was a trend for higher odds of HR-HPV detection and cervical abnormalities among lower income women compared with women in the highest income level. In addition, self-reported history of colposcopy was associated with a higher odds of both HR-HPV (odds ratio [OR]: 2.6; (95% confidence interval [CI]: 1.3, 5.5)) and abnormalities (OR: 4.1 (1.6, 10.5)) at baseline. Women under 45 years of age who were single as compared to married (OR: 3.7 (1.6, 8.6)), current hormone users (OR: 2.1 (1.0, 4.2)), and women reporting recent new sexual partners (OR: 17.7 (5.6, 84.0)), had a higher odds of HR-HPV detection only, whereas women with previous abnormalities (OR: 3.4 (1.3, 9.9)) had a higher odds of a current cytological abnormalities but not current HR-HPV detection.
HR-HPV detection in women 45 years and older was associated with being unmarried (OR: 2.8 (1.0, 7.5)), having a lower income as compared to $120,000 (range OR: 2.5 (0.0, 49.5)) to 5.7 (1.1, 57.4)), being a current smoker as compared to never smoking (OR: 3.5 (1.1, 10.0)), having 5 or more lifetime sex partners (OR: 3.4 (1.2, 11.8)), having a recent new sex partner (OR: 8.5 (1.3, 41.9)), and having a history of an abnormal Pap smear (OR: 2.6 (1.1, 7.1)). Only women reporting a history of 3 or more births had higher odds of abnormal cervical cytology at baseline. No risk factors were associated with both HR-HPV detection and cervical abnormalities in older women.
In women age 45 and older, having a low-risk HPV infection was associated with a 6-fold higher odds of cytologic abnormality compared to women who were HPV-negative (Table 5). This association was not seen in younger women. The odds of cytologic abnormalities increased for current smokers under 45, after controlling for HR-HPV (OR: 4.4 (1.0, 16.8)). The 5-fold higher odds of cytologic abnormality associated with 3 or more live births in women over 45 remained consistent after controlling for HR-HPV. The association between a recent new sex partner and cervical abnormalities decreased by 68% in younger women but only decreased 42% in older women after adjusting for HR-HPV. Although the adjusted estimate was slightly lower than the unadjusted estimate, the odds of current abnormalities remained higher among women under 45 years of age who reported previous colposcopy.
Table 5.
Correlates of cervical abnormalities independent of HR-HPV infection, stratified by age.
| Women <45 years | Women ≥ 45 years | |||||
|---|---|---|---|---|---|---|
| Abnormal [n(%)] |
uOR [95% CI] | aOR [95% CI] 1 | Abnormal [n(%)] |
uOR [95% CI] | aOR [95% CI] 1 | |
| HPV risk groups | ||||||
| None | 11 (5) | REF | 7 (2) | REF | ||
| Low-risk Only | 2 (8) | 1.7 (0.2, 8.7) | 5 (10) | 6.4 (1.5, 24.8) | ||
| Low-risk + High-risk | 5 (33) | 10.2 (2.3, 40.6) | 2 (17) | 11.0 (1.0, 68.8) | ||
| High-risk Only | 6 (22) | 5.9 (1.6, 19.6) | 2 (14) | 9.2 (0.8, 55.8) | ||
| Marital status | ||||||
| Married | 14 (8) | REF | REF | 10 (3) | REF | REF |
| Divorced/Separated/Widowed | 5 (9) | 1.2 (0.3, 3.9) | 0.9 (0.2, 3.0) | 3 (3) | 1.1 (0.2, 4.2) | 0.8 (0.1, 3.5) |
| Single | 5 (7) | 1.0 (0.3, 3.0) | 0.6 (0.1, 1.9) | 3 (4) | 1.3 (0.2, 5.3) | 1.2 (0.2, 4.8) |
| Race | ||||||
| White/Caucasian | 16 (8) | REF | REF | 14 (4) | REF | REF |
| Black/African-American | 6 (9) | 1.1 (0.3, 3.2) | 1.1 (0.3, 3.2) | 1 (1) | 0.3 (0.0, 2.3) | 0.3 (0.0, 2.2) |
| Other | 2 (7) | 0.9 (0.1, 4.0) | 0.8 (0.1, 2.8) | 1 (4) | 1.0 (0.0, 7.2) | 0.9 (0.0, 6.9) |
| Education completed | ||||||
| High School | 4 (7) | REF | REF | 3 (4) | REF | REF |
| Post high school | 10 (15) | 2.2 (0.6, 10.3) | 1.7 (0.4, 8.4) | 3 (3) | 0.6 (0.1, 4.9) | 0.5 (0.1, 4.1) |
| College | 3 (3) | 0.4 (0.1, 2.5) | 0.4 (0.1, 2.3) | 3 (2) | 0.6 (0.1, 4.2) | 0.5 (0.1, 4.2) |
| Post graduate | 7 (8) | 1.2 (0.3 (5.6) | 1.2 (0.3, 6.2) | 7 (5) | 1.1 (0.3, 7.0) | 1.1 (0.1, 4.1) |
| Yearly Income | ||||||
| >120,000 | 3 (4) | REF | REF | 6 (4) | REF | REF |
| 80–120,000 | 6 (8) | 2.2 (0.5, 14.2) | 1.9 (0.4, 23.5) | 2 (2) | 0.5 (0.1, 2.9) | 0.4 (0.0, 2.2) |
| 40–80,000 | 12 (14) | 4.1 (1.1, 23.7) | 3.2 (0.8, 19.1) | 3 (3) | 0.8 (0.1, 3.7) | 0.6 (0.1, 2.9) |
| <40,000 | 1 (4) | 1.2 (0.0, 15.5) | 1.1 (0.0, 15.6) | 3 (10) | 2.6 (0.4, 13.1) | 2.4 (0.4, 14.5) |
| unknown | 2 (5) | 1.3 (0.1, 11.8) | 0.9 (0.1, 8.8) | 2 (2) | 0.6 (0.1, 3.2) | 0.4 (0.0, 2.2) |
| Smoking history | ||||||
| Never | 14 (6) | REF | REF | 10 (3) | REF | REF |
| Former | 5 (10) | 1.6 (0.4, 5.0) | 1.9 (0.5, 6.6) | 5 (5) | 1.5 (0.4, 4.9) | 1.4 (0.4, 4.7) |
| Current smoker | 5 (16) | 2.9 (0.8, 9.4) | 4.4 (1.0, 16.8) | 1 (2) | 0.6 (0.0, 4.2) | 0.4 (0.0, 3.2) |
| Live Births | ||||||
| 0 | 5 (8) | REF | REF | 1 (1) | REF | REF |
| 1 | 3 (9) | 0.6 (0.1, 2.7) | 0.7 (0.1, 3.1) | 0 (0) | 1.4 (0.1, 19.4) | 1.4 (0.1, 19.8) |
| 2 | 6 (10) | 0.6 (0.2, 1.9) | 0.7 (0.2, 2.2) | 5 (4) | 2.0 (0.3, 21.2) | 2.0 (0.3, 21.6) |
| 3 or more | 10 (7) | 1.0 (0.2, 3.5) | 1.0 (0.2, 3.8) | 10 (5) | 5.5 (1.0, 55.3) | 5.9 (1.1, 60.8) |
| Exogenous Hormone Use | ||||||
| Never/ Former | 13 (7) | REF | REF | 15 (4) | REF | REF |
| Current | 11 (10) | 1.6 (0.6, 4.1) | 1.3 (0.5, 3.4) | 1 (1) | 0.3 (0.0, 1.8) | 0.3 (0.0, 1.8) |
| Lifetime sex partners | ||||||
| < 5 | 5 (5) | REF | REF | 10 (5) | REF | REF |
| ≥ 5 | 19 (9) | 1.9 (0.7, 6.7) | 1.7 (0.6, 6.3) | 6 (2) | 0.5 (0.1, 1.4) | 0.3 (0.1, 1.1) |
| Recent sex partners | ||||||
| Sex, no new partner(s) | 17 (7) | REF | REF | 12 (4) | REF | REF |
| No recent sex | 3 (8) | 1.2 (0.2, 4.3) | 1.2 (0.2, 4.8) | 2 (2) | 0.5 (0.1, 2.1) | 0.4 (0.1, 2.0) |
| Sex with new partner(s) | 3 (23) | 4.1 (0.7, 18.2) | 1.3 (0.2, 7.0) | 2 (20) | 6.7 (0.6, 39.2) | 3.9 (0.3, 26.3) |
| Ever previous abnormality | ||||||
| No | 7 (4) | REF | REF | 8 (3) | REF | REF |
| Yes | 17 (13) | 3.4 (1.3, 9.9) | 2.9 (1.1, 8.9) | 8 (4) | 1.1 (0.4, 3.5) | 0.9 (0.3, 2.9) |
| Time since last abnormal pap1 | ||||||
| No abnormal pap | 7 (4) | REF | REF | 8 (3) | REF | REF |
| 2–5 years | 4 (9) | 2.4 (0.5, 10.1) | 2.3 (0.4, 9.8) | 2 (3) | 0.9 (0.1, 4.6) | 0.8 (0.1, 4.4) |
| 6–10 years | 5 (19) | 5.6 (1.3, 22.6) | 5.4 (1.2, 22.9) | 0 (0) | 0.6 (0.0, 4.1) | 0.6 (0.0, 3.8) |
| >10 years | 4 (7) | 1.8, (0.4, 7.2) | 1.6 (0.3, 6.6) | 0 (0) | 0.2 (0.0, 1.4) | 0.2 (0.0, 1.4) |
| Ever previous colposcopy | ||||||
| No | 11 (5) | REF | REF | 11 (3) | REF | REF |
| Yes | 13 (17) | 4.1 (1.6, 10.5) | 3.2 (1.2, 8.6) | 5 (6) | 2.0 (0.5, 6.6) | 1.7 (0.4, 5.6) |
Adjusted for the presence of HR-HPV (N=744)
Abbreviations: N=number, %=percent, HR-HPV=high-risk human papillomavirus, uOR=unadjusted odds ratio, aOR=adjusted odds ratio, CI=confidence interval, REF=referent group
DISCUSSION
Current cervical cancer screening guidelines group together all women age 30 and above (2, 3, 14). Our data raise important questions about the homogeneity of the interpretation of HPV and Pap test results in this age group. Although the relative proportion of low and high-grade abnormalities did not change with age, we saw a loss of concordance between HR-HPV detection and cytological abnormalities with increasing age. This discordance cannot be attributed to an increase in the proportion of ASCUS relative to higher-grade lesions in older women. ASCUS accounted for 57% of cervical abnormalities in women 35–39 years, 70% in women 40–44, 67% in women 45–49, and 50% in women age 50–54 years. Consistent with our findings, a decrease in the prevalence of HPV among all grades of abnormal cytology was also observed with increasing age in the Kaiser Permanente screening cohort of nearly one million women age 30–64 years (15). For example, among women with ASCUS and LSIL, 52% and 89% of 30–34 year olds compared with 28% and 75% of 60–64 year olds had HPV infection, respectively. An age-specific decline in the prevalence of cervical abnormalities among HPV-positive women was also reported in the Kaiser Permanente screening cohort. Several studies have shown that HPV testing among older women with low-grade abnormalities detected by cytology can help to distinguish between true infections that carry a risk for progression to cervical precancer and other morphological changes that are not associated with risk of cancer (16–18). This is in contrast to the findings from a large US randomized controlled trial, which concluded that there was limited value for HR-HPV triage of low-grade lesions (19). The young age of participants in the ASCUS/LSIL Triage Study (mean, 27.9 years) limited the ability to make strong conclusions about HPV triage of LSIL in women over 30, although they did report a 2-fold lower HR-HPV DNA prevalence in the 18% of LSIL positive women who were over age 30 years.
Although the prevalence of HR-HPV decreased linearly with age among women with cytological abnormalities, the prevalence of any-HPV was U-shaped and highest among older women. This pattern would suggest that both LR-HPV and HR-HPV infection play a role in the detection of cervical abnormalities in older women. Indeed, there was a strong association between infection with only low-risk HPV types and cervical abnormalities in women over 45 years of age (OR: 6.4 (1.5, 24.8)) which was not observed in younger women (OR: 1.7 (0.2, 8.7)). This is consistent with two well-documented observations: the regression of the cervical transformation zone into the os in older women (20) and the higher prevalence of LR-HPV in vaginal compared with cervical epithelium (21).
An advantage of our study was the ability to compare the age-stratified correlates of HPV infection to the correlates of abnormal cytology. The correlates of HPV were generally similar in younger and older women. A notable exception was the association seen only in older women between HPV detection and a higher number of self-reported lifetime sex partners, suggesting that HPV in older women is more likely to represent long-term persistent infection (22, 23). The correlates of HPV were similar to the correlates of cervical abnormalities among younger women aged 30–44 years. However, in older women, the variables we expected to be correlated with both HPV and abnormalities, given the strong causal link between HPV and cervical abnormalities, were only seen for HPV infection. Although these findings are consistent with the observed decreased correlation between HR-HPV infection and cervical abnormalities in older women, the explanation for these differences is not clear.
The overall prevalence of cervical abnormalities in our population was low and no abnormalities were detected among the 116 women age 55–60 years. In addition, most abnormalities were low-grade (ASCUS and LSIL), which is likely due to the low-risk characteristics and high level of prior screening in this population. The relatively small sample size limited our ability to evaluate the significance of the loss of HPV-Pap correlation on the screening performance of either test at older ages. While the low number of abnormal cytology results limits our ability to make firm conclusions, our data suggest that the typical determinants for testing positive for two common markers of neoplasia used in screening (cytology and HPV DNA) may be different in 35–45 vs. 45–60 year old women, and suggest the need for a more detailed review of the specificity of these markers in perimenopausal women. The age-stratified analyses suggest that the abnormalities detected in older women are potentially related to LR-HPV infection or age-related morphological changes, as well as HR-HPV infection. However, we do not have pathologic confirmation to gain a clearer understanding of the differences in the sensitivity and specificity of HPV testing and cervical cytology in women over age 45 years. In the Kaiser Permanente screening cohort, the risk of CIN3+ among HPV−/Pap+ tended to be higher in older compared with younger women, while the risk of CIN3+ decreased with increasing age among HPV+/Pap− women (online supplement (10)).
With the consensus guidelines from the ASCCP recommending Pap smear screening with adjunctive HPV testing in women over 30 years of age, and increasing numbers of women asking for the test, HPV testing in women over 30 is on the rise. Still, controversy remains over the most appropriate use of HPV testing and cytology in older women. Recently, the U.S. Preventive Services Task Force did not include guidelines on the use of HPV testing in primary screening of perimenopausal and older women, citing the lack of evidence on which to base recommendations (14). We found that detection of HR-HPV and cytological abnormalities is not uniform among all women over 30 years of age, and neither is the correlation between these measures of risk of cervical precancer. These data suggest that ongoing surveillance of the performance of HPV and cytology in cervical cancer screening programs should consider a third age stratification at 45 years and above. Castle, et al., previously suggested a similar age-stratified approach for the use of HPV testing in the triage of women 45 to 50 years and older with low-grade abnormalities (15). The relevance of HPV and Pap test performance in older women will increase as cohorts of more highly exposed women age. Therefore, additional follow-up is needed in this cohort to observe the natural history of HPV infection and clarify the clinical relevance of positive HPV and Pap smear testing outcomes in older women.
Acknowledgments
Source of Funding: This work was supported by the US National Cancer Institute R01 CA123467 and the Institutional Research Cancer Epidemiology Fellowship funded by the National Cancer Institute T32 CA0009314. P.E. Gravitt is a member of the Women’s Health Scientific Advisory Board for Qiagen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: For the remaining authors none were declared.
PRESENTED IN PART: Poster presentation at the North American Menopause Society, Washington DC, September 21–24, 2011, “There is a Sustained Decrease in Pap Smear and HPV Concordance with Increasing Age: When Should we Stop Screening the Low Risk Perimenopausal Patient?”
REFERENCES
- 1.Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103(9):714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197(4):346–355. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 3.Clark JL, Lescano AG, Konda KA, Leon SR, Jones FR, Klausner JD, et al. Syndromic management and STI control in urban Peru. PLoS One. 2009;4(9):e7201. doi: 10.1371/journal.pone.0007201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moscicki AB, Shiboski S, Broering J, Powell K, Clayton L, Jay N, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132(2):277–284. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 5.Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health. 2008;43(4 Suppl):S5–S25. S e1–S e41. doi: 10.1016/j.jadohealth.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Watson M, Saraiya M, Benard V, Coughlin SS, Flowers L, Cokkinides V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer. 2008;113(10 Suppl):2855–2864. doi: 10.1002/cncr.23756. [DOI] [PubMed] [Google Scholar]
- 7.Peto J, Gilham C, Deacon J, Taylor C, Evans C, Binns W, et al. Cervical HPV infection and neoplasia in a large population-based prospective study: the Manchester cohort. Br J Cancer. 2004;91(5):942–953. doi: 10.1038/sj.bjc.6602049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102(5):315–324. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright TC, Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206(1):46, e1–e11. doi: 10.1016/j.ajog.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Katki HA, Kinney WK, Fetterman B, Lorey T, Poitras NE, Cheung L, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a populationbased study in routine clinical practice. Lancet Oncol. 2011;12(7):663–672. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marks MA, Viscidi RP, Chang K, Silver M, Burke A, Howard R, et al. Differences in the concentration and correlation of cervical immune markers among HPV positive and negative perimenopausal women. Cytokine. 2011;56(3):798–803. doi: 10.1016/j.cyto.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlee F, Hildesheim A, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38(1):357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coutlee F, Gravitt P, Kornegay J, Hankins C, Richardson H, Lapointe N, et al. Use of PGMY primers in L1 consensus PCR improves detection of human papillomavirus DNA in genital samples. J Clin Microbiol. 2002;40(3):902–907. doi: 10.1128/JCM.40.3.902-907.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Screening for Cervical Cancer: U.S. Preventive Services Task Force Recommendation Statement Draft. United States Preventative Services Task Force; 2011. http://www.uspreventiveservicestaskforce.org/uspstf11/cervcancer/cervcancerrs.htm. [Google Scholar]
- 15.Castle PE, Fetterman B, Thomas Cox J, Shaber R, Poitras N, Lorey T, et al. The age-specific relationships of abnormal cytology and human papillomavirus DNA results to the risk of cervical precancer and cancer. Obstet Gynecol. 2010;116(1):76–84. doi: 10.1097/AOG.0b013e3181e3e719. [DOI] [PubMed] [Google Scholar]
- 16.Ronco G, Cuzick J, Segnan N, Brezzi S, Carozzi F, Folicaldi S, et al. HPV triage for low grade (L-SIL) cytology is appropriate for women over 35 in mass cervical cancer screening using liquid based cytology. Eur J Cancer. 2007;43(3):476–480. doi: 10.1016/j.ejca.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Thrall MJ, Smith DA, Mody DR. Women >or=30 years of age with low grade squamous intraepithelial lesion (LSIL) have low positivity rates when cotested for high-risk human papillomavirus: should we reconsider HPV triage for LSIL in older women? Diagn Cytopathol. 2010;38(6):407–412. doi: 10.1002/dc.21209. [DOI] [PubMed] [Google Scholar]
- 18.Brismar-Wendel S, Froberg M, Hjerpe A, Andersson S, Johansson B. Age-specific prevalence of HPV genotypes in cervical cytology samples with equivocal or low-grade lesions. Br J Cancer. 2009;101(3):511–517. doi: 10.1038/sj.bjc.6605165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. J Natl Cancer Inst. 2000;92(5):397–402. doi: 10.1093/jnci/92.5.397. [DOI] [PubMed] [Google Scholar]
- 20.Castle PE, Jeronimo J, Schiffman M, Herrero R, Rodriguez AC, Bratti MC, et al. Age-related changes of the cervix influence human papillomavirus type distribution. Cancer Res. 2006;66(2):1218–1224. doi: 10.1158/0008-5472.CAN-05-3066. [DOI] [PubMed] [Google Scholar]
- 21.Castle PE, Rodriguez AC, Porras C, Herrero R, Schiffman M, Gonzalez P, et al. A comparison of cervical and vaginal human papillomavirus. Sex Transm Dis. 2007;34(11):849–855. doi: 10.1097/OLQ.0b013e318064c8c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castle PE, Schiffman M, Herrero R, Hildesheim A, Rodriguez AC, Bratti MC, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191(11):1808–1816. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- 23.Maucort-Boulch D, Plummer M, Castle PE, Demuth F, Safaeian M, Wheeler CM, et al. Predictors of human papillomavirus persistence among women with equivocal or mildly abnormal cytology. Int J Cancer. 2010;126(3):684–691. doi: 10.1002/ijc.24752. [DOI] [PubMed] [Google Scholar]


