Opinion statement
Glioblastoma multiforme (GBM) is the most common primary malignant tumor of the central nervous system (CNS) and one of the most lethal cancers in adults and children. Despite aggressive treatment with surgery, radiation, and chemotherapy, median survival is less than 15 months and overall survival is less than 10 % at 5 years. Development of therapeutics for malignant gliomas has been hampered by their natural complexity as well as protective mechanisms unique to the CNS. Better understanding of the pathogenesis of GBM is opening the path to novel, specific-targeted therapies. Recently, multiple immunotherapy approaches have been acquiring substantial indication of therapeutic efficacy with a very safe profile. Examples of the leading clinical approaches for GBM will be discussed in detail in this review.
Keywords: Glioblastoma, Glioblastoma multiforme, Malignant gliomas, Vaccine, Immunotherapy
Introduction
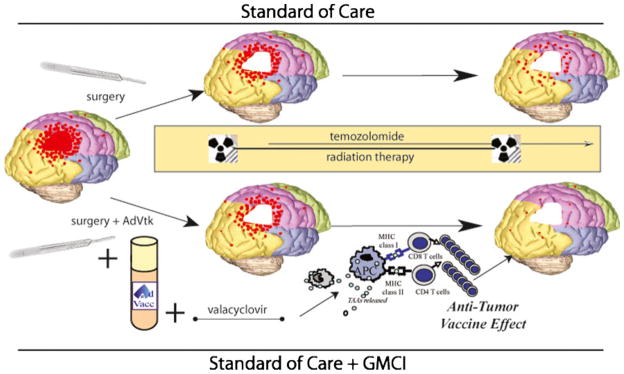
The incidence of brain tumors is increasing and treatment is usually unsuccessful, making this a growing, unmet medical need [1]. In the United States alone, 22,910 new cases and 13,700 deaths from primary nervous system tumors are expected in 2012 (American Cancer Society, 2012). Malignant gliomas represent more than 70 % of these, with a median age at diagnosis of 64 years [2]. Malignant gliomas include WHO Grade III malignant astrocytomas and grade IV tumors, of which GBM are most common [3]. GBM are differentiated histologically by the presence of microvascular proliferation and necrosis. In addition, they are characterized by finger-like infiltration of surrounding brain and even distant diffuse infiltration, making complete surgical resection and curative radiation therapy a challenge (Fig. 1) [4, 5].
Figure 1. Residual infiltrating tumor cells after standard of care treatments may be eliminated by tumor-specific T cells stimulated by immunotherapies, such as gene-mediated cytotoxic immunotherapy (GMCI).
Standard of care treatment for newly diagnosed GBM includes surgical resection followed by radiation and temozolomide chemotherapy. However, progression occurs in more than two thirds of patients within 1 year due to growth of residual, infiltrating tumor cells. Immunotherapies may increase the efficacy of the immune system to fight against this minimal residual disease. GMCI involves injection of AdV-tk to the tumor bed at the time of surgery followed by oral valacyclovir prodrug administration to induce immunogenic tumor cell death. Surgery, radiation, and the viral vector stimulate infiltration of antigen-presenting cells (APC). Tumor-associated antigens (TAA) are presented by MHC class I and II molecules on APCs stimulating tumor-specific CD4 and CD8 T cells. The HSV-tk protein expressed from the AdV-tk vector has a superantigen-like effect that further stimulates proliferation of T cells. Thus, the approach generates an antitumor vaccine effect that may compliment standard of care to improve outcomes for patients with GBM.
Genetic alterations often contribute to the pathogenesis of GBM and may provide insight for developing targeted therapies and targets for immune-mediated therapies. Approximately 5 % of patients with malignant glioma have a family history associated with syndromes, such as neurofibromatosis types 1 and 2, Li-Fraumeni syndrome (germline p53 mutations), and Turcot’s syndrome (intestinal polyposis and brain tumors) [6]. Loss of heterozygosity (LOH) in chromosome 10q is the most common genetic alteration [7]. Epidermal growth factor receptor (EGFR) amplification is found in 40–50 % of primary GBMs [8]. A constitutively active mutant receptor, EGFRvIII, is found in approximately half of EGFR amplified GBMs [8]. This mutant receptor is a potential therapeutic target and is being evaluated in late-stage clinical trials for a peptide-vaccine (described below). GBM have multiple genetic alterations that may serve as targets for recognition by the patients’ immune system.
Molecular factors also may predict prognosis or response to specific therapies. For patients receiving temozolomide chemotherapy, hypermethylation of the O6-methylguanine methyltransferase (MGMT) gene has been associated with improved survival [9]. A CpG island methylator phenotype (CIMP), a phenomenon previously reported in colorectal cancer, has been identified recently for gliomas and is associated with improved prognosis and IDH1 somatic mutations [10]. A gene expression-based molecular classification of GBM was recently reported identifying four subtypes with differing response characteristics: Proneural, Neural, Classical, and Mesenchymal [11]. Aggressive treatment with concurrent chemoradiation was associated with a significant survival advantage only in the Classical and Mesenchymal subtypes. The Mesenchymal class is characterized by necrosis and inflammatory infiltrates, which may have implications for the development of vaccine approaches. It is likely that better genomic characterization of these tumors will lead to better immunotherapeutic selection and targeting, especially for monovalent, single-antigen vaccine approaches.
It is clear that the immune system plays an important role in the development and prognosis of malignant gliomas. Patients with hyperactive immunity, such as atopy and elevated IgE levels, have decreased incidence and mortality from gliomas [6]. Increased levels of CD8+ T-cell infiltration in GBMs has been associated with improved survival [12]. Regulatory T cells (Treg), the “brakes” of an immune response, are found at a higher frequency in GBM than in lower-grade gliomas [13]. In fact, some suggest that chemotherapies, such as temozolomide, may enhance responses to immunotherapy by decreasing the frequency of Treg [14]. Therefore, malignant gliomas are good candidates for immunotherapy approaches.
Enlisting the immune system against cancer has been tried for more than a hundred years with little success. However, the field has evolved drastically in the past decade, and two recent approvals of therapeutic immunotherapy drugs have driven optimism for additional applications: Provenge, an autologous dendritic cell vaccine, for hormone-resistant recurrent prostate cancer [15] and ipilimumab, an antibody that blocks the CTLA4 T-cell regulatory pathway, for metastatic melanoma [16].
Many vaccine approaches are being evaluated for GBM, including 1) dendritic-cell–based vaccines, 2) autologous tumor cells, 3) peptide vaccines, and 4) gene-transfer mediated in situ vaccines. Progress in recent studies with these approaches is described below.
Standard treatment for GBM
Surgery
Surgical resection is the first line of treatment. The extent of resection is an important prognostic factor with a step-wise improvement in survival with more extensive resection [17, 18]. Recently, intraoperative MRI has been used to facilitate more extensive resection while preserving quality of life [19]. However, due to their infiltrative nature, tumor cells remain in the surrounding brain tissue after resection and often lead to tumor recurrence in a matter of months.
Radiation therapy
Due to the remaining tumor cells, the addition of radiation therapy to surgery was shown to increase survival from 3–4 months to 7–12 months [20, 21]. External beam radiation therapy is routinely used with a 2- to 3-cm margin based on studies indicating that most recurrences were within a few centimeters of the enhancing tumor [22]. Median progression-free survival is still less than 6 months [23].
Upon recurrence, GBM also may be treated with radiation, including stereotactic radiosurgery [24] or interstitial brachytherapy. However, both have limited efficacy and the potential for toxicity [25].
Pharmacologic treatment
Temozolomide is the primary chemotherapy used for GBM. It is an oral alkylating agent that was approved for use during (concomitant phase) and after (adjuvant phase) radiation based on a randomized Phase III trial comparing this regimen to radiation alone. The addition of temozolomide led to an improvement in median survival from 12.1 to 14.6 months and an improvement of 2-year survival from 10.9 % to 27.2 % [21]. Tumors with an unmethylated MGMT promoter and consequently high expression of the MGMT protein have a worse prognosis than those with low expression [26]. Efforts to overcome MGMT effects by dose intensification (RTOG 0525) have not been successful [27]. Implantable BCNU (carmustine)-polymer wafers (Gliadel, Eisai, Woodcliff Lake, NJ) were approved as up-front adjuvant treatment based on a modest increase in median survival over placebo (13.8 vs. 11.6 months) [28]. Temozolomide continues to be the most widely used chemotherapy approach for GBM.
For recurrent GBM, there is little that is effective and nothing that is curative. Chemotherapy results in median survivals of less than 6 months [29]. BCNU-wafers also are approved for recurrent malignant glioma patients undergoing surgery [30]. Bevacizumab, an anti-VEGF antibody designed as an anti-angiogenic agent, was approved for recurrent GBM based on an objective response rate of 28 %, decreased corticosteroid requirement, and a 6-month progression-free survival rate of 42.6 % [31]. Two additional Phase 3 trials are evaluating bevacizumab in up-front GBM. Interestingly, bevacizumab appears to change the pattern of recurrence by inhibiting local enhancing tumor recurrence while allowing diffuse infiltrative tumor growth [29].
Emerging immunotherapies for glioblastoma multiforme
Vaccine approaches are an attractive adjuvant therapy for solid tumors due to their potential to generate long-term immune surveillance against cancer cells. The critical components required for stimulating an effective antitumor immune response are target tumor antigens, antigen presenting cells, such as dendritic cells (DCs), and effector lymphocytes, which include T cells and B cells. Effector T cells include CD4+ helper cells, which produce cytokines that stimulate and regulate other immune components and CD8+ cytotoxic T cells (CTL), which can directly kill tumor cells. Innate immune components, such as natural killer (NK) cells, heat shock proteins, and Toll-like receptors also are important. Different forms of antigen delivery/exposure may be used in vaccine approaches to stimulate potent immune responses. Examples of approaches analyzed in clinical studies for GBM are highlighted below and listed in Table 1.
Table 1.
Clinical development of novel vaccines for glioblastoma multiforme
| Institution/Sponsor | Indication* | Restrictions Phase Status | Source of Information | ||
|---|---|---|---|---|---|
| Dendritic cells with autologous tumor | |||||
| University of California, Los Angeles | Primary malignant glioma | Resection | II | Recruiting | Clinicaltrials.gov |
| Northwest Biotherapeutics | Primary GBM | Resection | III | Recruiting | Clinicaltrials.gov |
| Cedars-Sinai Medical Center, Los Angeles | Primary and recurrent GBM | Resection | II | Completed | Wheeler et al, 2008 |
| Catholic University of Leuven, Belgium | Recurrent GBM | Resection | II | Completed | De Vleeschouwer et al, 2008 |
| Niigata University, Japan | Recurrent malignant gliomas | Resection | I/II | Completed | Yamanaka et al, 2005 |
| Duke University | Recurrent GBM | Resection | I | Recruiting | Clinicaltrials.gov |
| Dendritic Cells with Peptides | |||||
| University of Pittsburgh, Pennsylvania | Recurrent GBM | HLA-A2 | I/II | Completed | Okada et al, 2011 |
| Children’s Hospital of Pittsburgh | Pediatric primary or recurrent glioma | HLA-A2 | I | Recruiting | Clinicaltrials.gov |
| ImmunoCellular Therapeutics | Primary GBM | HLA-A1 or A2 | IIb | Recruiting | Clinicaltrials.gov |
| Autologous tumor cells | |||||
| Tokyo Women’s Medical University, Japan | Primary GBM | Resection | I/IIa | Completed | Muragaki et al, 2011 |
| Peptide | |||||
| Celldex Therapeutics | Primary GBM | EGFRvIII+ tumor | III | Recruiting | Clinicaltrials.gov |
| Celldex Therapeutics | Recurrent GBM | EGFRvIII + tumor | II | Recruiting | Clinicaltrials.gov |
| Cancer Research UK/immatics Biotechnologies GmbH | Primary GBM | HLA-A*02 | I | Recruiting | Clinicaltrials.gov |
| GMCI | |||||
| Advantagene Inc. | Primary malignant glioma | Accessible tumor | II | Active, not recruiting | Clinicaltrials.gov |
| Dana Farber Cancer Institute, Boston | Pediatric malignant glioma | Accessible tumor | I | Recruiting | Clinicaltrials.gov |
GBM glioblastoma multiforme; EGFRvIII endothelial growth factor receptor variant III; HLA-Human leukocyte antigen
Primary refers to first diagnosis prior to recurrence
Using dendritic cells loaded with autologous tumor or peptides
The concept is to preload antigen-presenting cells with tumor associated antigens and return them to the patient so that they can stimulate T cells that recognize those antigens. Multiple groups have evaluated DC approaches for gliomas [5]. Large numbers of peripheral blood mononuclear cells must be collected from the patient, usually via leukapheresis, cultured with cytokines, and then loaded with antigen. The antigen source may be DNA, RNA, or proteins, single antigens or autologous tumor cells or tumor cell proteins. For the latter two, surgery is required to obtain sufficient tumor cells, but, unlike single peptides, these approaches have the potential to elicit a polyvalent immune response against many of the patient’s own tumor associated antigens.
Results of studies using dendritic cells loaded with autologous tumor
Prins et al. (2011) at University of California, Los Angeles (UCLA) [32•]
Vaccine type
DCs loaded with autologous tumor lysate. Immunoadjuvant: either imiquimod or poly-ICLC, administered with the DC vaccine during the booster phase.
Study design
Phase I dose-escalation, adult newly diagnosed or recurrent GBM. Three dose levels were compared: 1, 5, and 10×106 DC/dose injected intradermally. Initial phase: three biweekly vaccinations. Booster phase: every 3 months up to 10 times or until tumor progression.
Patient population
N=23; 15 newly diagnosed and 8 recurrent. The mean age was 51 years (range, 26–74) and Karnofsky Performance Score (KPS) was 60–100.
Immune response
Serum IL-6 and TNF-α levels increased after DC vaccination and to greater extent after booster vaccines with adjuvant. CD3+ and CD8+ T-cell infiltration was increased after vaccination in tumors resected or biopsied at recurrence. This increase was associated with the mesenchymal gene expression signature but not with the dose of DC.
Clinical Outcomes
There were no grade 3 or 4 adverse events. The most common adverse events associated with the vaccinations were injection-site reactions and flu-like symptoms. Interestingly, patients with the mesenchymal gene expression signature (n=9) had significantly better survival than a randomly selected control mesenchymal group (n=82), whereas no difference was seen in patients with the proneural signature. These data suggest a potential differential responsiveness to vaccine therapy that may be related to gene expression, such as in the mesenchymal gene expression signature.
Wheeler et al. (2008) Cedars-Sinai Medical Center, Los Angeles [33]
Vaccine type
DCs loaded with autologous tumor lysate.
Study design
Phase II, adult newly diagnosed or recurrent GBM. The dose was 1 to 4 × 107 DCs injected subcutaneously. DC vaccination started approximately 15 weeks after surgery with 3 biweekly vaccinations and a fourth 6 weeks after the third.
Patient population
N=34; 11 newly diagnosed and 23 recurrent. Ages ranged from 22–74 years.
Immune response
Immunologic responders (17/32 patients tested) were defined as patients whose levels of interferon (IFN)-γ RNA expression from peripheral blood cells increased ≥1.5-fold compared with baseline.
Clinical outcomes
There were no grade 3 or 4 toxicities associated with the vaccinations. Time to progression (TTP) and overall survival (OS) was significantly longer in patients who had a positive immunological response (n=17) than in nonresponders (n =15). The immunologic responders and nonresponders had no significant differences in age, KPS, or extent of resection, but the responders did have a significantly higher number of newly diagnosed (47 %) compared with the nonresponders (20 %). In the recurrent patient population, 2-year survival was significantly higher for the immunologic responders (5/9, 56 %) than for the nonresponders (1/12, 8 %). In responders, there was a loose correlation between the level of IFN-γ expression and survival. For TTP, this correlation also existed for the interval of postvaccination recurrence to subsequent progression during which chemotherapy was administered, suggesting that immune responders may have an improved response to subsequent chemotherapy.
De Vleeschouwer et al. (2008) Catholic University of Leuven, Belgium [34]
Vaccine type
DCs loaded with autologous tumor lysate and matured with TNF-α, IL-1β, and PGE2.
Study design
Phase II; pediatric and adult patients with recurrent GBM. DCs were injected intradermally. Three cohorts with different vaccine schedules were compared: cohort A, 2 DC vaccines given at weeks 1 and 3 after surgery and then every 4 weeks until progression; cohort B, 5 DC vaccines every 2 weeks and then additional DC vaccines every 4 weeks until progression; cohort C, 4 weekly DC vaccinations and then additional boosts with tumor lysate without DCs every 4 weeks.
Patient population
N=56. Median age was 45 years (range, 7–77) years, and there was a trend toward a larger pediatric population on cohort A. Median KPS was 80 (range, 50–100).
Immune response
Delayed type hypersensitivity (DTH) skin tests using tumor lysate were positive in 9 of 17 patients after vaccination compared with 9 of 21 patients at diagnosis. There was no correlation reported between DTH results and PFS or OS.
Clinical outcomes
Adverse events were reported as mild except for one patient who developed grade 4 neurotoxicity associated with perilesional edema necessitating corticosteroid treatment. Median OS from time of reoperation for all cohorts (n=56) was 9.6 months; at 12, 24, and 36 months, OS was 37.4 %, 14 %, and 11.1 %, respectively. Comparison of outcomes for adult patients of the three cohorts revealed a significantly improved PFS and a trend toward better survival in cohort C.
Yamanaka et al. (2005) Niigata University School of Medicine, Japan [35]
Vaccine type
DCs loaded with autologous tumor lysate and the immunoadjuvant Keyhole Limpet Hemocyanin (KLH). In addition, in seven patients, half of the DCs were matured with penicillin-killed Streptococcus pyogenes (OK-432), a vaccine adjuvant approved in Japan that induces DC maturation via the toll-like receptor (TLR) pathway [36].
Study design
Phase I/II; adult recurrent malignant glioma. DCs were injected intradermally, close to a cervical lymph node, every 3 weeks for up to 10 vaccinations. In 11 patients, DCs with KLH also were injected intratumorally via an Ommaya reservoir.
Patient population
N=24, 18 with GBM and 6 with WHO grade III malignant gliomas. Mean age was 48.9 years (range, 20–80). The mean number of vaccinations was 7.4 (range, 1–22) intradermally and 4.6 (range, 1–18) intratumorally.
Immune response
DTH with tumor lysate was positive in 8 of 17 patients tested. Tumor lysate reactive IFN-γ producing T cells in peripheral blood (ELISPOT assay) were increased after vaccination in 6 of 16 patients tested.
Clinical outcomes
There were no significant adverse events related to the vaccinations. GBM patients who received DCs matured with OK-432 (n=7) had longer survival (p=0.027) than GBM patients who received DCs without OK-432 (n=11). GBM patients in the intratumoral/intradermal group (n=7) had longer survival (p=0.042) than GBM patients with only intradermal injection (n=11). In addition, GBM patients with a positive immunologic response to autologous tumor (DTH or ELISPOT assay) had significantly increased survival compared with those without a response. The GBM patients (n=18) were compared with a matched control group (n=27) and found to have increased median overall survival (480 vs. 400 days) and increased 2-year survival (23.5 % vs. 3.7 %), respectively.
Results of study using dendritic cells loaded with peptides
Peptides are an alternative source of antigens for DC-based vaccines. The peptides are synthesized and thus do not require autologous tumor collection and processing. However, the number of antigen targets for the immune response is limited, making tumor escape a potential problem. In addition, trials using peptide antigens usually require restriction to one or a few HLA types.
Okada et al. (2011) University of Pittsburgh [37••]
Vaccine type
α-type 1 polarized DCs (αDC1) loaded with synthetic HLA-A2 restricted peptides for 4 glioma-associated antigens (GAA). αDC1 are DCs matured with cytokines, in this case IL-1β, TNF-α, IFN-α, and IFN-γ, and polyinosinic:polycytidylic acid (poly I:C). αDC1 are able to produce high levels of interleukin-12 and induce type-1T-cell responses. An immunoadjuvant, polyI:C stabilized by lysine and carboxymethylcellulose (poly-ICLC) also was administered. Poly-ICLC is a TLR agonist that enhances vaccine efficacy in mouse glioma tumor models [38].
Study design
Phase I/II; adult recurrent malignant gliomas restricted to HLA-A2+ patients. Two dose levels: 1×107 and 3×107 αDC1/dose administered by intranodal injection every 2 weeks×4 and additional booster vaccinations until progression. Poly-ICLC (20 μg/kg) was administered by intramuscular (IM) injection twice per week for 8 weeks.
Patient population
N=22, 11 on each of the two dose levels, 13 with GBM, and 9 WHO grade III. All received at least one vaccination, 19 completed the initial four-vaccine schedule, 9 completed five additional booster vaccinations. Median age was 48 years (range, 28–71).
Immune response
T-cell responses to the peptides used in the vaccine were evaluated by IFN-γ ELISPOT and/or tetramer assay comparing the frequency of reactive T cells in peripheral blood before and after vaccination. Positive responses to at least one peptide were found after four vaccinations in six of ten and five of nine patients in dose levels 1 and 2, respectively. Three additional patients developed T-cell responses after booster vaccines. Upregulation of mRNA expression for type 1 cytokines and chemokines, specifically IFN-α1, CXCL10, and TLR3, was found in peripheral blood cells after the first and fourth DC vaccinations. IFN-γ increased only after the fourth vaccination. Serum protein levels of IFN-α, CXCL10, IL-15, MCP-1, and MIP-1β increased after vaccination. Three of five tumors evaluated after progression expressed mRNA for CXCL10, a chemokine important in trafficking of CD8+ T cells.
Clinical outcomes
There were no significant toxicities. Common side effects were injection site reactions and transient flu-like symptoms. Two patients with GBM had response on MRI. One had complete response at week 17 that was durable for at least 13 months. The other had partial response at week 9 and pseudo-progression after 2 booster vaccines when biopsy revealed intense infiltration of CD8+ T cells and CD68+ macrophages without mitotically active tumor; the patient subsequently developed recurrence. Both of these patients also had positive T-cell responses. Median time to progression was 4 months for GBM and 13 months for grade III gliomas. At the time of publication, 9 patients (4 GBM and 5 grade III) were progression-free for at least 12 months.
In summary, DCs mixed ex vivo with autologous tumors or peptides have consistently given encouraging results. Although there is high variability and conflicting data from immune response analyses, this approach is promising for future advances.
Using direct tumor cell or peptide immunization
Another approach to stimulate a vaccine effect is to use killed autologous tumor cells or peptides mixed with an adjuvant to stimulate the immune response. In this case, antigen presentation is done by antigen presenting cells at the injection site, putatively stimulated by the co-delivered adjuvant. This approach could avoid the extra logistics and variables of DC isolation and culture but may not be as reliable or potent.
Results of study using autologous tumor cells
Muragaki et al. (2011) Tokyo Women’s Medical University, Japan [39•]
Vaccine type
Autologous formalin-fixed tumor vaccine (AFTV) admixed with Bacillus-Calmette Guérin (BCG) derived adjuvant.
Study design
Phase I/IIa; newly diagnosed GBM in combination with radiation therapy. Standard fractionated radiotherapy (2 Gy per fraction to total dose of 60 Gy) was started 2–3 weeks after resection. When radiation reached 32–36 Gy, AFTV injections were given intradermally once per week for 3 weeks.
Patient population
N=24, 22 evaluable. Median age was 58 years (range, 18–70), median pre-operative KPS was 90, and 73 % had complete surgical resection.
Immune response
DTH tests were performed to AFTV without the BCG adjuvant at baseline and 2 weeks after the third vaccination.
Clinical outcomes
The most common vaccine-related adverse events were mild injection site reactions, but no adverse events greater than grade 1 were reported. Median OS was 21.4 months and actuarial 2-year survival rate was 40 %. Median PFS was 7.6 months. Patients with a positive DTH response (55 %) had significantly longer PFS: 13.9 months vs. 4.3 months. OS trended longer but did not meet statistical significance.
Results of studies using peptide vaccines
Sampson et al. (2010 and 2011) Duke University and MD Anderson Cancer Center [40••, 41••]
Vaccine type
EGFRvIII antigen peptide conjugated to KLH (PEPvIII-KLH) administered with GM-CSF.
Study design
Two Phase II studies; newly diagnosed adult GBM. Restricted to EGFR-vIII–expressing tumors that had gross total resection, without radiographic evidence of progression on MRI 2–4 weeks after completion of chemo-radiation and KPS ≥80.
First study—ACTIVATE: three vaccinations at 2-week intervals starting 4 weeks after completion of radiation and continued monthly until tumor progression without adjuvant temozolomide [40••].
Second study—ACT II: three vaccinations at 2-week intervals starting after chemoradiation completion and before adjuvant temozolomide. Subsequent vaccinations were given on day 21 of each 28-day cycle of temozolomide until tumor progression [41••]. Two cohorts comparing varying temozolomide doses: standard dose (STD, 200 mg/m2 per day for the first 5 days of each 28-day cycle) and dose-intensified (DI, 100 mg/m2 per day for 21 days of each 28-day cycle).
Patient population
ACTIVATE: N=21 of which 3 patients were not included in the analysis due to <95 % tumor resection. Median age was 52 years (range, 29–67).ACT II: N=22, 12 on the STD cohort and 10 on the DI cohort. All patients received at least one vaccination. Mean age was 57 years (range, 41–83).
Immune response
ACTIVATE: Of 14 patients tested for antibody response to the EGFRvIII peptide, 6 (43 %) were positive. Of 17 patients tested for DTH, 3 (18 %) were positive. A trend was observed in longer survival for those with positive antibody response and longer PFS and survival for those with positive DTH.
ACT II: All 22 patients developed positive antibody responses that increased over time to a higher titer in the DI cohort. At the time of the eighth vaccination, DTH responses were positive in seven of eight (87.5 %) of the DI cohort and zero of five in the STD cohort.
Clinical outcomes
ACTIVATE: One patient had a presumed severe allergic reaction, with perioral numbness and tingling. No other grade 3 or 4 adverse events related to vaccination were reported. Median PFS and OS were 14.2 and 26 months, respectively. The PFS and OS were not significantly different between patients with unmethylated and methylated MGMT.
ACT II: Four patients in the DI cohort had possible allergic reactions to the vaccinations. Sustained lymphopenia of grade 2 in the STD cohort and grade 3 in the DI cohort occurred. There was no significant difference in PFS or OS between the STD and DI cohorts. Median PFS and OS were 15.2 and 23.6 months, respectively.
In tumors recurring after vaccination and available for analysis of EGFRvIII-expression, 20 of 23 (87 %) had lost EGFRvIII-expression. This suggests that the vaccine successfully lead to elimination of EGFRvIII-expressing tumor cells but also demonstrates that a tumor vaccine with a single antigen can lead to selection of nonexpressing tumor cells.
Direct immunization with autologous cell or peptides with adjuvants have consistently given immune responses, but the translation to survival impact remains to be shown. The EGFRvIII study clearly demonstrated the efficacy of immunization as well as the risk of tumor escape if the vaccine is monovalent.
Using gene-mediated immunization
Gene transfer can be used to create a vaccine in-situ by direct transfer of a specific antigenic molecule, transfer of immune-modulating molecules, such as cytokines, or by the creation of conditions to generate a local immune response. The latter can generate a polyvalent immune response to autologous tumor without requiring ex vivo processing of patient’s cells [42••]. Gene-mediated cytotoxic immunotherapy (GMCI) is a specific example of generating the local conditions for a response (Fig. 1). It uses an adenoviral vector (AdV-tk) expressing the herpes simplex virus thymidine kinase gene (HSV-tk) followed by an antiherpetic prodrug, such as valacyclovir, in combination with standard of care debulking therapies, such as radiation or surgery [42••]. The HSV-tk protein participates in killing tumor cells and in the broad-spectrum activation of effector T cells through induction of cytokine expression. This generates a local immunostimulatory milieu that stimulates a systemic antitumor immune response to the released autologous tumor associated antigens. The approach has shown synergy with radiation, surgery, and chemotherapy in animal models. A Phase 3 trial was recently initiated in prostate cancer based on a threefold decrease in recurrence in a Phase 2 study. Studies in other tumor types, including malignant glioma, are ongoing.
Results of studies using gene transfer technology
Chiocca et al. (2011) OSU, Columbus, OH and The Methodist Hospital, Houston, TX [43••]
Vaccine type
Replication-defective adenoviral vector (AdV-tk) delivered by injection into the tumor bed at the time of surgery, followed by oral antiherpetic prodrug, valacyclovir.
Study design
Phase Ib, dose-escalation; adult newly diagnosed malignant gliomas. Three dose levels of AdV-tk were evaluated, 3×1010, 1×1011, and 3×1011 vector particles, followed by 14 days of valacyclovir. Radiation was started 1 week after AdV-tk injection and temozolomide was administered after completing valacyclovir.
Patient population
Thirteen patients were enrolled; 12 were evaluable, 10 with GBM and 2 WHO grade III. Three patients each on dose levels 1 and 2 and six on dose level 3. Median age was 59.5 years (range, 41–72), and median KPS was 90 (range, 70–100).
Immune response
In four of four cases analyzed, significant CD3+ T cell CD68+ macrophages infiltration was found after AdV-tk injection. These were predominantly CD8+ in one case analyzed for T-cell subsets.
Clinical outcomes
There were no dose-limiting toxicities and no complications related to starting radiation within 7 days of surgery. Survival was 33 % at 2 years and 25 % at 3 years. There did not appear to be a correlation between MGMT methylation and survival; the longest surviving GBM patient had unmethylated MGMT and survived for 46.4 months. Three patients developed pseudoprogression that gradually resolved. Quality of life assessed by FACT-Br questionnaire was stable or improved after treatment.
Gene transfer technology creates the potential for generating an in situ patient specific, polyvalent vaccine. If successful, this could greatly simplify the logistics of vaccine production compared with autologous DC or killed-tumor vaccines and make it more difficult for the tumor to escape immune surveillance.
Other approaches in development
Additional clinical trials are ongoing with the approaches described as well as some for which data are not yet published (Table 1). Two pediatric studies are in progress: one at Children’s Hospital of Pittsburgh, using dendritic cells loaded with peptides similar to the Okada et al. adult study; the other is at Dana Farber Cancer Institute/Boston Children’s Hospital using the described GMCI approach, similar to the Chiocca et al. adult study. At Duke University, a Phase I study is evaluating DCs loaded with autologous brain tumor stem cell mRNA and ImmunoCellular Therapeutics has a Phase II study underway with DCs loaded with a set of synthetic peptides. Cancer Research UK along with Immatics Biotechnologies are testing a multipeptide vaccine, IMA950, in two Phase I studies. Additional approaches for gliomas not considered vaccines but using similar mechanisms or components are adoptive T-cell therapy and replication conditional viruses used to kill tumor cells (virotherapy).
Future success in therapy for GBM will likely require a combination approach adding to current multimodality therapies. Immunotherapy has the potential to harness the immune system as an additional weapon but is unlikely to be successful as a monotherapy. Its application is most likely to succeed as complimentary to standard of care, especially since its toxicity is minimal. The studies described above provide encouraging data on immune responses and clinical outcomes. Randomized, controlled trials are required to assess efficacy.
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
Contributor Information
Laura K. Aguilar, Email: lkaguilar@advantagene.com.
Mariel Arvizu, Email: marvizu@advantagene.com.
Estuardo Aguilar-Cordova, Email: eaguilar@advantagene.com.
E. Antonio Chiocca, Email: EACHIOCCA@PARTNERS.ORG.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977–2000. Cancer. 2004;101:2293–9. doi: 10.1002/cncr.20621. [DOI] [PubMed] [Google Scholar]
- 2.Kohler B, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Canc Inst. 2011;103:714–36. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59:1169–80. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]
- 5.Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol. 2011;2011:732413. doi: 10.1155/2011/732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 7.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–53. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sathornsumetee S, et al. Molecularly targeted therapy for malignant glioma. Cancer. 2007;110:13–24. doi: 10.1002/cncr.22741. [DOI] [PubMed] [Google Scholar]
- 9.Hegi ME, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26:4189–99. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 10.Noushmehr H, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Canc Cell. 2010;17:510–22. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhaak RGW, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Canc Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang I, et al. CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J Clin Neurosci. 2010;17:1381–5. doi: 10.1016/j.jocn.2010.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heimberger AB, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14:5166–72. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 14.Jordan JT, et al. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Canc Immunol Immunother. 2008;57:123–31. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 16.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stummer W, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 18.Sanai N, Polley M-Y, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 19.Kubben PL, et al. Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol. 2011;12:1062–70. doi: 10.1016/S1470-2045(11)70130-9. [DOI] [PubMed] [Google Scholar]
- 20.Walker MD, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333–43. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 21.Stupp R, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 22.Halperin EC, Bentel G, Heinz ER, Burger PC. Radiation therapy treatment planning in supratentorial glioblastoma multiforme: an analysis based on post mortem topographic anatomy with CT correlations. Int J Radiat Oncol Biol Phys. 1989;17:1347–50. doi: 10.1016/0360-3016(89)90548-8. [DOI] [PubMed] [Google Scholar]
- 23.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 24.Butowski NA, Sneed PK, Chang SM. Diagnosis and treatment of recurrent high-grade astrocytoma. J Clin Oncol. 2006;24:1273–80. doi: 10.1200/JCO.2005.04.7522. [DOI] [PubMed] [Google Scholar]
- 25.Chen AM, et al. Phase I trial of gross total resection, permanent iodine-125 brachytherapy, and hyper-fractionated radiotherapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2007;69:825–30. doi: 10.1016/j.ijrobp.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 26.Hegi ME, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 27.Ahluwalia MS. American Society of Clinical Oncology 2011 CNS tumors update. Expert Rev Anticancer Ther. 2011;11:1495–7. doi: 10.1586/era.11.151. [DOI] [PubMed] [Google Scholar]
- 28.Westphal M, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norden D, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–87. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 30.Perry J, Chambers A, Spithoff K, Laperriere N. Gliadel wafers in the treatment of malignant glioma: a systematic review. Curr Oncol. 2007;14:189–94. doi: 10.3747/co.2007.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman HS, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 32•.Prins RM, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–15. doi: 10.1158/1078-0432.CCR-10-2563. This study describes a correlation between gene expression profile and response to vaccination with dendritic cells loaded with tumor lysate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheeler CJ, et al. Vaccination Elicits Correlated Immune and Clinical Responses in Glioblastoma Multiforme Patients. Cancer Res. 2008;68:5955–64. doi: 10.1158/0008-5472.CAN-07-5973. [DOI] [PubMed] [Google Scholar]
- 34.De Vleeschouwer S, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098–104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- 35.Yamanaka R, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–7. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- 36.Ryoma Y, et al. Biological effect of OK-432 (picibanil) and possible application to dendritic cell therapy. Anticancer Res. 2004;24:3295–301. [PubMed] [Google Scholar]
- 37••.Okada H, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–6. doi: 10.1200/JCO.2010.30.7744. This paper describes results of a trial using dendritic cells loaded with synthetic peptides for glioma antigens and an immunoadjuvant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu X, et al. Toll like receptor-3 ligand poly-ICLC promotes the efficacy of peripheral vaccinations with tumor antigen-derived peptide epitopes in murine CNS tumor models. J Transl Med. 2007;5:10. doi: 10.1186/1479-5876-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Muragaki Y, et al. Phase I/IIa trial of autologous formalin-fixed tumor vaccine concomitant with fractionated radiotherapy for newly diagnosed glioblastoma. Clinical article. J Neurosurg. 2011;115:248–55. doi: 10.3171/2011.4.JNS10377. This article describes results of a trial using autologous tumor cells as a vaccine for GBM. [DOI] [PubMed] [Google Scholar]
- 40••.Sampson JH, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–9. doi: 10.1200/JCO.2010.28.6963. This paper describes results of immune response and clinical outcome in a Phase II trial with an EGFRvIII peptide vaccine. The interesting observation is reported that the majority of tumors recurring after vaccination had lost EGFRvIII expression is reported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Sampson JH, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13:324–33. doi: 10.1093/neuonc/noq157. This study demonstrates the ability to induce immune responses that eliminate tumor cells even when vaccine is administered in the setting of lymphopenia induced by temozolomide chemotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Aguilar LK, Guzik BW, Aguilar-Cordova E. Cytotoxic immunotherapy strategies for cancer: mechanisms and clinical development. J Cell Biochem. 2011;112:1969–77. doi: 10.1002/jcb.23126. This article provides a review of the immune effects of the AdV-tk approach for cancer. [DOI] [PubMed] [Google Scholar]
- 43••.Chiocca EA, et al. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29:3611–9. doi: 10.1200/JCO.2011.35.5222. This article describes results of a trial using AdV-tk with radiation in newly diagnosed malignant glioma. [DOI] [PMC free article] [PubMed] [Google Scholar]