Abstract
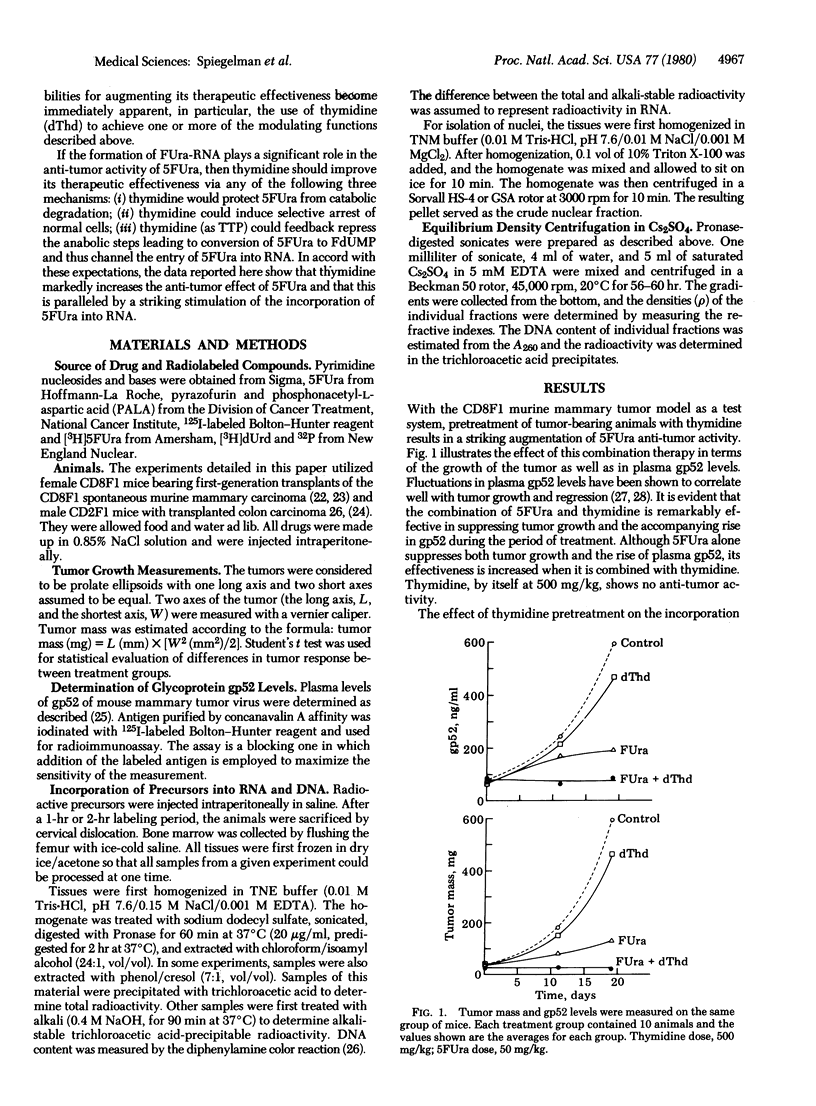
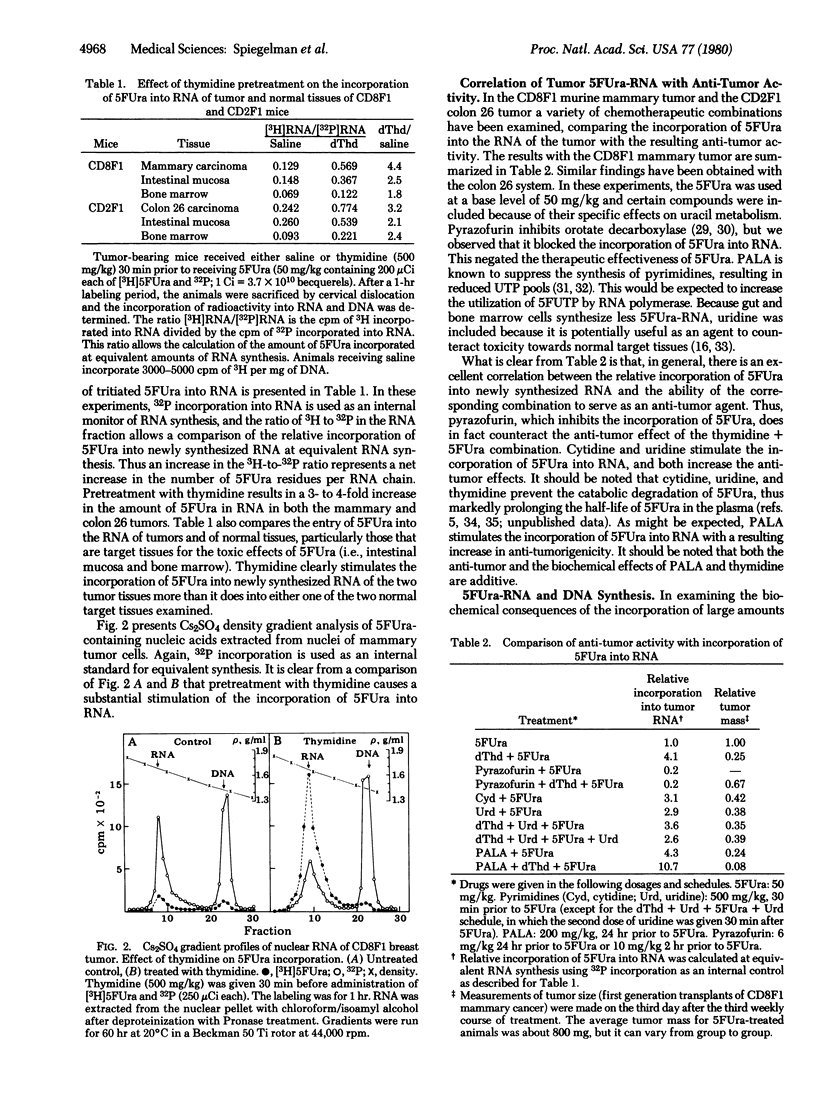
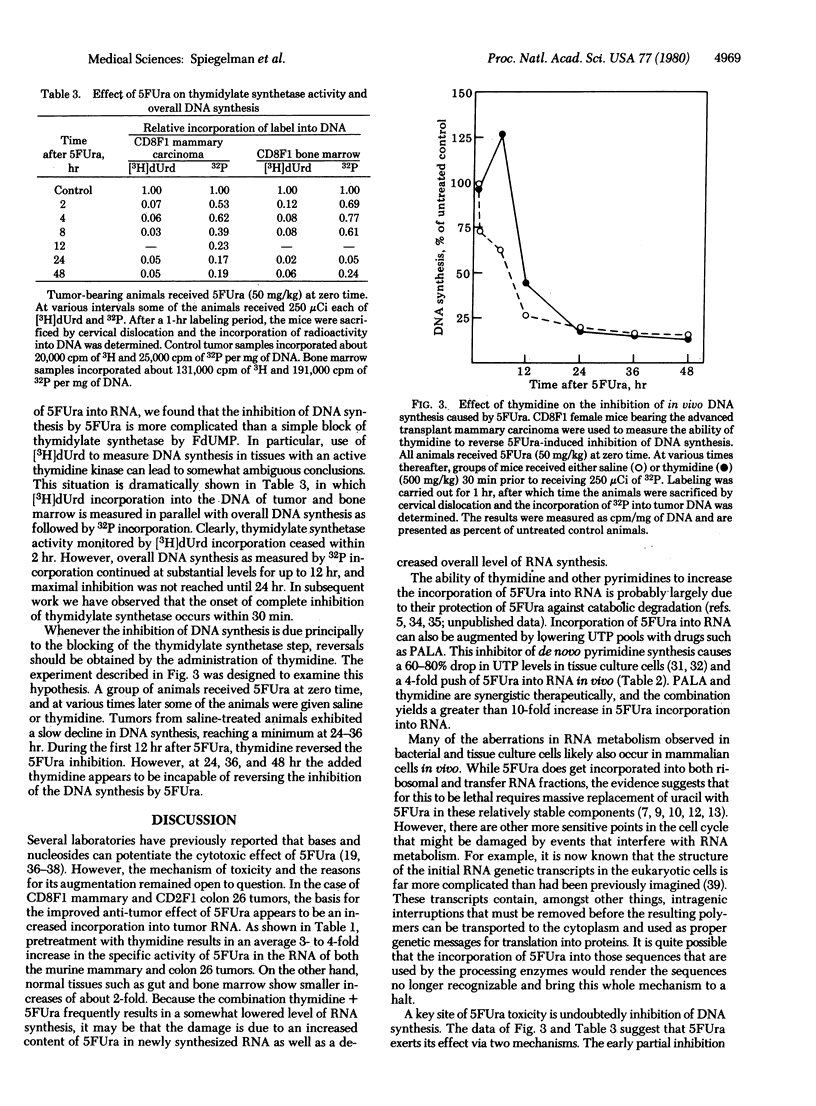
The experiments described here illustrate the use of metabolic modulation to improve the therapeutic effectiveness of 5-fluorouracil (5FUra) in two murine tumor systems (CD8F1 mannary carcinoma and CD2F1 colon tumor 26). The manipulations chosen were based on the assumption that a major fraction of the anti-tumor activity of 5FUra is due to its incorporation into RNA and that the resulting 5FUra-RNA creates difficulty for a variety of cellular mechanisms requiring RNA processing and function. This hypothesis leads to the prediction that thymidine would promote the anti-neoplastic effect of 5FUra due to the following possible interactions: (i) sparing 5FUra from catabolic degradation by saturating the relevant enzymes wit thymidine; (ii) selective arrest of normal cells due to feedback inhibition of robonucleotide reductase by the accumulating thymidine triphosphate (TTP); and (iii) the high levels of TTP would also be expected to repress the anabolic conversion of 5FUra to the deoxy derivatives, thus preserving it for entry into RNA. The data show that thymidine (and certain other nucleosides) does in fact markedly stimulate the incorporation of 5FUra into nuclear RNA and that this event is paralleled by a striking icrease in anti-tumor activity. Kinetic analysis reveals that, although the injection of 5FUra leads to an immediate cessation of thymidylate synthetase activity, DNA synthesis continues at a lower rate for 12 hr and then ceases completely. At this point, in contrast to the earlier partial inhibition, the addition of thymidine fails to restore the ability of the tumor cells to synthesize DNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURCHENAL J. H. OETTGEN HF, REPPERT JA, COLEY V: Studies on the synergism of fluorinated pyrimidines and certain pyrimidine and purine derivatives against transplanted mouse leukemia. Cancer Chemother Rep. 1960 Feb;6:1–5. [PubMed] [Google Scholar]
- Brockman R. W., Shaddix S. C., Rose L. M. Biochemical aspects of chemotherapy of mouse colon carcinoma: fluoropyrimidines and pyrazofurin. Cancer. 1977 Nov;40(5 Suppl):2681–2691. doi: 10.1002/1097-0142(197711)40:5+<2681::aid-cncr2820400941>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Burstin S. J., Basilico C. Transformation by polyoma virus alters expression of a cell mutation affecting cycle traverse. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2540–2544. doi: 10.1073/pnas.72.7.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadman E. C., Dix D. E., Handschumacher R. E. Clinical, biological, and biochemical effect of pyrazofurin. Cancer Res. 1978 Mar;38(3):682–688. [PubMed] [Google Scholar]
- Glazer R. I., Peale A. L. The effect of 5-fluorouracil on the synthesis of nuclear RNA in L1210 cells in vitro. Mol Pharmacol. 1979 Jul;16(1):270–277. [PubMed] [Google Scholar]
- HEIDELBERGER C., CHAUDHURI N. K., DANNEBERG P., MOOREN D., GRIESBACH L., DUSCHINSKY R., SCHNITZER R. J., PLEVEN E., SCHEINER J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957 Mar 30;179(4561):663–666. doi: 10.1038/179663a0. [DOI] [PubMed] [Google Scholar]
- Heidelberger C. Fluorinated pyrimidines. Prog Nucleic Acid Res Mol Biol. 1965;4:1–50. doi: 10.1016/s0079-6603(08)60783-7. [DOI] [PubMed] [Google Scholar]
- Jato J., Windheuser J. J. 5-Fluorouracil and derivatives in cancer chemotherapy. 3. In vivo enhancement of antitumor activity of 5-fluorouracil (FU) and 5-fluoro-2'-deoxyuridine (FUDR). J Pharm Sci. 1973 Dec;62(12):1975–1978. doi: 10.1002/jps.2600621215. [DOI] [PubMed] [Google Scholar]
- Johnson R. K., Swyryd E. A., Stark G. R. Effects of N-(phosphonacetyl)-L-aspartate on murine tumors and normal tissues in vivo and in vitro and the relationship of sensitivity to rate of proliferation and level of aspartate transcarbamylase. Cancer Res. 1978 Feb;38(2):371–378. [PubMed] [Google Scholar]
- KAPLAN A. S., BEN-PORAT T. The action of 5-fluorouracil on the nucleic acid metabolism of pseudorabies virus-infected and noninfected rabbit kidney cells. Virology. 1961 Jan;13:78–92. doi: 10.1016/0042-6822(61)90034-4. [DOI] [PubMed] [Google Scholar]
- MUKHERJEE K. L., HEIDELBERGER C. Studies on fluorinated pyrimidines. IX. The degradation of 5-fluorouracil-6-C14. J Biol Chem. 1960 Feb;235:433–437. [PubMed] [Google Scholar]
- Martin D. S., Stolfi R. L., Sawyer R. C., Nayak R., Spiegelman S., Young C. W., Woodcock T. An overview of thymidine. Cancer. 1980 Mar 15;45(5 Suppl):1117–1128. doi: 10.1002/1097-0142(19800315)45:5+<1117::aid-cncr2820451316>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Moyer J. D., Handschumacher R. E. Selective inhibition of pyrimidine synthesis and depletion of nucleotide pools by N-(phosphonacetyl)-L-aspartate. Cancer Res. 1979 Aug;39(8):3089–3094. [PubMed] [Google Scholar]
- Pardee A. B., James L. J. Selective killing of transformed baby hamster kidney (BHK) cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4994–4998. doi: 10.1073/pnas.72.12.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P. Processing of RNA. Annu Rev Biochem. 1976;45:605–629. doi: 10.1146/annurev.bi.45.070176.003133. [DOI] [PubMed] [Google Scholar]
- Ritzi E., Baldi A., Spiegelman S. The purification of a gs antigen of the murine mammary tumor virus and its quantitation by radioimmunoassay. Virology. 1976 Nov;75(1):188–197. doi: 10.1016/0042-6822(76)90017-9. [DOI] [PubMed] [Google Scholar]
- Ritzi E., Martin D. S., Stolfi R. L., Spiegelman S. Plasma levels of a viral protein as a diagnostic signal for the presence of mammary tumor: the effect of tumor removal. J Exp Med. 1977 Apr 1;145(4):999–1013. doi: 10.1084/jem.145.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzi E., Martin D. S., Stolfi R. L., Spiegelman S. Plasma levels of a viral protein as a diagnostic signal for the presence of tumor : the murine mammary tumor model. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4190–4194. doi: 10.1073/pnas.73.11.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelli G., Valeriote F. In vivo enhancement of 5-fluorouracil cytotoxicity to AKR leukemia cells by thymidine in mice. J Natl Cancer Inst. 1978 Sep;61(3):843–847. [PubMed] [Google Scholar]
- Santelli G., Valeriote F. In vivo potentiation of 5-fluorouracil cytotoxicity against AKR leukemia by purines, pyrimidines, and their nucleosides and deoxynucleosides. J Natl Cancer Inst. 1980 Jan;64(1):69–72. [PubMed] [Google Scholar]
- Spiegelman S., Nayak R., Sawyer R., Stolfi R., Martin D. Potentiation of the anti-tumor activity of 5FU by thymidine and its correlation with the formation of (5FU)RNA. Cancer. 1980 Mar 15;45(5 Suppl):1129–1134. doi: 10.1002/1097-0142(19800315)45:5+<1129::aid-cncr2820451317>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Stolfi R. L., Martin D. S., Fugmann R. A. Spontaneous murine mammary adenocarcinoma: model system for evaluation of combined methods of therapy. Cancer Chemother Rep. 1971 Jun;55(3):239–251. [PubMed] [Google Scholar]
- Tobey R. A., Petersen D. F., Anderson E. C. Differential response of two derivatives of the BHK21 hamster cell to thymidine. J Cell Physiol. 1967 Jun;69(3):341–343. doi: 10.1002/jcp.1040690310. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. S., Cihak A., Pitot H. C. Inhibition of ribosomal ribonucleic acid maturation in rat liver by 5-fluoroorotic acid resulting in the selective labeling of cytoplasmic messenger ribonucleic acid. J Biol Chem. 1971 Nov;246(21):6418–6427. [PubMed] [Google Scholar]
- Wilkinson D. S., Crumley J. The mechanism of 5-fluorouridine toxicity in Novikoff hepatoma cells. Cancer Res. 1976 Nov;36(11 Pt 1):4032–4038. [PubMed] [Google Scholar]
- Wilkinson D. S., Pitot H. C. Inhibition of ribosomal ribonucleic acid maturation in Novikoff hepatoma cells by 5-fluorouracil and 5-fluorouridine. J Biol Chem. 1973 Jan 10;248(1):63–68. [PubMed] [Google Scholar]
- Wilkinson D. S., Tlsty T. D., Hanas R. J. The inhibition of ribosomal RNA synthesis and maturation in Novikoff hepatoma cells by 5-fluorouridine. Cancer Res. 1975 Nov;35(11 Pt 1):3014–3020. [PubMed] [Google Scholar]
- Willén R., Stenram U. RNA synthesis in the liver of rats treated with 5-fluorouracil. Arch Biochem Biophys. 1967 Mar;119(1):501–503. doi: 10.1016/0003-9861(67)90483-3. [DOI] [PubMed] [Google Scholar]
- Zechel K. Initiation of DNA synthesis by RNA. Curr Top Microbiol Immunol. 1978;82:71–112. doi: 10.1007/978-3-642-46388-4_3. [DOI] [PubMed] [Google Scholar]



