Abstract
Abstract
Even a short blockade of oxygen flow in brain may lead to the inhibition of oxidative phosphorylation and depletion of cellular ATP, which results in profound deficiencies in cellular function. Following ischemia, dying, injured, and hypoxic cells release soluble purine-nucleotide and -nucleoside pools. Growing evidence suggests that purine nucleosides might act as trophic factors in the CNS and PNS. In addition to equilibrative nucleoside transporters (ENTs) regulating purine nucleoside concentrations intra- and extracellularly, specific extracellular receptor subtypes for these compounds are expressed on neurons, glia, and endothelial cells, mediating stunningly diverse effects. Such effects range from induction of cell differentiation, apoptosis, mitogenesis, and morphogenetic changes, to stimulation of synthesis and/or release of cytokines and neurotrophic factors under both physiological and pathological conditions. Multiple signaling pathways regulate the critical balance between cell death and survival in hypoxia–ischemia. A convergent pathway for the regulation of multiple modalities involved in O2 sensing is the mitogen activated protein kinase (p42/44 MAPK) or (ERK1/2 extracellular signal-regulated kinases) pathway terminating in a variety of transcription factors, for example, hypoxia-inducible factor 1α. In this review, the coherence of purine nucleoside-related pathways and MAPK activation in the endogenous neuroprotective regulation of the nervous system's development and neuroplasticity under hypoxic stress will be discussed.
Keywords: adenosine, guanosine, hypoxia, inosine, neuron, purine nucleosides
Hypoxia in brain
In acute neurological conditions such as stroke severe injuries to the CNS occur (Honig and Rosenberg 2000) and stroke is the second most common cause of death and a major cause of long-term disability worldwide (Macrez et al. 2011). Hippocampus and cerebellar cortex are particularly sensitive to ischemia (Yue et al. 1997). Hypoxic–ischemic insult generally causes necrosis, although in most cases there exists also a process of delayed and apoptotic type injury in the region (penumbra) surrounding the area of most severe damage (Honig and Rosenberg 2000; Yuan and Yankner 2000; Schaller et al. 2003; Lo 2008). Lately, it was considered that this degeneration might be better regarded as an 'apoptosis-necrosis cell death continuum' (Northington et al. 2011). Neurons in the adult mammalian CNS, which are injured by stroke normally fail or have only limited ability to regenerate axons, which causes long lasting disabilities in sensory, motor, or cognitive functions (Benowitz and Carmichael 2010). In addition, cell death in the brain leads to the subsequent release of endogenous molecules termed 'damage-associated molecular patterns' from dying cells, triggering further cascades of inflammatory events that both have deleterious but also beneficial effects (Sitkovsky et al. 2004; Chen and Nunez 2010). As an immediate result, a disrupted microcirculation leads to local tissue hypoxia associated with an impaired adenosine 5′-triphosphate (ATP) production and energy status of neurons and glia. This is the basis of further insults including increased calcium, release of glutamate, synthesis of enzymes involved in free radical production and the accumulation of leukocytes (Barone and Feuerstein 1999; Lipton 1999; White et al. 2000; Hertz 2008) (Fig. 1). In the hope to improve clinical outcome after stroke, remarkable progress in understanding its pathophysiology has been made in the past 10 years and basic research yielded numerous pharmacologic agents leading to the identification of more than 1000 molecules with brain-protective effects from experimental models and to the implementation of more than 250 clinical trials. However, none has so far successfully completed phase III clinical development and the only acute pharmacological treatment approved to date is tissue plasminogen activator and aspirin, other antiplatelets, and anticoagulants are used as preventative therapy (Young et al. 2007; Ginsberg 2009; Moskowitz et al. 2010; Albers et al. 2011; Macrez et al. 2011).
Figure 1.
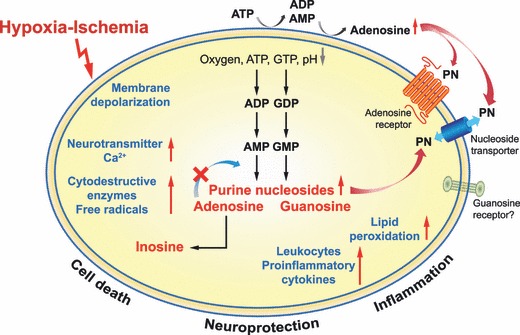
Biochemistry of ischemia–reperfusion injury. Hypoxic–ischemic brain injury starts with the insult but extends into a recovery-reperfusion period (Barone and Feuerstein 1999; Lipton 1999; White et al. 2000; Hertz 2008; Macrez et al. 2011). In case of prolonged ischemia, restricted blood flow leads to a reduction in ATP, causing severe impairment of cellular function by disruption of ATP-dependent processes. A key incidence is the increase in intracellular calcium, which is responsible for the release of neurotransmitters such as glutamate and the activation of many cytocidal enzymes. Activated endonucleases then lead to DNA damage and apoptosis. Though restoration of seized blood flow and oxygen delivery is essential for organ survival, damage is potentially amplified during this period by oxygen sensitive mechanisms, for example, by the activity of pro-inflammatory cytokines (Barone and Feuerstein 1999; Lipton 1999; White et al. 2000; Hertz 2008; Macrez et al. 2011). In parallel, hypoxia leads to the decreased production and enhanced breakdown of purine nucleotides to purine nucleosides (PN) (Jurkowitz et al. 1998; Sitkovsky et al. 2004; Fredholm et al. 2007; Fredholm 2010), which may enter/leave cells via bidirectional nucleoside transporters (ENTs) or in the case of adenosine and inosine directly bind to adenosine receptors (Fredholm et al. 1994, 2001a; Schulte and Fredholm 2003b). To date it is not clear, whether the protective effect of guanosine is at least partly arbitrated by adenosine or adenosine receptors (Ciccarelli et al. 2000; D'Alimonte et al. 2007), or is mediated by its own specific G-coupled receptors (Traversa et al. 2003; Rathbone et al. 2008).
Purine nucleosides in hypoxia
Following hypoxia–ischemia, dying, injured, and hypoxic cells release soluble purine- nucleotide and -nucleoside pools (Ciccarelli et al. 1999; Dale et al. 2000; Latini and Pedata 2001), normally regulated by ENTs, ectonucleotidases and ecto-adenosine deaminase (Delaney et al. 1998; Zimmermann et al. 1998; Dunwiddie and Masino 2001; Frenguelli et al. 2003; Fredholm et al. 2011; Ipata et al. 2011; Zhang et al. 2011). Purine nucleoside-mediated effects in hypoxia are therefore exceptionally interesting due to their endogenous regulatory mechanisms in stress situations. Growing evidence suggests that purine nucleosides, which may remain elevated for days after the insult (Uemura et al. 1991), might also act as trophic factors in both the CNS and PNS (Neary et al. 1996; Rathbone et al. 1999). In addition to ENTs regulating purine nucleoside concentrations, specific extracellular receptor subtypes for these compounds are expressed on neurons, glia, and endothelial cells, where they mediate strikingly different effects. Such effects range from induction of cell differentiation, apoptosis, mitogenesis, and morphogenetic changes, to stimulation of synthesis and/or release of cytokines and neurotrophic factors under both physiological and pathological conditions (Fields and Burnstock 2006; Burnstock 2008). Nucleosides, for example, adenosine, inosine and guanosine are therefore likely to be involved in the regulation of the nervous system's development and plasticity (Neary et al. 1996).
Adenosine is formed by stepwise dephosphorylation of ATP (Zimmermann 2000) and to a minor extent from hydrolysis of S-adenosyl homocysteine (Deussen et al. 1989). Normally, it is present in body fluids in concentrations 20–200 nM but in response to stress, for example, hypoxia, ischemia, inflammation and trauma, elevated levels of adenosine, up to 300 μM are produced and released (Fredholm et al. 2001a; Fredholm 2007, 2010; Burnstock 2008; Lopes et al. 2011). This observed increase in extracellular adenosine is due to a decreased production of intracellular ATP, accumulation of AMP, enhanced dephosphorylation of adenine nucleotides to adenosine by cytosolic-5′-nucleotidase and inhibition of adenosine kinase (Sitkovsky et al. 2004; Sitkovsky 2009) and liberation from cells via nucleoside transporters (Pastor-Anglada et al. 2001). However, extracellular adenosine accumulates through the activities of local tissue hypoxia-up-regulated ectonucleotidase activity (Braun et al. 1998) (Fig. 1). In conditions of profound hypoxia these could depict significant sources of extracellular adenosine, which was shown in other cell systems to generate immunosuppressive loops (Ohta and Sitkovsky 2001; Sitkovsky et al. 2004; Deaglio et al. 2007; Fredholm 2007; Sitkovsky 2009).
Adenosine then acts as a powerful endogenous neuroprotectant and intra- and intercellular messenger during ischemia-induced energy failure (Dunwiddie and Masino 2001; Fredholm et al. 2005b) by decreasing neuronal metabolism and increasing cerebral blood flow and there is reduction in the release of excitotoxic neurotransmitters, attenuation of NMDA receptors, vasorelaxation, and anti-inflammatory effects (Sciotti et al. 1992; Yawo and Chuhma 1993; Soricelli et al. 1995; Johansson et al. 2001; Ohta and Sitkovsky 2001; Li et al. 2006; Kusano et al. 2010). But most importantly, there is, at least in animal models, an impressive reduction of neuronal damage and mortality (von Lubitz 1999). Adenosine also showed neuroprotective effects in in vitro models of hypoxic neuronal cells (Bocklinger et al. 2004; Heftberger et al. 2005; Tomaselli et al. 2005a; b; Tomaselli et al. 2008; zur Nedden et al. 2008).
Adenosine effects are mediated by specific receptors (Rudolphi et al. 1992; Sweeney 1997; Kobayashi et al. 1998; von Lubitz 1999; Fredholm et al. 2001a). In brain, high membrane adenosine receptor expression levels are found (Fredholm et al. 2005b; Hasko et al. 2005; Wei et al. 2010), and stimulation of adenosine receptors was hypothesized to result in an effective treatment of stroke (Dunwiddie and Masino 2001; Laubach et al. 2011). As discussed before, extracellular adenosine accumulates in inflamed areas with damaged microcirculation, diminished blood supply, and low oxygen tension. Under such conditions, adenosine serves as a marker of collateral immune damage and supports the prevention of additional injury through inhibition of activated immune cells (Sitkovsky et al. 2004; Sitkovsky 2009). Adenosine deaminase (converting adenosine to inosine), adenosine kinase (phosphorylates adenosine to 5′-AMP) and nucleoside transporters, are responsible for an extremely short half-life of adenosine in circulation (Fredholm et al. 2001a, 2011; Eltzschig 2009) and therefore some of its effects, are apparently due to its metabolites as was reported, for example, for inosine (Haun et al. 1996).
Inosine is formed by deamination of adenosine, mainly at high intracellular concentrations, which are associated with hypoxia, ischemia and other forms of cellular stress (Hasko et al. 2004). Inosine may be formed intra- and extracellularly and shunted across the cell membrane via ENTs (Pastor-Anglada et al. 2001) (Fig. 1). Inosine concentrations up to 6 μM have been detected in human myocardial ischemia, and many times higher concentrations may be observed in experimental models of ischemia–reperfusion injury (Hasko et al. 2004). Initially, inosine did not attract the same interest as adenosine. Yet, inosine was shown to effect neuronal (Benowitz et al. 1998; Litsky et al. 1999; Bocklinger et al. 2004; Heftberger et al. 2005; Tomaselli et al. 2005a, b, 2008; zur Nedden et al. 2008) and glial (Haun et al. 1996; Jurkowitz et al. 1998) cell viability and neurite outgrowth in cells subjected to glucose deprivation and/or mitochondrial respiratory chain inhibition or challenged with low oxygen. Moreover, inosine was shown to stimulate neurons to extend new projections to denervated areas in adult rats with unilateral cortical infarcts (Chen et al. 2002). Inosine was also shown to exert multiple anti-inflammatory effects such as reduction of the production of pro-inflammatory cytokines such as tumor necrosis factor-alpha, macrophage inflammatory protein-2 and Il-6 (Hasko et al. 2004). These findings, coupled with the fact that inosine has very low toxicity, suggested that this agent may be useful in the treatment of inflammatory/ischemic diseases, and might help to restore essential circuitry after injury to the CNS (Jurkowitz et al. 1998; Benowitz et al. 1999, 2002; Chen et al. 2002; Hasko et al. 2004; Zai et al. 2009; Benowitz and Carmichael 2010).
Guanosine is metabolized from guanosine 5′-triphosphate (GTP) and guanosine 5′-monophosphate (GMP) (Schmidt et al. 2007) and is present in the brain under both physiological and pathological conditions (Uemura et al. 1991). In analogy to ATP, GTP concentrations decrease in ischemic tissue (Kinouchi et al. 1990) and guanosine concentrations showed significant increases at 2 h to 7 days (Uemura et al. 1991). Guanine derivatives may reach threefold higher levels than adenine-derivatives in cell injuries like hypoxia and hypoglycemia (Ciccarelli et al. 1999). Guanine-based purines are released from neurons and astrocytes (Rathbone et al. 2008). As discussed (Rathbone et al. 2008), extracellular guanosine stimulates mitosis, synthesis of trophic factors, and cell differentiation, including neuritogenesis, is neuro- and glia-protective, and reduces apoptosis (Gysbers and Rathbone 1996a, b; Benowitz et al. 1998; Jurkowitz et al. 1998; Rathbone et al. 1998, 2008, 2011; Litsky et al. 1999; Ciccarelli et al. 2000; Frizzo et al. 2002; Bau et al. 2005; Tomaselli et al. 2005b; Ballerini et al. 2006; Jiang et al. 2007; Schmidt et al. 2007; Chang et al. 2008; Oleskovicz et al. 2008; Su et al. 2010; Thauerer et al. 2010; Dal-Cim et al. 2011).
Purine nucleoside receptors
Adenosine receptors (AR) belong to the superfamily of G-protein-coupled receptors characterized by seven transmembrane helices (Palmer and Stiles 1995). There are four G-protein-coupled ARs, namely A1R, A2AR, A2BR and A3R, all of them expressed in brain, with A1R and A2AR being the physiologically more important subtypes (Fredholm et al. 2001a, 2005a; Abbracchio et al. 2009; Wei et al. 2010). ARs, via its alpha subunit, either stimulate (Gs) or inhibit (Gi) adenylate cyclase, the enzyme that catalyzes the formation of cAMP, whereby A1R and A3R interact with Gi/ Go proteins, and A2A and A2B with Gs (van Calker et al. 1979; Zhou et al. 1992; Palmer and Stiles 1997; Fredholm et al. 2001a). In addition to the classical adenylate cyclase–cAMP-protein kinase A signaling pathway, it is now apparent that other pathways, such as phospholipase C, Ca2+- and mitogen-activated protein kinases (MAPKs), are also relevant (Linden 1991; Abbracchio et al. 1995; Fredholm et al. 2001a; Schulte and Fredholm 2003b; Tomaselli et al. 2008).
The A1 high affinity receptor albeit expressed throughout the body reaches highest levels in brain especially in neurons of cortex, hippocampus, cerebellum and dorsal horn of spinal cord, eye, adrenal gland, and atria (Fredholm et al. 2005b; Wei et al. 2010) at pre-synaptic and post-synaptic sites (Rebola et al. 2003). A1R stimulation generally suppresses neuronal activity and efficiently controls the release of all the classical neurotransmitters (glutamate, acetylcholine and serotonin), leading to the idea that A1Rs mainly fulfill a synaptic neuromodulatory role, particularly in excitatory nerve terminals in the brain (Dunwiddie and Masino 2001; Cunha 2005; Fredholm et al. 2005a; b; Wei et al. 2010). The expression of the high affinity A2AR is highest in brain in dopamine-rich regions, the striato-pallidal GABAergic neurons and olfactory bulb (Peterfreund et al. 1996; Wei et al. 2010). Evidence indicates that activation of A2AR exerts damaging as well as protective effects in brain ischemia (Dai and Zhou 2011). The preferred partner of A2AR-mediated activation is Gs, except in striatum, where A2AR interacts with Golf, whereby both result in coupling to its canonical protein kinase A (PKA)-activating pathway (Fredholm et al. 2007). Stimulation of the A2AR activates the Ras/RAF-1/MEK/MAPK signaling through PKA-dependent and PKA-independent pathways via Src-and Sos-mediated mechanisms (Schulte and Fredholm 2003b). Hypoxic conditions were shown to up-regulate A1R and A2AR (Kobayashi and Millhorn 1999; Lai et al. 2005; Podhraski et al. 2005). Selective AR agonists and antagonists were created and extensively reviewed recently (Lopes et al. 2011; Muller and Jacobson 2011).
Adenosine is a full agonist at all these receptors, whereas inosine can act as a partial agonist in functional assays at A1R and A3R (Jin et al. 1997; Fredholm et al. 2001a; Hasko et al. 2004) and initiate intracellular signaling events (Jin et al. 1997; Hasko et al. 2000, 2004; Fredholm et al. 2001b). Recent findings showed inosine-mediated stimulatory effects in the predominantly A2AR-positive neuronal PC12 cell line (zur Nedden et al. 2008; Tomaselli et al. 2008). However, it remains to be seen whether these systemic immunomodulatory effects are the consequences of direct binding of inosine to A2AR. Even more controversial is the question, whether the protective effect of guanosine is at least partly arbitrated by adenosine or its receptors (Ciccarelli et al. 2000; D'Alimonte et al. 2007), or is mediated by its own specific G-coupled receptors (Traversa et al. 2003; Rathbone et al. 2008). Recently, results again suggest that guanosine, 6-thioguanosine, and their derivatives activate a G-protein-coupled receptor that is different from the well-characterized AR (Volpini et al. 2011). Our own data suggest at least a supporting role for the A2AR in guanosine-mediated signal transduction in neurite formation ((Thauerer et al. 2010) and B. Thauerer, unpublished data).
Nucleoside transporters
During metabolic stress like hypoxia, intracellular adenosine is formed at the expense of ATP, leaves cells via nucleoside transporters and activate ARs (Fredholm et al. 2005a). Bidirectional transporters allow purine nucleosides to gain access to the intracellular space (Pastor-Anglada et al. 2001; Parkinson et al. 2005, 2011; King et al. 2006; Takahashi et al. 2010; Sebastiao 2011). Alternatively, ATP may be released from cells by cell lysis, exocytosis, transporters or channels, and dephosphorylated extracellularly to adenosine (Neary 2005). The increase in inosine during hypoxia was reported to be largely due to, either extracellular degradation of adenosine (Frenguelli et al. 2003), or else to the intracellular formation of inosine and subsequent release by ENTs (Parkinson and Xiong 2004). Likewise guanosine was shown to be transported into neurons and astrocytes by nucleoside transporters (Nagasawa et al. 2007).
Purine nucleoside-mediated neuroprotection and neuroregeneration
Evaluation of the effects of AR agonists and antagonists in stroke models indicates that adenosine acting through A1R has neuroprotective effects (Rudolphi et al. 1992; Sweeney 1997; von Lubitz 1999), probably by control of glutamate release and inhibiting excitatory synaptic neurotransmission in the brain during hypoxia (Wei et al. 2010). In contrast, activation of A2AR may enhance neuronal damage, as mice lacking these receptors exhibited reduced damage following focal ischemia (Chen et al. 1999). Results by others (Kobayashi et al. 1998), however indicated that hypoxia-induced membrane responses of PC12 cells are likely to be mediated via activation of the A2AR.
Administration of adenosine to the brain at times of stroke was shown to ameliorate damage (Kitagawa et al. 2002), and transgenic over-expression of adenosine kinase, leads to increased vulnerability to ischemia-induced cell death (Pignataro et al. 2007). It is now at large believed and confirmed by genetic knockout models, that elevated extracellular adenosine levels exert an overall neuroprotective effect in injured brain; however, because of complex organ- and injury-type specific responses precise predictions are still difficult (Wei et al. 2010). Correspondingly, stroke animals receiving inosine pre-treatment demonstrated a higher level of locomotor activity and less cerebral infarction (Shen et al. 2005). Also guanosine was shown to prolong rat survival and decrease both neurological deficits and tissue damage resulting from middle cerebral artery occlusion (MCAo) (Chang et al. 2008). Other strategies to improve outcome after stroke-induced injuries are considering a potential-reinnervation of brain regions that are devoid of their normal inputs. Along this line, adenosine and guanosine were shown to inhibit injury-induced axonal degeneration in cultured dorsal root ganglion (DRG) neurons (Press and Milbrandt 2009). Also inosine, proved to alter gene expression in neurons and enhance the ability of neurons to form new connections on the side of the spinal cord that lost its normal innervation, and to restore skilled behavior formerly mediated by the damaged area, thus revealing their potential to modulate circuit remodeling that might recover lost functions (Zai et al. 2009). Together these findings give rise to the hope of new therapeutical approaches for the improvement of hypoxia/ischemia-induced plasticity and to lessen neuronal damage in stroke (Zai et al. 2009; Benowitz and Carmichael 2010).
Hypoxia signaling
Oxygen sensing and adaptation is achieved by a variety of molecules whose effects are complexly interwoven, and sophisticated mechanisms have evolved that regulates gene expression and the critical balance between cell death and survival during hypoxia/ischemia (Seta et al. 2002). Those include the Ca2+-calmodulin pathway, the 3′–5′ adenosine monophosphate (cAMP)-PKA pathway, the MAPK pathway, the stress-activated protein kinase (also known as p38 kinase) pathway, and the phosphatidylinositol 3-kinase-Akt pathway (Conrad et al. 2001; Bickler and Donohoe 2002; Seta et al. 2002; Seta and Millhorn 2004; Semenza 2007; Majmundar et al. 2010; Henke et al. 2011; Koeppen et al. 2011). For in vitro hypoxia studies, the sympathetic ganglion-like clonal rat pheochromocytoma (PC12) cells (Greene and Tischler 1976), which are O2-sensitive (Zhu et al. 1996; Seta et al. 2002), are widely used as a model system. PC12-cells express abundant A2A adenosine receptors (Hide et al. 1992; van der Ploeg et al. 1996; Arslan et al. 1999), which have been shown to affect these cellular responses to hypoxia (Kobayashi et al. 1998; Kobayashi and Millhorn 1999). Numerous studies in this cell line, but also in other neuronal cell models, for example, primary cerebellar granule neurons have shown that purine nucleosides have neuroprotective functions in cells, which were subjected to chemical- (Litsky et al. 1999; Bocklinger et al. 2004; Heftberger et al. 2005; Tomaselli et al. 2005a; b) and physiological- (Kobayashi and Millhorn 1999; Frizzo et al. 2002; Chang et al. 2008; zur Nedden et al. 2008; Oleskovicz et al. 2008; Thomazi et al. 2008; Tomaselli et al. 2008; Thauerer et al. 2010; Dal-Cim et al. 2011) hypoxia (Table 1). Results in these models confirmed an important role for A1R as well as A2AR, because purine-mediated rescue was inhibited by A1R (in primary cerebellar granule neurons) or A2AR-antagonists (in neuronal PC12 cells) respectively (Heftberger et al. 2005; Tomaselli et al. 2005a).
Table 1.
Key molecules in Purine nucleoside-mediated signal transduction in hypoxic neuronal cells. This table summarizes in vitro data, collected from neuronal/hypoxia experiments. Data are separated in the effects of purine nucleosides (adenosine, inosine and guanosine) on (i) viability and (ii) neurite outgrowth
| Purine nucleoside | Experimental model | Proposed key molecule | Reference |
|---|---|---|---|
| Viability studies | |||
| Adenosine–NECA | PC12 cells, 1% O2 | Ca2+ homeostasis | Kobayashi and Millhorn (1999) |
| Adenosine | Cerebellar granule neurons, rotenone | Bocklinger et al. (2004) | |
| Adenosine | Cerebellar granule neurons, rotenone | AR (DPCPX), ENT (NBTI) | Heftberger et al. (2005) |
| Adenosine | PC12 cells, rotenone | AR (CSC) | Tomaselli et al. (2005a) |
| Adenosine | PC12 cells, rotenone | ENT (NBTI) | Tomaselli et al. (2005a) |
| Adenosine | PC12 cells, rotenone | PI3K (LY294002) | Tomaselli et al. (2005a) |
| Adenosine | PC12 cells, rotenone | MAPK (PD098059, U0126) | Tomaselli et al. (2005b) |
| Adenosine | PC12 cells, 1% O2 | MAPK (PD098059, siRNA) | Tomaselli et al. (2008) |
| Adenosine | PC12 cells, 1% O2 | HIF-1α (siRNA) | zur Nedden et al. (2008) |
| Adenosine | Cerebellar granule neurons, 1% O2 | MAPK (siRNA) | Tomaselli et al. (2008) |
| Adenosine | Cerebellar granule neurons, 1% O2 | HIF-1α (siRNA) | zur Nedden et al. (2008) |
| Inosine | Murine spinal cord, rotenone | Litsky et al. (1999) | |
| Inosine | Cerebellar granule neurons, rotenone | Bocklinger et al. (2004) | |
| Inosine | Cerebellar granule neurons, rotenone | AR (DPCPX), ENT (NBTI) | Heftberger et al. (2005) |
| Inosine | PC12 cells, rotenone | AR (CSC) | Tomaselli et al. (2005a) |
| Inosine | Cerebellar granule neurons, 1% O2 | MAPK (siRNA) | Tomaselli et al. (2008) |
| Inosine | Cerebellar granule neurons, 1% O2 | HIF-1α (siRNA) | zur Nedden et al. (2008) |
| Inosine | PC12 cells, 1% O2 | MAPK (PD098059) | Tomaselli et al. (2008) |
| Inosine | PC12 cells, 1% O2 | HIF-1α (siRNA) | zur Nedden et al. (2008) |
| Guanosine | Murine spinal cord, rotenone | Purine nucleoside phosphorylase | Litsky et al. (1999) |
| Guanosine | Cortical slices, OGD | Frizzo et al. (2002) | |
| Guanosine | Cerebellar granule neurons, rotenone | Bocklinger et al. (2004) | |
| Guanosine | Cerebellar granule neurons, rotenone | ENT (NBTI) | Heftberger et al. (2005) |
| Guanosine | PC12 cells, rotenone | AR (CSC) | Tomaselli et al. (2005a) |
| Guanosine | SH-SY5Y cells, OGD | Chang et al. (2008) | |
| Guanosine | Hippocampal slices, OGD-reox. | Thomazi et al. (2008) | |
| Guanosine | Hippocampal slices, OGD-reox. | PKA, PKC, MEK, PI3K | Oleskovicz et al. (2008) |
| Guanosine | PC12 cells, 1% O2 | PRK 1(siRNA) | Thauerer et al. (2010) |
| Guanosine | Cerebellar granule neurons, 1% O2 | PRK 1(siRNA) | Thauerer et al. (2010) |
| Guanosine | Hippocampal slices, OGD-reox. | Ca2+-activated K+ channels, PI3K, AKT | Dal-Cim et al. (2011) |
| Neurite studies | |||
| Adenosine | Cerebellar granule neurons, rotenone | Bocklinger et al. (2004) | |
| Adenosine | Cerebellar granule neurons, rotenone | Heftberger et al. (2005) | |
| Adenosine | PC12 cells, 1% O2 | MAPK (PD098059, siRNA) | Tomaselli et al. (2008) |
| Adenosine | PC12 cells, 1% O2 | HIF-1α (siRNA) | zur Nedden et al. (2008) |
| Adenosine | PC12 cells, 1% O2 | AR (SCH-58261) | Thauerer et al. (2010) and unpublished data |
| Inosine | Cerebellar granule neurons, rotenone | Bocklinger et al. (2004) | |
| Inosine | Cerebellar granule neurons, rotenone | Heftberger et al. (2005) | |
| Inosine | PC12 cells, 1% O2 | MAPK (PD098059, siRNA) | Tomaselli et al. (2008) |
| Inosine | PC12 cells, 1% O2 | HIF-1α (siRNA) | zur Nedden et al. (2008) |
| Inosine | Dorsal root ganglion neurons | Mstb3, MAPK | Lorber et al. (2009) |
| Inosine | PC12 cells, 1% O2 | AR (SCH-58261) | Thauerer et al. (2010) and unpublished data |
| Guanosine | Cerebellar granule neurons, rotenone | Bocklinger et al. (2004) | |
| Guanosine | Cerebellar granule neurons, rotenone | Heftberger et al. (2005) | |
| Guanosine | PC12 cells, 1% O2 | PRK 1 (siRNA) | Thauerer et al. (2010) |
| Guanosine | Cerebellar granule neurons, 1% O2 | PRK 1 (siRNA) | Thauerer et al. (2010) |
| Guanosine | PC12 cells, 1% O2 | AR (SCH-58261) | Thauerer et al. (2010) and unpublished data |
In hypoxic PC12 cells, viability is predominantly rescued by adenosine, whereas guanosine is more supportive of neurite outgrowth (Kobayashi et al. 1998; Tomaselli et al. 2005a). Hypoxia-induced membrane responses of PC12 cells are likely to be mediated via activation of the A2A adenosine receptors, and elevation of cAMP and inhibition of the A2A receptor itself induced death of PC12 cells (Kobayashi et al. 1998; Arslan et al. 1999; Tomaselli et al. 2005a). Therefore, it is not surprising that PKA is required for the A2 receptor modulation of both voltage-sensitive potassium IK(V) and calcium ICa currents in PC12 cells (Kobayashi et al. 1998) and that pharmacological inhibition of protein kinase A (PKA) with H89 superinduced chemical hypoxia-mediated cell death and inhibited the rescue of hypoxic PC12 cells by purine nucleosides (Tomaselli et al. 2005a). The role of nucleoside transport varies for different purine nucleosides and cell types. In PC12 cells, the inhibition of nucleoside transport with S-(4-nitrobenzyl)-6-thioinosine caused an increase in adenosine-mediated rescue of viability (Tomaselli et al. 2005a), presumably due to increased A2A receptor-mediated signaling (Parkinson et al. 2000). Our own results therefore confirm the hypothesis that adenosine mainly acts via adenosine receptor-mediated signaling mechanisms, whereas many aspects of the mechanisms involved in inosine- and guanosine-based protection still remain unclear. However, A1R-expressing primary cerebellar granule neurons were more effectively rescued by the adenosine metabolite inosine, than adenosine and guanosine (Bocklinger et al. 2004; Heftberger et al. 2005), whereby adenosine- and inosine-mediated rescue was sensitive to an A1R antagonist (8-cyclopentyl-1, 3-dipropylxanthine) whereas guanosine was largely unaffected (Heftberger et al. 2005). Nucleoside transport is apparently important for adenosine-, inosine- and guanosine-mediated rescue of hypoxic A1R positive cerebellar granule neurons (Heftberger et al. 2005).
The p42/44 mitogen-activated protein kinase (MAPK) pathway, serine–threonine kinases constitute a convergent pathway for the regulation of multiple modalities involved in O2 sensing (Seta et al. 2002). They are part of a signaling module that transduces signals from the cell membrane to the nucleus in response to a vast range of external stimuli (Irving and Bamford 2002; Colucci-D'Amato et al. 2003; Cheung and Slack 2004; Wada and Penninger 2004) and regulate proliferation, neuronal survival, differentiation, long-term memory and synaptic plasticity and apoptosis (Boulton et al. 1991; Segal and Greenberg 1996; Alessandrini et al. 1999; Johnson and Lapadat 2002; Sweatt 2004; Wada and Penninger 2004). Emerging evidence suggests that MAPKs are highly related to processes that promote neuron survival (e.g. by neuroprotective growth factors (Nicole et al. 2001), and plasticity (Impey et al. 1999). Stimulation of the adenosine receptors A1R, A2A- and A2BR was shown to activate MAPK (Sexl et al. 1997; Dickenson et al. 1998; Arslan and Fredholm 2000; Fredholm et al. 2001a; Charles et al. 2003; Schulte and Fredholm 2003a; b; Tomaselli et al. 2008). Authors reflect on different activation pathways of MAPK, as diverse as coupling of ARs to G12/13 proteins instead of Gs or Gs- and cAMP-independent affects on MAPK involving the Ras module (Sexl et al. 1997; Seidel et al. 1999; Schulte and Fredholm 2003b). Along this line another group (Faure et al. 1994) showed transiently expressed A1R in COS-7 cells mediated MAPK activation via release of βγ subunits. Although it appears from these and from many other cases (Hetman and Gozdz 2004), that MAPK activation is neuroprotective, mediating the effects of several extrinsic survival signals, MAPK activation in hypoxia/ischemia still remains a controversial issue. MAPKs are activated by small increases in calcium during survivable degrees of hypoxia (Minet et al. 2000; Bickler and Donohoe 2002) and studies in perinatal cerebral hypoxia–ischemia showed MAPK activation in neurons, mainly in cells displaying signs of damage (Wang et al. 2003). Authors therefore debate, whether MAPK is either trying, unsuccessfully, to rescue cells, or actually contributing to harmful cell signals (Wang et al. 2003). Recent results, however, suggested a vital role of the MAPK pathway in purine nucleoside-mediated protection of neuronal cells and primary neurons following hypoxic insult (Tomaselli et al. 2005b, 2008). In cells subjected to hypoxia, an increased phosphorylation of MAPK, was detected, that was further increased upon addition of purine nucleosides. Vice versa, upon blocking this pathway with a pharmacological inhibitor of MEK-1 (PD098059) viability and neurite outgrowth were decreased (Tomaselli et al. 2008). Further evidence came from experiments with small interference RNA constructs. Knockdown of MAPK severely affected purine nucleoside-mediated rescue of hypoxic PC12 cells and cerebellar granule neurons (Tomaselli et al. 2008; Fig. 2).
Figure 2.
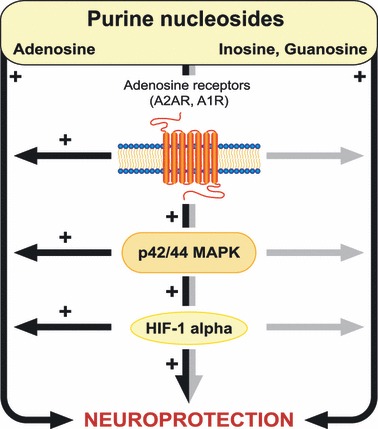
Key-signaling modules in purine-mediated protection of hypoxic neurons.In brain mainly A1 and A2A G-protein coupled adenosine receptors are expressed (Fredholm et al. 2005a; b; Wei et al. 2010). Two models were established, PC12 cells (predominantly A2AR-positive) and cerebellar granule neurons (A1R-positive), in which the significance of the purine nucleosides adenosine (left side) and inosine and guanosine (right side) in protection of hypoxic neurons was proved (Bocklinger et al. 2004; Heftberger et al. 2005; Tomaselli et al. 2005a, b, 2008; zur Nedden et al. 2008; Thauerer et al. 2010). The importance of A1R and A2AR was confirmed using specific receptor antagonists (Heftberger et al. 2005; Tomaselli et al. 2005a). Purine nucleoside-mediated neuroprotection also critically involves the activation of mitogen-activated protein kinases (MAPKs; Tomaselli et al. 2008), hypoxia-inducible factor-1 (HIF-1α) (zur Nedden et al. 2008), and their interconnection (B. Thauerer, unpublished data). The adenosine receptor MAPK-HIF-1α module plays therefore a dominant role in adenosine-mediated protection (black bars), and takes also part in inosine- and guanosine-mediated neuroprotection of hypoxic neuronal cells (grey bars).
MAPK was shown to positively modulate the hypoxia-inducible factor-1 alpha (HIF-1α) by phosphorylation control (Minet et al. 2000). HIF-1α, a transcription factor that plays an essential role in cellular and systemic responses to hypoxia, appears especially interesting and relevant for hypoxia induced signaling. HIF-1α is a heterodimer composed of a 120-kDa HIF-1α subunit complexed to a 91- to 94-kDa HIF-1-β subunit. Under hypoxic conditions HIF-1α is stabilized and constitutes a key role in the cellular defense against hypoxic injury, including the regulation of genes involved in energy metabolism, angiogenesis, and apoptosis (Sitkovsky and Lukashev 2005; Semenza 2011). Direct HIF-1 target genes are involved in energy metabolism and cell viability and thus HIF-1 is causally involved in human disease pathophysiology such as cerebral ischemia (Semenza 2000). Adenosine was hypothesized to have the ability to engage HIF-1 activation towards the cellular and systemic responses to hypoxia it mediates (Sitkovsky 2009). Fitting to these data, it was later reported that the nuclear HIF-1α signal in neuronal cells is increased by adenosine and that HIF-1α is apparently critical for purine-mediated neuroprotection (zur Nedden et al. 2008). Authors conclude from their results that the adenosine receptor/MAPK/HIF-1α pathway is tightly interwoven as proven by pharmacological inhibition or siRNA knockdown and plays a critical role for adenosine- and to a lesser degree also for inosine and guanosine-mediated neuro-protection (Fig. 2).
Next to the clear-cut effects of adenosine receptor-mediated activation of MAPK-HIF-1α, neuroprotection of hypoxic neuronal cells apparently does involve other pathways that deserve future attention. Amongst purine nucleosides guanosine attracted attention for its strong neurite-stimulating capacity (Bau et al. 2005; Jiang et al. 2007; Schmidt et al. 2007; Chang et al. 2008; Rathbone et al. 2008; Thauerer et al. 2010). Neuroprotective effects of guanosine were reported to involve an augmentation of glutamate uptake modulated by K+ channels and the activation of the phosphatidylinositol 3-kinase/Akt pathway (Dal-Cim et al. 2011). Recently, another protein kinase, namely protein kinase N alpha (PKNα)/protein kinase C-related kinase1 (PRK1) (Mellor and Parker 1998; Mukai 2003), made a name of itself in purine-mediated neuroprotection (Tomaselli et al. 2005a; Thauerer et al. 2010). PRK1 is a lipid-activated serine/threonine protein kinase and a member of the protein kinase C superfamily (Mellor and Parker 1998; Mukai 2003) of potential key regulators orchestrating physiological responses, and is involved in regulation of the actin cytoskeleton (Modha et al. 2008). PRK1 is activated by interacting with the Rho and Rac families of small G-proteins and arachidonic acid, or by caspase cleavage (Takahashi et al. 1998; Lu and Settleman 1999; Mukai 2003). Adenosine, inosine and guanosine up-regulated its activity in hypoxic neuronal cells (Tomaselli et al. 2005a; Thauerer et al. 2010). Vice versa, loss of functional PRK1 initiated a significant loss of viability and inhibition of neurite formation (Thauerer et al. 2010), which apparently involved a disturbance of the F-actin-associated cytoskeleton and the expression of the plasticity protein growth-associated protein-43 (Thauerer et al. 2010). An up-regulation of growth-associated protein-43 was also reported for inosine (Petrausch et al. 2000). To what extent inosine's ability to induce neurite outgrowth (Zurn and Do 1988; Benowitz et al. 1998) is due to the activity of Mst3b, a Ste-20-like purine-sensitive protein kinase (Irwin et al. 2006; Lorber et al. 2009) or on PRK1 remains to be shown (Table 1).
Conclusion
Hypoxic–ischemic brain injury starts with the insult but extends into a recovery–reperfusion period (Barone and Feuerstein 1999; Lipton 1999; White et al. 2000; Hertz 2008; Macrez et al. 2011). In case of prolonged ischemia, restricted blood flow leads to a reduction in ATP, causing severe impairment of cellular function by disruption of ATP-dependent processes. Brain exposure to hypoxia in ischemia/reperfusion injuries often causes devastating and irreversible loss of function (Chen et al. 2002) and is linked to long term neurological shortages (Berger and Garnier 1999; El-Khodor and Boksa 2000). In parallel hypoxia leads to the decreased production and enhanced breakdown of purine nucleotides to purine nucleosides (Jurkowitz et al. 1998; Sitkovsky et al. 2004; Fredholm et al. 2007; Fredholm 2010). Earlier studies showed that in response to hypoxia, adenosine is produced intracellularly and released into the medium (Meghji et al. 1989; Lloyd et al. 1993; Parkinson and Xiong 2004; Takahashi et al. 2010), from where it triggers different actions through the activation of ARs (Fredholm et al. 1994, 2001a; Schulte and Fredholm 2003b). Growing evidence suggests that purine nucleotides and nucleosides might act as trophic factors in both the central and peripheral nervous systems and are involved in the regulation of the nervous system's development and plasticity (Neary et al. 1996). Adenosine was reported to act as a powerful endogenous neuroprotectant during ischemia-induced energy failure by decreasing neuronal metabolism and increasing cerebral blood flow, and by playing a variety of different roles as an intra- and intercellular messenger (Dunwiddie and Masino 2001; Fredholm et al. 2005a; b). Guanosine and inosine the like, were shown to induce neurite outgrowth (Benowitz et al. 1998; Rathbone et al. 2008) and in vivo studies demonstrated inosine's ability to stimulate neurons to extend new projections to denervated areas in adult rats with unilateral cortical infarcts (Chen et al. 2002). In vitro studies confirmed the amazing neuroprotective capability of purine nucleosides in several neuronal hypoxia systems e.g PC12 cells and cerebellar granule neurons (Bocklinger et al. 2004; Heftberger et al. 2005; Tomaselli et al. 2005a, b, 2008; zur Nedden et al. 2008; Thauerer et al. 2010) and prompted the investigation of purine-mediated hypoxia sensitive signaling. Amongst the multiple pathways and sophisticated mechanisms that have evolved and regulate gene expression during hypoxia, the MAPK module constitutes a convergent pathway for the regulation of multiple modalities involved in O2 sensing (Seta et al. 2002). Stimulation of the A1R, A2A- and A2BR was shown to activate MAPK (Sexl et al. 1997; Dickenson et al. 1998; Arslan and Fredholm 2000; Fredholm et al. 2001a; Charles et al. 2003; Schulte and Fredholm 2003a; b; Tomaselli et al. 2008) and recent results suggested a vital role of the MAPK pathway plays in purine nucleoside-mediated protection of neuronal cells following hypoxic insult (Tomaselli et al. 2005b, 2008). These results are very relevant to understand the mechanisms by which purine nucleosides modulate neuronal signaling and should support the therapeutic approaches investigated by other groups, which claim that brief ischemia activates MAPK whereas its blockade inhibits ischemic tolerance (Meller et al. 2005). MAPK activation may thus act as a defensive mechanism that helps to compensate for deleterious effects of a damaging insult (Hetman and Gozdz 2004). Amongst MAPK-associated (Minet et al. 2000) downstream effector molecules the transcription factor HIF-1α appears to be most interesting. Adenosine was hypothesized to collaborate with HIF-1α in triggering the production of immunosuppressive molecules (Sitkovsky 2009). Likewise adenosine augmented hypoxia-mediated HIF-1α translocation to the nucleus and HIF-1α was shown to be critical for purine-mediated neuroprotection (zur Nedden et al. 2008).
Many stroke patients fail clinical time windows for acute effective treatment, hence making approaches that promote repair and recovery essential for integrated stroke therapy (Moskowitz et al. 2010). Growing evidence suggests that the biological processes underlying stroke are driven by the interaction of neurons, glia, vascular cells, and matrix components; all actively participating in tissue injury and repair and therefore trophic factor treatments that amplify and augment endogenous processes of neuroplasticity are pre-destined to support recovery (Moskowitz et al. 2010). Furthermore, the detection of continuous neurogenesis in the adult mammalian brain has encouraged a new perception of the plasticity of the mature nervous system (Ming and Song 2011). Thus, as data on the competence of purine nucleosides to support neuroprotection and regeneration accumulate, increasing levels of pro survival proteins may be a promising new strategy to reduce cell damage after ischemia (Cao et al. 2002). In light of recent developments for adenosine in epilepsy (Van Dycke et al. 2011), purine nucleoside augmentation techniques or localized delivery may facilitate possible approaches for neuroprotection and/or enhanced neuroregeneration in stroke.
Acknowledgments
This work was supported by a grant of the Austrian FWF grants P19578-B05 and T421-B18. We are grateful to Dr C. Bandtlow for helpful discussion and support.
Glossary
- AR
adenosine receptor 1, 2A, 2B, 3
- ENT
equilibrative nucleoside transporter
- Gi
inhibiting G-protein
- Gs
stimulating G-protein
- HIF-1α
hypoxia-inducible factor-1 alpha
- MAPK
p42/44 mitogen-activated protein kinase, also ERK1/2
- PI3-K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
References
- Abbracchio MP, Brambilla R, Ceruti S, Kim HO, von Lubitz DK, Jacobson KA, Cattabeni F. G protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol. Pharmacol. 1995;48:1038–45. [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, Hurn P, Liebeskind DS, Nogueira RG, Saver JL. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42:2645–50. doi: 10.1161/STROKEAHA.111.618850. [DOI] [PubMed] [Google Scholar]
- Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc. Natl Acad. Sci. USA. 1999;96:12866–9. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan G, Fredholm BB. Stimulatory and inhibitory effects of adenosine A(2A) receptors on nerve growth factor-induced phosphorylation of extracellular regulated kinases 1/2 in PC12 cells. Neurosci. Lett. 2000;292:183–6. doi: 10.1016/s0304-3940(00)01461-0. [DOI] [PubMed] [Google Scholar]
- Arslan G, Kull B, Fredholm BB. Signaling via A2A adenosine receptor in four PC12 cell clones. Naunyn. Schmiedebergs. Arch. Pharmacol. 1999;359:28–32. doi: 10.1007/pl00005319. [DOI] [PubMed] [Google Scholar]
- Ballerini P, Ciccarelli R, Di Iorio P, et al. Guanosine effect on cholesterol efflux and apolipoprotein E expression in astrocytes. Purinergic Signal. 2006;2:637–49. doi: 10.1007/s11302-006-9011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J. Cereb. Blood Flow Metab. 1999;19:819–34. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Bau C, Middlemiss PJ, Hindley S, Jiang S, Ciccarelli R, Caciagli F, Diiorio P, Werstiuk ES, Rathbone MP. Guanosine stimulates neurite outgrowth in PC12 cells via activation of heme oxygenase and cyclic GMP. Purinergic Signal. 2005;1:161–72. doi: 10.1007/s11302-005-6214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Carmichael ST. Promoting axonal rewiring to improve outcome after stroke. Neurobiol. Dis. 2010;37:259–66. doi: 10.1016/j.nbd.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Jing Y, Tabibiazar R, Jo SA, Petrausch B, Stuermer CA, Rosenberg PA, Irwin N. Axon outgrowth is regulated by an intracellular purine-sensitive mechanism in retinal ganglion cells. J. Biol. Chem. 1998;273:29626–34. doi: 10.1074/jbc.273.45.29626. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Goldberg DE, Madsen JR, Soni D, Irwin N. Inosine stimulates extensive axon collateral growth in the rat corticospinal tract after injury. Proc. Natl Acad. Sci. USA. 1999;96:13486–90. doi: 10.1073/pnas.96.23.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Goldberg DE, Irwin N. Inosine stimulates axon growth in vitro and in the adult CNS. Prog. Brain Res. 2002;137:389–99. doi: 10.1016/s0079-6123(02)37030-4. [DOI] [PubMed] [Google Scholar]
- Berger R, Garnier Y. Pathophysiology of perinatal brain damage. Brain Res. Brain Res. Rev. 1999;30:107–34. doi: 10.1016/s0165-0173(99)00009-0. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Donohoe PH. Adaptive responses of vertebrate neurons to hypoxia. J. Exp. Biol. 2002;205:3579–86. doi: 10.1242/jeb.205.23.3579. [DOI] [PubMed] [Google Scholar]
- Bocklinger K, Tomaselli B, Heftberger V, Podhraski V, Bandtlow C, Baier-Bitterlich G. Purine nucleosides support the neurite outgrowth of primary rat cerebellar granule cells after hypoxia. Eur. J. Cell Biol. 2004;83:51–4. doi: 10.1078/0171-9335-00362. [DOI] [PubMed] [Google Scholar]
- Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–75. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Braun N, Zhu Y, Krieglstein J, Culmsee C, Zimmermann H. Upregulation of the enzyme chain hydrolyzing extracellular ATP after transient forebrain ischemia in the rat. J. Neurosci. 1998;18:4891–900. doi: 10.1523/JNEUROSCI.18-13-04891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat. Rev. Drug Discov. 2008;7:575–90. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- van Calker D, Muller M, Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J. Neurochem. 1979;33:999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- Cao YJ, Shibata T, Rainov NG. Liposome-mediated transfer of the bcl-2 gene results in neuroprotection after in vivo transient focal cerebral ischemia in an animal model. Gene Ther. 2002;9:415–9. doi: 10.1038/sj.gt.3301676. [DOI] [PubMed] [Google Scholar]
- Chang R, Algird A, Bau C, Rathbone MP, Jiang S. Neuroprotective effects of guanosine on stroke models in vitro and in vivo. Neurosci. Lett. 2008;431:101–5. doi: 10.1016/j.neulet.2007.11.072. [DOI] [PubMed] [Google Scholar]
- Charles MP, Adamski D, Kholler B, Pelletier L, Berger F, Wion D. Induction of neurite outgrowth in PC12 cells by the bacterial nucleoside N6-methyldeoxyadenosine is mediated through adenosine A2a receptors and via cAMP and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2003;304:795–800. doi: 10.1016/s0006-291x(03)00666-1. [DOI] [PubMed] [Google Scholar]
- Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 2010;10:826–37. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 1999;19:9192–200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc. Natl Acad. Sci. USA. 2002;99:9031–6. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EC, Slack RS. Emerging role for ERK as a key regulator of neuronal apoptosis. Sci. STKE. 2004;2004:PE45. doi: 10.1126/stke.2512004pe45. [DOI] [PubMed] [Google Scholar]
- Ciccarelli R, Di Iorio P, Giuliani P, D'Alimonte I, Ballerini P, Caciagli F, Rathbone MP. Rat cultured astrocytes release guanine-based purines in basal conditions and after hypoxia/hypoglycemia. Glia. 1999;25:93–8. [PubMed] [Google Scholar]
- Ciccarelli R, Di Iorio P, D'Alimonte I, Giuliani P, Florio T, Caciagli F, Middlemiss PJ, Rathbone MP. Cultured astrocyte proliferation induced by extracellular guanosine involves endogenous adenosine and is raised by the co-presence of microglia. Glia. 2000;29:202–11. [PubMed] [Google Scholar]
- Colucci-D'Amato L, Perrone-Capano C, di Porzio U. Chronic activation of ERK and neurodegenerative diseases. BioEssays. 2003;25:1085–95. doi: 10.1002/bies.10355. [DOI] [PubMed] [Google Scholar]
- Conrad PW, Conforti L, Kobayashi S, Beitner-Johnson D, Rust RT, Yuan Y, Kim HW, Kim RH, Seta K, Millhorn DE. The molecular basis of O2-sensing and hypoxia tolerance in pheochromocytoma cells. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001;128:187–204. doi: 10.1016/s1096-4959(00)00326-2. [DOI] [PubMed] [Google Scholar]
- Cunha RA. Neuroprotection by adenosine in the brain: from A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005;1:111–34. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai SS, Zhou YG. Adenosine 2A receptor: a crucial neuromodulator with bidirectional effect in neuroinflammation and brain injury. Rev. Neurosci. 2011;22:231–9. doi: 10.1515/RNS.2011.020. [DOI] [PubMed] [Google Scholar]
- Dal-Cim T, Martins WC, Santos AR, Tasca CI. Guanosine is neuroprotective against oxygen/glucose deprivation in hippocampal slices via large conductance Ca(2)+-activated K+ channels, phosphatidilinositol-3 kinase/protein kinase B pathway activation and glutamate uptake. Neuroscience. 2011;183:212–20. doi: 10.1016/j.neuroscience.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Dale N, Pearson T, Frenguelli BG. Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice. J. Physiol. 2000;14:3–55. doi: 10.1111/j.1469-7793.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alimonte I, Flati V, D'Auro M, et al. Guanosine inhibits CD40 receptor expression and function induced by cytokines and beta amyloid in mouse microglia cells. J. Immunol. 2007;178:720–31. doi: 10.4049/jimmunol.178.2.720. [DOI] [PubMed] [Google Scholar]
- Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney SM, Shepel PN, Geiger JD. Levels of endogenous adenosine in rat striatum I. Regulation by ionotropic glutamate receptors, nitric oxide and free radicals. J. Pharmacol. Exp. Ther. 1998;285:561–7. [PubMed] [Google Scholar]
- Deussen A, Lloyd HG, Schrader J. Contribution of S-adenosylhomocysteine to cardiac adenosine formation. J. Mol. Cell. Cardiol. 1989;21:773–82. doi: 10.1016/0022-2828(89)90716-5. [DOI] [PubMed] [Google Scholar]
- Dickenson JM, Blank JL, Hill SJ. Human adenosine A1 receptor and P2Y2-purinoceptor-mediated activation of the mitogen-activated protein kinase cascade in transfected CHO cells. Br. J. Pharmacol. 1998;124:1491–9. doi: 10.1038/sj.bjp.0701977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- El-Khodor BF, Boksa P. Transient birth hypoxia increases behavioral responses to repeated stress in the adult rat. Behav. Brain Res. 2000;107:171–5. doi: 10.1016/s0166-4328(99)00119-9. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111:904–15. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure M, Voyno-Yasenetskaya TA, Bourne HR. cAMP and beta gamma subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J. Biol. Chem. 1994;269:7851–4. [PubMed] [Google Scholar]
- Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat. Rev. Neurosci. 2006;7:423–36. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–23. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine receptors as drug targets. Exp. Cell Res. 2010;316:1284–8. doi: 10.1016/j.yexcr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–56. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001a;53:527–52. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem. Pharmacol. 2001b;61:443–8. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int. Rev. Neurobiol. 2005a;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu. Rev. Pharmacol. Toxicol. 2005b;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chern Y, Franco R, Sitkovsky M. Aspects of the general biology of adenosine A2A signaling. Prog. Neurobiol. 2007;83:263–76. doi: 10.1016/j.pneurobio.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol. Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenguelli BG, Llaudet E, Dale N. High-resolution real-time recording with microelectrode biosensors reveals novel aspects of adenosine release during hypoxia in rat hippocampal slices. J. Neurochem. 2003;86:1506–15. doi: 10.1046/j.1471-4159.2003.01957.x. [DOI] [PubMed] [Google Scholar]
- Frizzo ME, Lara DR, Prokopiuk Ade S, Vargas CR, Salbego CG, Wajner M, Souza DO. Guanosine enhances glutamate uptake in brain cortical slices at normal and excitotoxic conditions. Cell. Mol. Neurobiol. 2002;22:353–63. doi: 10.1023/A:1020728203682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke. 2009;40:S111–4. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl Acad. Sci. USA. 1976;73:2424–8. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysbers JW, Rathbone MP. Neurite outgrowth in PC12 cells is enhanced by guanosine through both cAMP-dependent and -independent mechanisms. Neurosci. Lett. 1996a;220:175–8. doi: 10.1016/s0304-3940(96)13253-5. [DOI] [PubMed] [Google Scholar]
- Gysbers JW, Rathbone MP. GTP and guanosine synergistically enhance NGF-induced neurite outgrowth from PC12 cells. Int. J. Dev. Neurosci. 1996b;14:19–34. doi: 10.1016/0736-5748(95)00083-6. [DOI] [PubMed] [Google Scholar]
- Hasko G, Kuhel DG, Nemeth ZH, Mabley JG, Stachlewitz RF, Virag L, Lohinai Z, Southan GJ, Salzman AL, Szabo C. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J. Immunol. 2000;164:1013–9. doi: 10.4049/jimmunol.164.2.1013. [DOI] [PubMed] [Google Scholar]
- Hasko G, Sitkovsky MV, Szabo C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol. Sci. 2004;25:152–7. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Hasko G, Pacher P, Vizi ES, Illes P. Adenosine receptor signaling in the brain immune system. Trends Pharmacol. Sci. 2005;26:511–6. doi: 10.1016/j.tips.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun SE, Segeleon JE, Trapp VL, Clotz MA, Horrocks LA. Inosine mediates the protective effect of adenosine in rat astrocyte cultures subjected to combined glucose-oxygen deprivation. J. Neurochem. 1996;67:2051–9. doi: 10.1046/j.1471-4159.1996.67052051.x. [DOI] [PubMed] [Google Scholar]
- Heftberger V, Tomaselli B, Podhraski V, Baier-Bitterlich G. Purine nucleoside mediated protection of primary cerebellar granule cells after hypoxic insult. In Focus on Neurochemistry Research, (Nova Science Publ.) 2005;IX:229–47. [Google Scholar]
- Henke RM, Dastidar RG, Shah A, Cadinu D, Yao X, Hooda J, Zhang L. Hypoxia elicits broad and systematic changes in protein subcellular localization. Am. J. Physiol. Cell Physiol. 2011;301:C913–28. doi: 10.1152/ajpcell.00481.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L. Bioenergetics of cerebral ischemia: a cellular perspective. Neuropharmacology. 2008;55:289–309. doi: 10.1016/j.neuropharm.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Hetman M, Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur. J. Biochem. 2004;271:2050–5. doi: 10.1111/j.1432-1033.2004.04133.x. [DOI] [PubMed] [Google Scholar]
- Hide I, Padgett WL, Jacobson KA, Daly JW. A2A adenosine receptors from rat striatum and rat pheochromocytoma PC12 cells: characterization with radioligand binding and by activation of adenylate cyclase. Mol. Pharmacol. 1992;41:352–9. [PMC free article] [PubMed] [Google Scholar]
- Honig LS, Rosenberg RN. Apoptosis and neurologic disease. Am. J. Med. 2000;108:317–30. doi: 10.1016/s0002-9343(00)00291-6. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Storm DR. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron. 1999;23:11–4. doi: 10.1016/s0896-6273(00)80747-3. [DOI] [PubMed] [Google Scholar]
- Ipata PL, Camici M, Micheli V, Tozz MG. Metabolic network of nucleosides in the brain. Curr. Top. Med. Chem. 2011;11:909–22. doi: 10.2174/156802611795347555. [DOI] [PubMed] [Google Scholar]
- Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J. Cereb. Blood Flow Metab. 2002;22:631–47. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Irwin N, Li YM, O'Toole JE, Benowitz LI. Mst3b, a purine-sensitive Ste20-like protein kinase, regulates axon outgrowth. Proc. Natl Acad. Sci. USA. 2006;103:18320–5. doi: 10.1073/pnas.0605135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Bendjelloul F, Ballerini P, D'Alimonte I, Nargi E, Jiang C, Huang X, Rathbone MP. Guanosine reduces apoptosis and inflammation associated with restoration of function in rats with acute spinal cord injury. Purinergic Signal. 2007;3:411–21. doi: 10.1007/s11302-007-9079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Shepherd RK, Duling BR, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J. Clin. Invest. 1997;100:2849–57. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc. Natl Acad. Sci. USA. 2001;98:9407–12. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Jurkowitz MS, Litsky ML, Browning MJ, Hohl CM. Adenosine, inosine, and guanosine protect glial cells during glucose deprivation and mitochondrial inhibition: correlation between protection and ATP preservation. J. Neurochem. 1998;71:535–48. doi: 10.1046/j.1471-4159.1998.71020535.x. [DOI] [PubMed] [Google Scholar]
- King AE, Ackley MA, Cass CE, Young JD, Baldwin SA. Nucleoside transporters: from scavengers to novel therapeutic targets. Trends Pharmacol. Sci. 2006;27:416–25. doi: 10.1016/j.tips.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, Imaizumi S, Yoshimoto T, Motomiya M. Phenytoin affects metabolism of free fatty acids and nucleotides in rat cerebral ischemia. Stroke. 1990;21:1326–32. doi: 10.1161/01.str.21.9.1326. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Mori A, Shimada J, Mitsumoto Y, Kikuchi T. Intracerebral adenosine infusion improves neurological outcome after transient focal ischemia in rats. Neurol. Res. 2002;24:317–23. doi: 10.1179/016164102101199819. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Millhorn DE. Stimulation of expression for the adenosine A2A receptor gene by hypoxia in PC12 cells A potential role in cell protection. J. Biol. Chem. 1999;274:20358–65. doi: 10.1074/jbc.274.29.20358. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Conforti L, Pun RY, Millhorn DE. Adenosine modulates hypoxia-induced responses in rat PC12 cells via the A2A receptor. J. Physiol. 1998;508(Pt 1):95–107. doi: 10.1111/j.1469-7793.1998.095br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen M, Eckle T, Eltzschig HK. The hypoxia-inflammation link and potential drug targets. Curr. Opin. Anaesthesiol. 2011;24:363–9. doi: 10.1097/ACO.0b013e32834873fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano Y, Echeverry G, Miekisiak G, Kulik TB, Aronhime SN, Chen JF, Winn HR. Role of adenosine A2 receptors in regulation of cerebral blood flow during induced hypotension. J. Cereb. Blood Flow Metab. 2010;30:808–15. doi: 10.1038/jcbfm.2009.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai DM, Tu YK, Liu IM, Cheng JT. Increase of adenosine A1 receptor gene expression in cerebral ischemia of Wistar rats. Neurosci. Lett. 2005;387:59–61. doi: 10.1016/j.neulet.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J. Neurochem. 2001;79:463–84. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- Laubach VE, French BA, Okusa MD. Targeting of adenosine receptors in ischemia-reperfusion injury. Expert. Opin. Ther. Targets. 2011;15:103–18. doi: 10.1517/14728222.2011.541441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Oskouian RJ, Day YJ, Rieger JM, Liu L, Kern JA, Linden J. Mouse spinal cord compression injury is reduced by either activation of the adenosine A2A receptor on bone marrow-derived cells or deletion of the A2A receptor on non-bone marrow-derived cells. Neuroscience. 2006;141:2029–39. doi: 10.1016/j.neuroscience.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Linden J. Structure and function of A1 adenosine receptors. Faseb J. 1991;5:2668–76. doi: 10.1096/fasebj.5.12.1916091. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 1999;79:1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Litsky ML, Hohl CM, Lucas JH, Jurkowitz MS. Inosine and guanosine preserve neuronal and glial cell viability in mouse spinal cord cultures during chemical hypoxia. Brain Res. 1999;821:426–32. doi: 10.1016/s0006-8993(99)01086-0. [DOI] [PubMed] [Google Scholar]
- Lloyd HG, Lindstrom K, Fredholm BB. Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation or energy depletion. Neurochem. Int. 1993;23:173–85. doi: 10.1016/0197-0186(93)90095-m. [DOI] [PubMed] [Google Scholar]
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat. Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Sebastiao AM, Ribeiro JA. Adenosine and related drugs in brain diseases: present and future in clinical trials. Curr. Top. Med. Chem. 2011;11:1087–101. doi: 10.2174/156802611795347591. [DOI] [PubMed] [Google Scholar]
- Lorber B, Howe ML, Benowitz LI, Irwin N. Mst3b, an Ste20-like kinase, regulates axon regeneration in mature CNS and PNS pathways. Nat. Neurosci. 2009;12:1407–14. doi: 10.1038/nn.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Settleman J. The Drosophila Pkn protein kinase is a Rho/Rac effector target required for dorsal closure during embryogenesis. Genes Dev. 1999;13:1168–80. doi: 10.1101/gad.13.9.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lubitz DK. Adenosine and cerebral ischemia: therapeutic future or death of a brave concept? Eur. J. Pharmacol. 1999;371:85–102. doi: 10.1016/s0014-2999(99)00135-1. [DOI] [PubMed] [Google Scholar]
- Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, Vivien D. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10:471–80. doi: 10.1016/S1474-4422(11)70066-7. [DOI] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghji P, Tuttle JB, Rubio R. Adenosine formation and release by embryonic chick neurons and glia in cell culture. J. Neurochem. 1989;53:1852–60. doi: 10.1111/j.1471-4159.1989.tb09252.x. [DOI] [PubMed] [Google Scholar]
- Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan JQ, Henshall DC, Simon RP. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J. Cereb. Blood Flow Metab. 2005;25:234–46. doi: 10.1038/sj.jcbfm.9600024. [DOI] [PubMed] [Google Scholar]
- Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem. J. 1998;332(Pt 2):281–92. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minet E, Arnould T, Michel G, Roland I, Mottet D, Raes M, Remacle J, Michiels C. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett. 2000;468:53–8. doi: 10.1016/s0014-5793(00)01181-9. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modha R, Campbell LJ, Nietlispach D, Buhecha HR, Owen D, Mott HR. The Rac1 polybasic region is required for interaction with its effector PRK1. J. Biol. Chem. 2008;283:1492–500. doi: 10.1074/jbc.M706760200. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–98. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai H. The structure and function of PKN, a protein kinase having a catalytic domain homologous to that of PKC. J. Biochem. (Tokyo) 2003;133:17–27. doi: 10.1093/jb/mvg019. [DOI] [PubMed] [Google Scholar]
- Muller CE, Jacobson KA. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim. Biophys. Acta. 2011;1808:1290–308. doi: 10.1016/j.bbamem.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa K, Kawasaki F, Tanaka A, Nagai K, Fujimoto S. Characterization of guanine and guanosine transport in primary cultured rat cortical astrocytes and neurons. Glia. 2007;55:1397–404. doi: 10.1002/glia.20550. [DOI] [PubMed] [Google Scholar]
- Neary JT. Protein kinase signaling cascades in CNS trauma. IUBMB Life. 2005;57:711–8. doi: 10.1080/15216540500319143. [DOI] [PubMed] [Google Scholar]
- Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–8. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- zur Nedden S, Tomaselli B, Baier-Bitterlich G. HIF-1 alpha is an essential effector for purine nucleoside-mediated neuroprotection against hypoxia in PC12 cells and primary cerebellar granule neurons. J. Neurochem. 2008;105:1901–14. doi: 10.1111/j.1471-4159.2008.05275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicole O, Ali C, Docagne F, Plawinski L, MacKenzie ET, Vivien D, Buisson A. Neuroprotection mediated by glial cell line-derived neurotrophic factor: involvement of a reduction of NMDA-induced calcium influx by the mitogen-activated protein kinase pathway. J. Neurosci. 2001;21:3024–33. doi: 10.1523/JNEUROSCI.21-09-03024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northington FJ, Chavez-Valdez R, Martin LJ. Neuronal cell death in neonatal hypoxia-ischemia. Ann. Neurol. 2011;69:743–58. doi: 10.1002/ana.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–20. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- Oleskovicz SP, Martins WC, Leal RB, Tasca CI. Mechanism of guanosine-induced neuroprotection in rat hippocampal slices submitted to oxygen-glucose deprivation. Neurochem. Int. 2008;52:411–8. doi: 10.1016/j.neuint.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Palmer TM, Stiles GL. Adenosine receptors. Neuropharmacology. 1995;34:683–94. doi: 10.1016/0028-3908(95)00044-7. [DOI] [PubMed] [Google Scholar]
- Palmer TM, Stiles GL. Structure-function analysis of inhibitory adenosine receptor regulation. Neuropharmacology. 1997;36:1141–7. doi: 10.1016/s0028-3908(97)00128-7. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Xiong W. Stimulus- and cell-type-specific release of purines in cultured rat forebrain astrocytes and neurons. J. Neurochem. 2004;88:1305–12. doi: 10.1046/j.1471-4159.2003.02266.x. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Zhang YW, Shepel PN, Greenway SC, Peeling J, Geiger JD. Effects of nitrobenzylthioinosine on neuronal injury, adenosine levels, and adenosine receptor activity in rat forebrain ischemia. J. Neurochem. 2000;75:795–802. doi: 10.1046/j.1471-4159.2000.0750795.x. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Xiong W, Zamzow CR. Astrocytes and neurons: different roles in regulating adenosine levels. Neurol. Res. 2005;27:153–60. doi: 10.1179/016164105X21878. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Damaraju VL, Graham K, Yao SY, Baldwin SA, Cass CE, Young JD. Molecular biology of nucleoside transporters and their distributions and functions in the brain. Curr. Top. Med. Chem. 2011;11:948–72. doi: 10.2174/156802611795347582. [DOI] [PubMed] [Google Scholar]
- Pastor-Anglada M, Casado FJ, Valdes R, Mata J, Garcia-Manteiga J, Molina M. Complex regulation of nucleoside transporter expression in epithelial and immune system cells. Mol. Membr. Biol. 2001;18:81–5. doi: 10.1080/096876800110033783. [DOI] [PubMed] [Google Scholar]
- Peterfreund RA, MacCollin M, Gusella J, Fink JS. Characterization and expression of the human A2a adenosine receptor gene. J. Neurochem. 1996;66:362–8. doi: 10.1046/j.1471-4159.1996.66010362.x. [DOI] [PubMed] [Google Scholar]
- Petrausch B, Tabibiazar R, Roser T, Jing Y, Goldman D, Stuermer CA, Irwin N, Benowitz LI. A purine-sensitive pathway regulates multiple genes involved in axon regeneration in goldfish retinal ganglion cells. J. Neurosci. 2000;20:8031–41. doi: 10.1523/JNEUROSCI.20-21-08031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J. Cereb. Blood Flow Metab. 2007;27:1–5. doi: 10.1038/sj.jcbfm.9600334. [DOI] [PubMed] [Google Scholar]
- van der Ploeg I, Ahlberg S, Parkinson FE, Olsson RA, Fredholm BB. Functional characterization of adenosine A2 receptors in Jurkat cells and PC12 cells using adenosine receptor agonists. Naunyn. Schmiedebergs. Arch. Pharmacol. 1996;353:250–60. doi: 10.1007/BF00168626. [DOI] [PubMed] [Google Scholar]
- Podhraski V, Tomaselli B, Baier-Bitterlich G. Adenosine receptor expression in PC12-cells during normoxic and hypoxic conditions. J. Neurochem. Suppl. 2005;2:210–266. [Google Scholar]
- Press C, Milbrandt J. The purine nucleosides adenosine and guanosine delay axonal degeneration in vitro. J. Neurochem. 2009;109:595–602. doi: 10.1111/j.1471-4159.2009.06002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbone MP, Middlemiss P, Andrew C, Caciagli F, Ciccarelli R, Di Iorio P, Huang R. The trophic effects of purines and purinergic signaling in pathologic reactions of astrocytes. Alzheimer Dis. Assoc. Disord. 1998;12(Suppl. 2):S36–45. doi: 10.1097/00002093-199803001-00006. [DOI] [PubMed] [Google Scholar]
- Rathbone MP, Middlemiss PJ, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F. Trophic effects of purines in neurons and glial cells. Prog. Neurobiol. 1999;59:663–90. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Rathbone M, Pilutti L, Caciagli F, Jiang S. Neurotrophic effects of extracellular guanosine. Nucleosides Nucleotides Nucl. Acids. 2008;27:666–72. doi: 10.1080/15257770802143913. [DOI] [PubMed] [Google Scholar]
- Rathbone MP, Saleh TM, Connell BJ, Chang R, Su C, Worley B, Kim M, Jiang S. Systemic administration of guanosine promotes functional and histological improvement following an ischemic stroke in rats. Brain Res. 2011;1407:79–89. doi: 10.1016/j.brainres.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Rebola N, Pinheiro PC, Oliveira CR, Malva JO, Cunha RA. Subcellular localization of adenosine A(1) receptors in nerve terminals and synapses of the rat hippocampus. Brain Res. 2003;987:49–58. doi: 10.1016/s0006-8993(03)03247-5. [DOI] [PubMed] [Google Scholar]
- Rudolphi KA, Schubert P, Parkinson FE, Fredholm BB. Neuroprotective role of adenosine in cerebral ischaemia. Trends Pharmacol. Sci. 1992;13:439–45. doi: 10.1016/0165-6147(92)90141-r. [DOI] [PubMed] [Google Scholar]
- Schaller B, Graf R, Jacobs AH. Ischaemic tolerance: a window to endogenous neuroprotection? Lancet. 2003;362:1007–8. doi: 10.1016/S0140-6736(03)14446-7. [DOI] [PubMed] [Google Scholar]
- Schmidt AP, Lara DR, Souza DO. Proposal of a guanine-based purinergic system in the mammalian central nervous system. Pharmacol. Ther. 2007;116:401–16. doi: 10.1016/j.pharmthera.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Schulte G, Fredholm BB. The G(s)-coupled adenosine A(2B) receptor recruits divergent pathways to regulate ERK1/2 and p38. Exp. Cell Res. 2003a;290:168–76. doi: 10.1016/s0014-4827(03)00324-0. [DOI] [PubMed] [Google Scholar]
- Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell. Signal. 2003b;15:813–27. doi: 10.1016/s0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Sciotti VM, Roche FM, Grabb MC, Van Wylen DG. Adenosine receptor blockade augments interstitial fluid levels of excitatory amino acids during cerebral ischemia. J. Cereb. Blood Flow Metab. 1992;12:646–55. doi: 10.1038/jcbfm.1992.89. [DOI] [PubMed] [Google Scholar]
- Sebastiao AM. Neuronal ENT1 takes up synaptic adenosine even under hypoxia/ischemia. J. Neurochem. 2011;118:1–3. doi: 10.1111/j.1471-4159.2011.07263.x. [DOI] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu. Rev. Neurosci. 1996;19:463–89. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Seidel MG, Klinger M, Freissmuth M, Holler C. Activation of mitogen-activated protein kinase by the A(2A)-adenosine receptor via a rap1-dependent and via a p21(ras)-dependent pathway. J. Biol. Chem. 1999;274:25833–41. doi: 10.1074/jbc.274.36.25833. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–91. [PubMed] [Google Scholar]
- Semenza GL. Life with oxygen. Science. 2007;318:62–4. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim. Biophys. Acta. 2011;1813:1263–8. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seta KA, Millhorn DE. Functional genomics approach to hypoxia signaling. J. Appl. Physiol. 2004;96:765–73. doi: 10.1152/japplphysiol.00836.2003. [DOI] [PubMed] [Google Scholar]
- Seta KA, Spicer Z, Yuan Y, Lu G, Millhorn DE. Responding to hypoxia: lessons from a model cell line. Sci. STKE. 2002;2002:RE11. doi: 10.1126/stke.2002.146.re11. [DOI] [PubMed] [Google Scholar]
- Sexl V, Mancusi G, Holler C, Gloria-Maercker E, Schutz W, Freissmuth M. Stimulation of the mitogen-activated protein kinase via the A2A-adenosine receptor in primary human endothelial cells. J. Biol. Chem. 1997;272:5792–9. doi: 10.1074/jbc.272.9.5792. [DOI] [PubMed] [Google Scholar]
- Shen H, Chen GJ, Harvey BK, Bickford PC, Wang Y. Inosine reduces ischemic brain injury in rats. Stroke. 2005;36:654–9. doi: 10.1161/01.STR.0000155747.15679.04. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol. 2009;30:102–8. doi: 10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat. Rev. Immunol. 2005;5:712–21. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 2004;22:657–82. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- Soricelli A, Postiglione A, Cuocolo A, De Chiara S, Ruocco A, Brunetti A, Salvatore M, Ell PJ. Effect of adenosine on cerebral blood flow as evaluated by single-photon emission computed tomography in normal subjects and in patients with occlusive carotid disease. A comparison with acetazolamide. Stroke. 1995;26:1572–6. doi: 10.1161/01.str.26.9.1572. [DOI] [PubMed] [Google Scholar]
- Su C, Picard P, Rathbone MP, Jiang S. Guanosine-induced decrease in side population of lung cancer cells: lack of correlation with ABCG2 expression. J. Biol. Regul. Homeost. Agents. 2010;24:19–25. [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 2004;14:311–7. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Sweeney MI. Neuroprotective effects of adenosine in cerebral ischemia: window of opportunity. Neurosci. Biobehav. Rev. 1997;21:207–17. doi: 10.1016/s0149-7634(96)00011-5. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Mukai H, Toshimori M, Miyamoto M, Ono Y. Proteolytic activation of PKN by caspase-3 or related protease during apoptosis. Proc. Natl Acad. Sci. U S A. 1998;95:11566–71. doi: 10.1073/pnas.95.20.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Otsuguro K, Ohta T, Ito S. Adenosine and inosine release during hypoxia in the isolated spinal cord of neonatal rats. Br. J. Pharmacol. 2010;161:1806–16. doi: 10.1111/j.1476-5381.2010.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauerer B, zur Nedden S, Baier-Bitterlich G. Vital role of protein kinase C-related kinase in the formation and stability of neurites during hypoxia. J. Neurochem. 2010;113:432–46. doi: 10.1111/j.1471-4159.2010.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomazi AP, Boff B, Pires TD, Godinho G, Battu CE, Gottfried C, Souza DO, Salbego C, Wofchuk ST. Profile of glutamate uptake and cellular viability in hippocampal slices exposed to oxygen and glucose deprivation: developmental aspects and protection by guanosine. Brain Res. 2008;1188:233–40. doi: 10.1016/j.brainres.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Tomaselli B, Podhraski V, Böck G, Baier-Bitterlich G. Early cellular responses of purine nucleoside-mediated protection of hypoxia-induced injuries of neuronal PC12 cells. Am. J. Biochem. Biotechnol. 2005a;2:161–167. [Google Scholar]
- Tomaselli B, Podhraski V, Heftberger V, Bock G, Baier-Bitterlich G. Purine nucleoside-mediated protection of chemical hypoxia-induced neuronal injuries involves p42/44 MAPK activation. Neurochem. Int. 2005b;46:513–21. doi: 10.1016/j.neuint.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Tomaselli B, Nedden SZ, Podhraski V, Baier-Bitterlich G. p42/44 MAPK is an essential effector for purine nucleoside-mediated neuroprotection of hypoxic PC12 cells and primary cerebellar granule neurons. Mol. Cell. Neurosci. 2008;38:559–68. doi: 10.1016/j.mcn.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Traversa U, Bombi G, Camaioni E, Macchiarulo A, Costantino G, Palmieri C, Caciagli F, Pellicciari R. Rat brain guanosine binding site Biological studies and pseudo-receptor construction. Bioorg. Med. Chem. 2003;11:5417–25. doi: 10.1016/j.bmc.2003.09.043. [DOI] [PubMed] [Google Scholar]
- Uemura Y, Miller JM, Matson WR, Beal MF. Neurochemical analysis of focal ischemia in rats. Stroke. 1991;22:1548–53. doi: 10.1161/01.str.22.12.1548. [DOI] [PubMed] [Google Scholar]
- Van Dycke A, Raedt R, Vonck K, Boon P. Local delivery strategies in epilepsy; a focus on adenosine. Seizure. 2011;20:376–82. doi: 10.1016/j.seizure.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Volpini R, Marucci G, Buccioni M, Dal Ben D, Lambertucci C, Lammi C, Mishra RC, Thomas A, Cristalli G. Evidence for the existence of a specific g protein-coupled receptor activated by guanosine. ChemMedChem. 2011;6:1074–80. doi: 10.1002/cmdc.201100100. [DOI] [PubMed] [Google Scholar]
- Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–49. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhu C, Qiu L, Hagberg H, Sandberg M, Blomgren K. Activation of ERK1/2 after neonatal rat cerebral hypoxia-ischaemia. J. Neurochem. 2003;86:351–62. doi: 10.1046/j.1471-4159.2003.01838.x. [DOI] [PubMed] [Google Scholar]
- Wei CJ, Li W, Chen JF. Normal and abnormal functions of adenosine receptors in the central nervous system revealed by genetic knockout studies. Biochim. Biophys. Acta. 2010;1808:1358–79. doi: 10.1016/j.bbamem.2010.12.018. [DOI] [PubMed] [Google Scholar]
- White BC, Sullivan JM, DeGracia DJ, O'Neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J. Neurol. Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Yawo H, Chuhma N. Preferential inhibition of omega-conotoxin-sensitive presynaptic Ca2+ channels by adenosine autoreceptors. Nature. 1993;365:256–8. doi: 10.1038/365256a0. [DOI] [PubMed] [Google Scholar]
- Young AR, Ali C, Duretete A, Vivien D. Neuroprotection and stroke: time for a compromise. J. Neurochem. 2007;103:1302–9. doi: 10.1111/j.1471-4159.2007.04866.x. [DOI] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–9. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Yue X, Mehmet H, Penrice J, Cooper C, Cady E, Wyatt JS, Reynolds EO, Edwards AD, Squier MV. Apoptosis and necrosis in the newborn piglet brain following transient cerebral hypoxia-ischaemia. Neuropathol. Appl. Neurobiol. 1997;23:16–25. [PubMed] [Google Scholar]
- Zai L, Ferrari C, Subbaiah S, Havton LA, Coppola G, Strittmatter S, Irwin N, Geschwind D, Benowitz LI. Inosine alters gene expression and axonal projections in neurons contralateral to a cortical infarct and improves skilled use of the impaired limb. J. Neurosci. 2009;29:8187–97. doi: 10.1523/JNEUROSCI.0414-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Xiong W, Albensi BC, Parkinson FE. Expression of human equilibrative nucleoside transporter 1 in mouse neurons regulates adenosine levels in physiological and hypoxic-ischemic conditions. J. Neurochem. 2011;118:4–11. doi: 10.1111/j.1471-4159.2011.07242.x. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Li C, Olah ME, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc. Natl Acad. Sci. USA. 1992;89:7432–6. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WH, Conforti L, Czyzyk-Krzeska MF, Millhorn DE. Membrane depolarization in PC-12 cells during hypoxia is regulated by an O2-sensitive K+ current. Am. J. Physiol. 1996;271:C658–65. doi: 10.1152/ajpcell.1996.271.2.C658. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn. Schmiedebergs. Arch. Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- Zimmermann H, Braun N, Kegel B, Heine P. New insights into molecular structure and function of ectonucleotidases in the nervous system. Neurochem. Int. 1998;32:421–5. doi: 10.1016/s0197-0186(97)00126-5. [DOI] [PubMed] [Google Scholar]
- Zurn AD, Do KQ. Purine metabolite inosine is an adrenergic neurotrophic substance for cultured chicken sympathetic neurons. Proc. Natl Acad. Sci. USA. 1988;85:8301–5. doi: 10.1073/pnas.85.21.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]


