Abstract
Stress often disrupts behavior and can lead to psychiatric illness. Considerable evidence suggests that corticotropin-releasing factor (CRF) plays an important role in regulating the effects of stress. CRF administration produces stress-like effects in humans and laboratory animals, and CRF levels are elevated in individuals with stress-related illness. Recent work indicates that κ-opioid receptor (KOR) antagonists can block CRF effects, raising the possibility that at least some of the effects of stress are mediated via KORs. Here we examined the effects of CRF on performance in the 5-choice serial reaction time task (5CSRTT), a test used to quantify attention in rodents, as well as functional interactions between CRF and KORs. Male Sprague-Dawley rats were trained in the 5CSRTT and then each was implanted with an intracerebroventricular (ICV) cannula. After recovery and restabilization of performance, they received a single intraperitoneal (IP) injection of vehicle or JDTic (10 mg/kg), a KOR antagonist with long-lasting (>14 days) effects. In subsequent sessions, rats received ICV infusions of CRF (0.25–1.0 μg) or vehicle and were tested 60 min later. CRF dose-dependently disrupted performance as reflected by decreases in correct responding, increases in omission errors, increases in latencies to respond correctly, and increases in time to complete the session. JDTic attenuated each of these CRF-induced deficits while having no effects on its own. The persistent ability of JDTic to disrupt KOR function was confirmed using the tail immersion assay. These findings indicate that KOR antagonists can prevent acute stress-related effects that degrade performance in tasks requiring attention.
Keywords: stress, CRF, cognition, attention, κ-antagonist, rat
INTRODUCTION
Stress can have disruptive effects on behavior, cognition, and motivation (Campeau et al, 2011; Knoll and Carlezon, 2010). Exposure to severe or repeated stress can cause or exacerbate psychiatric illnesses including anxiety and depressive disorders such as posttraumatic stress disorder (PTSD) (Keane et al, 2006; Keller et al, 2007; Kessler et al, 2010). Stress-related illnesses are debilitating and burdensome because they tend to be persistent, resistant to treatment, and comorbid with substance abuse disorders (Chilcoat and Breslau, 1998; Greenberg et al, 1999; Koob and Kreek, 2007). Currently, there are no treatments available that reliably block the effects of stress or have broad efficacy in reversing the long-term effects of prior stress exposure.
There is considerable evidence that corticotropin-releasing factor (CRF) plays an important role in regulating stress effects. CRF is a neuropeptide that is released in the brain in response to stress (Koob, 1999). Administration of CRF produces many of the same physiological and behavioral effects as stress in people and laboratory animals (Hauger et al, 2009), and people with stress-related psychiatric illness have higher levels of CRF in cerebrospinal fluid (CSF) and blood (Bremner et al, 1997; Sautter et al, 2003; de Kloet et al, 2008). Although much is known about the neural mechanisms by which CRF regulates stress (Bangasser and Valentino, 2012), it has remained difficult to develop clinically effective antistress agents that act directly at CRF receptors (Zorrilla and Koob, 2010).
Accumulating evidence suggests that important aspects of the stress-related effects of CRF are mediated by κ-opioid receptors (KORs) (Bruchas and Chavkin, 2010; Knoll and Carlezon, 2010), the receptor at which the endogenous opioid dynorphin acts (Chavkin et al, 1982). For example, the prototypical KOR antagonist nor-binaltorphimine (nor-BNI) blocks CRF-induced dysphoria in the place conditioning test (Land et al, 2008) and reductions in open arm time in the elevated plus maze (Bruchas et al, 2009). Our group has shown in preliminary tests that JDTic, another highly selective KOR antagonist that is structurally unrelated to nor-BNI (Carroll et al, 2004), also blocks CRF-induced elevations in acoustic startle behavior (Van't Veer et al, 2011). The observations that CRF-induced phosphorylation of KORs is blocked by KOR antagonists (Land et al, 2008) and CRF-induced anxiety behavior is reduced in dynorphin knockout mice (Bruchas et al, 2009) provide molecular evidence for links between CRF and KOR systems. Interactions between these systems have been thoroughly characterized within the raphe nucleus (Bruchas et al, 2011) but may also occur in other brain regions (Pliakas et al, 2001; Newton et al, 2002; Shirayama et al, 2004; Muschamp et al, 2011b; Knoll et al, 2011). The notion that KOR antagonists block the effects of stress fits well with other evidence that these agents have antidepressant-like (Pliakas et al, 2001; Newton et al, 2002; Mague et al, 2003; Shirayama et al, 2004) and anxiolytic-like effects, including the ability to block acquisition of fear-potentiated startle (Knoll et al, 2007, 2011), a procedure often used to study PTSD (Mahan and Ressler, 2012). In addition, KOR agonists can produce key behavioral signs of stress (McLaughlin et al, 2003, 2006; Mague et al, 2003; Todtenkopf et al, 2004). When considered together, these findings raise the possibility that pretreatment with KOR antagonists could reduce or prevent the effects of stress, representing an alternative approach to modulating the behavior-disrupting effects of CRF.
The present studies were designed to examine how CRF affects a subset of cognitive behaviors in rodents, and whether pretreatment with a selective KOR antagonist (JDTic) mitigates any stress-like effects. Cognitive behavior was quantified using the 5-choice serial reaction time task (5CSRTT), a food-motivated test that is analogous to the continuous performance task used to study attention in humans (Rosvold et al, 1956; Robbins, 2002). The 5CSRTT yields metrics that quantify attention, reaction time, motivation, and impulsivity (Robbins, 2002; Paine et al, 2007; Nemeth et al, 2010). Stress is known to degrade performance in tasks requiring attention or concentration in humans (Campeau et al, 2011), and poor concentration is one of the diagnostic criteria for stress-related psychiatric illnesses such as PTSD (American Psychiatric Association, 2000). Previous work has demonstrated that JDTic produces long-lasting (>14 days) disruptions of KOR function (Carroll et al, 2004) and that the behavioral effects of JDTic and nor-BNI are virtually identical (Knoll et al, 2007; Knoll and Carlezon, 2010). To confirm that a single injection of JDTic produced disruption of KOR function for the duration of our tests in the 5CSRTT, we examined the ability of the KOR agonist U50,488 to produce antinociceptive effects in the tail immersion assay (Smith and French, 2002).
MATERIALS AND METHODS
Rats
A total of 14 male Sprague-Dawley rats (Charles River, Raleigh, NC; 250–275 g at the start of the experiment) were used. Rats were housed two per cage upon arrival and kept on a 12 : 12-h light/dark cycle (lights on at 0700 h) and given 1 week to acclimate with free access to food (Purina Rat Chow; Ralston Purina, St Louis, MO) and water. Beginning 2 days before the start of training, the rats were food restricted to 85% of their free-feeding weight. Experiments were conducted in accordance with National Institutes of Health and McLean Hospital guidelines for the care and use of laboratory animals.
5CSRTT
The apparatus and training have been described previously (Paine et al, 2007). Briefly, the operant chambers (Med-Associates, St Albans, VT) were contained within sound-attenuating cubicles. One wall contained five apertures capable of LED illumination and outfitted with infrared detectors to record nose-pokes. The opposite wall contained a food reward receptacle also capable of illumination and nose-poke detection that was connected to a pellet dispenser. Rats were handled for 3 days before the start of training. During the next 3 days, rats were trained to retrieve food pellets (45 mg; Bio-Serv, Frenchtown, NJ) from the food magazine. Rats were then trained to nose-poke in one of five spatial locations within 5 s of the presentation of a brief stimulus light (0.5 s). A timely response in this aperture resulted in delivery of one food pellet. Incorrect nose-pokes in the other apertures resulted in a 5-s timeout. Similarly, failing to respond (omission) or responding during the 5-s intertrial interval (premature response) resulted in a 5-s timeout. Sessions were 90 trials or 30 min, whichever came first. Performance measures of primary interest were: % correct ((correct responses/(correct+incorrect+omitted responses)) × 100), accuracy ((correct responses/(correct+incorrect responses)) × 100), % omissions ((total omissions/number of trials) × 100; trials in which no response was emitted), latency to make a correct response (the time from the stimulus onset to a correct response; a putative indicator of speed of processing or decision making), reward latency (the time from a correct response to the collection of the food pellet; a putative indicator of motivation), premature responses (responses during the ITI; a putative indicator of impulsivity), and time to complete the task (a putative indicator of overall performance capabilities). The criteria to advance to the next stages of the experiments were >60% correct responses and <20% omissions for 5 consecutive days.
Stereotaxic Surgery
Upon meeting performance criteria, rats underwent surgery to implant an intracerebroventricular (ICV) cannula. Each rat was anesthetized with an intraperitoneal (IP) injection of pentobarbital (65 mg/kg) supplemented with subcutaneous atropine (0.25 mg/kg) to minimize bronchial secretions, and placed in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA). For each rat, a stainless steel guide cannula (23 gauge, Plastics One, Roanoke, VA) with a dummy stylet extending 1.5 mm beyond the tip was lowered into the right lateral ventricle at coordinates relative to bregma; anteroposterior =−0.8 mm, mediolateral =1.3 mm, and lowered −3.5 mm ventral to dura. Dental acrylic (Stoelting, Wood Dale, IL) secured the cannula to screws (Plastics One) attached to the skull. Rats were housed individually after surgery to recover for 5–7 days, and then tested until their performance had restabilized to baseline levels (±10%) while also fulfilling the basic response criteria (>60% correct responding, <20% omissions). Microinfusions were performed by removing the dummy stylet and replacing it with a 30-gauge infusion stylet (Plastics One) attached to a Hamilton microsyringe (10 μl) by polyethylene tubing. ICV infusions of CRF (0.25, 0.5, or 1.0 μg) or vehicle (artificial CSF (aCSF); Harvard Apparatus, Holliston, MA) were performed over a 2-min period at a rate of 0.5 μl/min, with an additional 2 min of diffusion time before the stylet was removed and the dummy stylet was replaced. Testing began 60 min after infusion.
5CRSTT Studies: Drugs and Design
CRF was purchased from American Peptide (Sunnyvale, CA) and dissolved in aCSF. JDTic was synthesized at Research Triangle Institute (Research Triangle Park, NC) and dissolved in 0.9% saline; 10 mg/kg (based on the salt form of the drug) was selected because this dose produces strong anxiolytic-like effects in rats (Knoll et al, 2007). Rats first received an infusion of aCSF to ensure the infusion procedure did not affect performance, and to obtain data to serve as baseline. After 48 h, the rats received an injection of either JDTic (10 mg/kg, IP; n=7) or vehicle (1.0 ml/kg, IP, n=7). A 24-h pretreatment period was used before beginning behavioral testing to optimize KOR selectivity (Carroll et al, 2004; Knoll et al, 2007). Rats were subsequently tested with CRF in the following order: 0 (aCSF), 0.5, 1.0, and 0.25 μg. Rats did not receive subsequent treatments until their performance had restabilized to baseline levels (±10%), while also fulfilling the basic response criteria (>60% correct responding, <20% omissions).
Tail Immersion Assay: Drugs and Design
After the final test in the 5CSRTT, the ability of a single injection of JDTic to produce long-lasting disruptions of KOR function was assessed by quantifying KOR agonist-induced analgesia in the tail immersion assay (Smith and French, 2002). A stopwatch was used to measure the latency at which each rat removed its tail from a 52 °C (±1 °C) water bath. A baseline measurement was obtained before treatment with the KOR agonist (±)-trans-U50,488 methanesulfonate (15 mg/kg, IP, dissolved in 0.9% saline; dose based on the salt form of the drug). Latencies were re-assessed 60 min after KOR agonist treatment. A cutoff time of 15 s was used to prevent tissue damage to the tail.
Histological Analysis
After the tail immersion assay, rats were overdosed with pentobarbital (130 mg/kg, IP) and perfused with 0.9% saline followed by 4% paraformaldehyde. Brains were kept overnight in 30% glycerol before sectioning (40 μm). ICV cannula placements were verified in histological analyses by an observer unaware of the treatment conditions. Data from rats in which the tip of the cannula was found to be embedded in brain tissue adjacent to the lateral ventricle, rather than being located within the lateral ventricle itself, were excluded from the statistical analyses as this could affect the quality of the ICV infusion.
Statistics
For the 5CSRTT, two-way (treatment × session) analyses of variance (ANOVAs) with repeated measures were used to compare the effects of an ICV infusion alone (baseline) with the effects of the ICV infusion plus the pretreatment (saline or JDTic). Separate two-way (pretreatment × treatment) ANOVAs with repeated measures were used to compare the effects of various doses of CRF in saline- or JDTic-treated mice. Analyses were performed for each individual metric. For the tail immersion assay, a two-way (pretreatment × treatment) ANOVA with repeated measures was used to examine the effects of prior treatment with JDTic on U50,488-induced antinociception, and a t-test was used to examine group differences in the timing of the tail immersion assay. Significant interactions in the ANOVAs were further analyzed using Newman–Keuls post-hoc tests, whereas significant main effects in the absence of interactions were further analyzed using Simple Main Effects tests.
RESULTS
Three rats (1 vehicle and 2 JDTic) were excluded because histological analyses revealed that the tips of their guide cannula had been embedded in tissue adjacent to the lateral ventricle. The tips of the ICV cannulas for the remaining six vehicle-treated rats and five JDTic-treated rats were located entirely within the lateral ventricle (Figure 1) and thus data from these rats were included in the final statistical analyses.
Figure 1.
Representative micrograph of ICV cannula track in cresyl violet-stained tissue. Rats were excluded if the tip of the cannula was embedded in the brain tissue surrounding the lateral ventricle (LV).
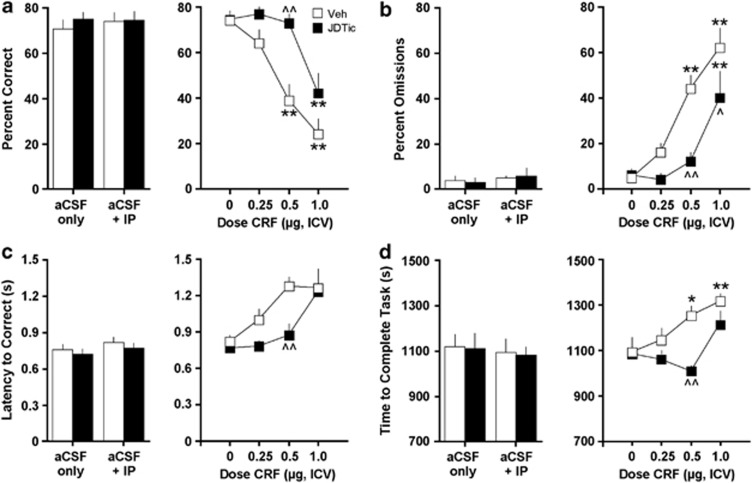
CRF produced JDTic-sensitive disruptions in performance in four of the 5CSRTT metrics: percent correct responding, percent omission errors, latency to make a correct response, and time to complete the task (Figure 2). For correct responding, administration of JDTic did not produce any effects on its own (Figure 2a, left panel). However, the effects of CRF depended on an interaction of pretreatment (saline or JDTic) and treatment (CRF dose) (F(3, 27)=3.60, P<0.05; Figure 2a, right panel). The post-hoc analyses of within-subject effects revealed that CRF significantly reduced the percentage of correct responding at 0.5 and 1.0 μg doses (all P-values <0.01) in saline-treated rats, but only at the 1.0 μg dose (P<0.01) in JDTic-treated rats. Between-group analyses revealed that correct responding was significantly higher in JDTic-treated rats than in saline-treated rats at the 0.5 μg dose of CRF (P<0.01). Similarly, for omission errors, JDTic did not produce any effects on its own (Figure 2b, left panel), but the effects of CRF depended on a pretreatment × treatment interaction (F(3, 27)=3.33, P<0.05; Figure 2b, right panel). The post-hoc analyses of within-subject effects revealed that CRF significantly increased the percentage of omitted responses at 0.5 and 1.0 μg doses (all P-values <0.01) in saline-treated rats, but only at the 1.0 μg dose (P<0.01) in JDTic-treated rats. Between-group analyses revealed that the percentage of omitted responses was lower in JDTic-treated rats than in saline-treated rats at the 0.5 μg (P<0.01) and 1.0 μg (P<0.05) doses of CRF. Whereas JDTic on its own did not have any effects on latency to make correct responses (Figure 2c, left panel), the effects of CRF depended on the main effects of pretreatment (F(1, 9)=5.82, P<0.05) and treatment (F(3, 27)=9.48, P<0.01; Figure 2c, right panel). The post-hoc analyses of between-group effects using Simple Main Effects tests revealed that the latencies to respond correctly were lower in JDTic-treated rats than in saline-treated rats at the 0.5 μg dose of CRF (F(1, 9)=10.9, P<0.01). JDTic on its own also did not have any effects on time to complete the task (ie, to finish the test session; Figure 2d, left panel), but the effects of CRF depended on a pretreatment × treatment interaction (F(3, 27)=3.00, P<0.05; Figure 2d, right panel). The post-hoc analyses of within-subject effects revealed that CRF significantly increased the time to complete the task at the 0.5 μg (P<0.05) and 1.0 μg doses (P<0.01) in saline-treated rats, but not at any of the doses tested in the JDTic-treated rats. Between-group analyses revealed that time to complete the task was shorter in JDTic-treated rats than in saline-treated rats at the 0.5 μg dose of CRF (P<0.01).
Figure 2.
Effects of JDTic pretreatment on the ability of CRF to affect performance in the 5CSRTT. Left panel represents effects of pretreatment alone on baseline (a) percent correct responses, (b) percent omissions, (c) latency to correct responses (in seconds), and (d) latency to complete the task (in seconds); right panel represents effects after various doses of CRF. *P<0.05, **P<0.01 within-group comparisons, ^P<0.05, ^^P<0.01 between-group comparisons, Newman–Keuls post-hoc t-tests.
In addition (Table 1), CRF produced effects on other metrics that were not affected by JDTic treatment: there were main effects on accuracy (F(3, 27)=3.56, P<0.05), latencies to collect the reward (food pellet) (F(3, 27)=5.87, P<0.01), and number of premature responses (F(3, 27)=3.33, P<0.05).
Table 1. Additional 5CSRTT Metrics.
| Metric | Group | Pretreat | aCSF | CRF 0.25 | CRF 0.5 | CRF 1.0 | Statisticsa |
|---|---|---|---|---|---|---|---|
| Accuracy | Vehicle | 74.0±4.6 | 77.7±4.2 | 75.8±4.4 | 69.0±6.1 | 58.2±6.6 | F(3, 27)=3.56 |
| JDTic | 76.2±2.4 | 78.2±4.3 | 81.6±4.9 | 82.8±2.3 | 67.8±2.8 | P<0.05 | |
| Reward latency | Vehicle | 1.53±0.10 | 1.65±0.14 | 1.93±0.33 | 2.09±0.41 | 2.35±0.36 | F(3, 27)=5.87 |
| JDTic | 1.52±0.15 | 1.48±0.11 | 1.59±0.16 | 1.63±0.17 | 2.40±0.30 | P<0.01 | |
| Premature | Vehicle | 21.8±5.8 | 20.8±10.2 | 15.4±4.1 | 10.8±1.6 | 9.7±1.8 | F(3, 27)=3.33 |
| JDTic | 21.8±6.8 | 17.2±2.9 | 16.4±3.7 | 4.4±2.0 | 7.6±1.7 | P<0.05 |
Main effects of treatment (CRF dose). No main effects of pretreatment or interactions.
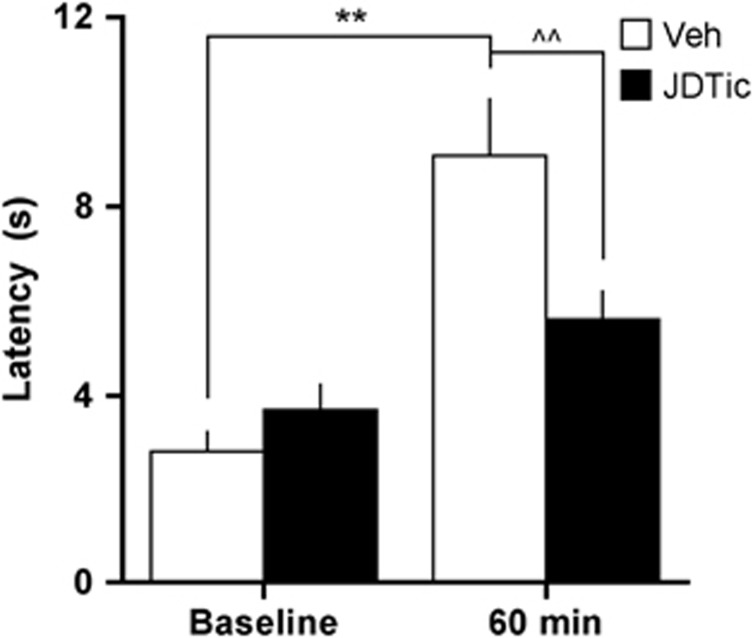
In the tail immersion assay, latencies to remove the tail from the hot water depended upon a significant pretreatment × treatment interaction (F(1, 9)=9.34, P<0.05; Figure 3). The post-hoc analyses of within-subject effects revealed that latencies were significantly higher 60 min after U50,488 (15 mg/kg, IP) in the saline-treated group (P<0.01) but not in the JDTic-treated group. Between-group analyses revealed no group differences at baseline but significantly higher latencies in the saline-treated rats 60 min after U50,488 treatment (P<0.01). Rats in the JDTic-treated group received the tail immersion test 11.0 (±1.6) days after pretreatment, whereas rats in the saline-treated group received it 15.8 (±6.4) days after pretreatment. This difference was not statistically significant (t(9)=1.76, not significant); much of the variability in the saline-treated group was attributable to a rat that was resistant to restabilization after treatment with the 1.0 μg dose of CRF.
Figure 3.
Effects of JDTic pretreatment on latency to withdraw the tail (in seconds) in the tail immersion assay at baseline or 60 min after administration of the KOR-selective agonist U50,488 (15 mg/kg, IP). **P<0.01 within-group comparisons, ^^P<0.01 between-group comparisons, Newman–Keuls post-hoc t-tests.
DISCUSSION
We report three important findings. First, we show that administration of CRF, an approach known to mimic effects of stress in humans and laboratory animals (Bangasser and Valentino, 2012), can produce dramatic disruptions of performance in rats as measured in the 5CSRTT. This finding is novel, considering that previous work suggests that CRF can enhance performance in the 5CSRTT at lower doses (0.1 μg) and shorter pretreatment times (20 min) (Ohmura et al, 2009), and suggests an inverted U-shaped function of CRF on cognitive behavior. Second, we show that pretreatment with a single injection of JDTic, a highly selective KOR antagonist with long-lasting effects (Carroll et al, 2004), can reduce or prevent numerous acute stress-related effects that degrade performance in tasks requiring attention. Finally, we confirm that the behavioral effects of JDTic are persistent in rats, producing a virtually complete blockade of KOR function for at least 11 days after administration. These findings are broadly consistent with previous work indicating that KOR antagonists can block the effects of stress (Bruchas and Chavkin, 2010; Knoll and Carlezon, 2010), but extend it to a more complex aspect of cognitive behavior (attention/concentration) using a procedure that is directly analogous to that used to measure attention in humans (Robbins, 2002).
The primary indicator that CRF disrupted performance in these studies is a decrease in the percentage of correct responses. CRF also increased the percentage of trials in which the rats failed to respond (omission errors) and increased the latency to make a correct response, an effect that may reflect reduced speed of processing or decision making (Robbins, 2002; Paine et al, 2007; Nemeth et al, 2010). The fact that CRF increased the time required to complete the task (ie, collect 90 rewards before the end of the 30-min test session) likely reflects the accumulation of 5-s timeout periods after omitted responses and the small but significant increases in decision-making time over the course of the session. Although some of these 5CSRTT metrics are clearly related (eg, latencies to respond and time to complete the task), previous work demonstrates that key metrics can vary independently and that different drug classes can produce different patterns of alterations in responding (Paine et al, 2007; Nemeth et al, 2010). Pretreatment with JDTic attenuated each of these CRF effects. JDTic was most effective at intermediate doses of CRF that caused significant disruptions of behavior, but its effects were less evident at high doses of CRF that caused more profound stress-like behavior, suggesting lower efficacy in preventing the acute effects of extreme amounts or degrees of stress. Importantly, JDTic did not have any effects of its own on any of these measures. Although some of the JDTic effects might be suggestive of behavioral activation, stimulant effects have not been observed with this class of drugs. For example, we did not observe any effects of KOR antagonists on locomotor activity at doses that produce antidepressant-like or anxiolytic-like effects (Mague et al, 2003; Knoll et al, 2007), or alterations in reward-driven behavior or response capabilities at doses that block the prodepressive-like effects of KOR agonists on motivation (Todtenkopf et al, 2004). JDTic also blocks (rather than primes) stress-induced reinstatement of cocaine self-administration in rats (Beardsley et al, 2005), another indicator of a lack of effects that would raise concerns about stimulant effects or abuse potential of this class of drugs. The fact that JDTic did not attenuate the effects of CRF on accuracy, time to collect the reward, or premature responses reinforces the notion that individual 5CSRTT metrics are not inextricably linked to one another. The CRF-induced reductions in accuracy reflect gradual increases in ‘commission errors', where the rat responds but at the incorrect aperture. JDTic caused nominal reductions in the accuracy-disrupting effects of CRF, but these effects were not statistically significant. Overall, this pattern of effects (increases in omission errors but not commission errors) resembles that seen in this test following administration of ketamine (Nemeth et al, 2010), a drug known to disrupt attention in humans (Knott et al, 2011). Both omission and commission errors can have significant adverse consequences during times of stress.
The mechanisms by which KOR antagonists prevent the effects of stress in general or of CRF specifically are not fully understood. There is no evidence from published studies that KOR antagonists bind to CRF receptors. Although it would be speculative to attribute our effects to actions in any particular brain area or circuit, there are some obvious candidates. As one example, there is evidence that KOR antagonists produce antistress effects via interactions with the intracellular signaling molecule p38α MAPK (Bruchas et al, 2011). Stress produces increases in the activity (phosphorylation) of p38α MAPK within the dorsal raphe nucleus (DRN), which sends serotonin (5HT)-containing projections to forebrain areas critical for modulating fear and anxiety responses (Lowry et al, 2005). This effect is mimicked by administration of U50,488, which produces dysphoria (see Carlezon et al, 2009), and is blocked by KOR antagonism. Activation of p38α MAPK leads to increases in surface expression of 5HT transporters and, in turn, decreases in extracellular levels of 5HT. Indeed, central administration of CRF inhibits DRN neurons and produces decreases in 5HT release (Price et al, 1998; Kirby et al, 2000). Thus, it is possible that the effects of JDTic reported here may be due to inhibition of CRF-induced decreases in 5HT via blockade of CRF-regulating KORs within the DRN (see Bruchas et al, 2011; Muschamp et al, 2011a). Another possibility is that actions in striatal regions may contribute to these effects. Lesions of the striatum, an area rich in dopamine (DA), degrade 5CSRTT performance (Rogers et al, 2001). Previous work has shown that systemic DA receptor antagonism decreases premature responses and increases omissions and response latencies in the 5CSRTT (Harrison et al, 1997). Likewise, depletion of DA from the striatum increases both response latencies and omissions (Cole and Robbins, 1989; Baunez and Robbins, 1999), a pattern of effects similar to that which we observed after CRF administration. CRF stimulates dynorphin release (Nikolarakis et al, 1986; Song and Takemori, 1992), which can in turn inhibit DA release via KOR activation at terminals of midbrain DA neurons (Donzanti et al, 1992; Svingos et al, 1999), creating a hypo-dopaminergic state. Additionally, there is evidence that the DRN sends direct projections to the striatum that regulates DA release: activation of 5HT receptors in the striatum increases extracellular concentrations of DA (Benloucif and Galloway, 1991; Benloucif et al, 1993), whereas CRF reduces 5HT release in the striatum (Price et al, 1998), raising the possibility that CRF-induced decreases in 5HT may reduce DA activity in this region. Blockade of KORs with JDTic either at DA nerve terminals or within the DRN may attenuate reductions in DA activity, thereby improving performance in the 5CSRTT. These possibilities are not mutually exclusive; indeed, each may be only one of several mechanisms acting in concert, considering that stress can elevate dynorphin expression in areas including the ventral striatum (nucleus accumbens (NAc)) and hippocampus, and that microinjections of KOR antagonists into these regions is sufficient to produce antidepressant-like effects (Pliakas et al, 2001; Newton et al, 2002; Shirayama et al, 2004; Muschamp et al, 2011b). The NAc is of particular interest because it is embedded within a complex circuitry that can influence the function of other brain areas implicated in motivation and emotion, such as the frontal cortex and amygdala (see Carlezon and Thomas, 2009). It is not yet known if all of these effects can be tied together within a single neural circuitry model. Clearly, a broad scope of additional work is needed to further characterize CRF/KOR interactions and determine if the effects in the various behavioral tests are attributable to a uniform neural substrate or circuit.
JDTic (10 mg/kg) was administered once, 24 h before testing began, because this drug is known to have a slow onset and long duration of action (Carroll et al, 2004; Knoll et al, 2007; Knoll and Carlezon, 2010). Indeed, data from the present study confirm that JDTic can disrupt responsiveness to a KOR-selective agonist for at least 11 days. The mechanism of this effect is not fully understood, but may involve ligand-directed signaling (also known as biased agonism), a process by which a drug can act as an antagonist of some downstream intracellular signaling pathways while simultaneously acting as an agonist at others. The long-lasting effects of KOR antagonists in general may be related to their ability to activate c-Jun N-terminal kinase-1 (JNK), leading to a de-coupling of KORs from their intracellular signaling cascades (Melief et al, 2010; Melief et al, 2011), rather than long-term persistence of these drugs in the brain (Munro et al, 2012). From a drug development perspective, such long-lasting effects may be ultimately desirable once safety and efficacy are established, but they complicate early-phase clinical studies in humans (Carlezon et al, 2009). Next-generation KOR antagonists that block p38α MAPK without stimulating JNK may be optimal as therapeutic agents.
These new findings supplement a growing body of evidence suggesting that KOR antagonists can block the effects of stress. These agents produce antidepressant-like effects in models that depend upon stressful experiences such as inescapable swimming or shock to trigger a depressive-like state (Pliakas et al, 2001; Newton et al, 2002; Mague et al, 2003). They also produce anxiolytic-like effects in models where stress produces a resistance to exploring open spaces (elevated plus maze) or persistent fear behaviors in the presence of cues associated with prior pain or trauma (fear conditioning) (Knoll et al, 2007). The fact that KOR antagonists produce both these antidepressant- and anxiolytic-like behaviors together gives them a unique profile, as acute administration of standard antidepressants tends to produce anxiogenic effects in rodent models that may reflect those often seen early in antidepressant treatment regimens in humans (Knoll et al, 2007).
There are currently no methods to prevent the immediate effects of stress or the subsequent development of anxiety or depressive disorders. In situations where stress can be predicted, the ability to intervene with a preventive measure in advance of stress exposure may promote short-term safety and long-term health. The present studies indicate that pretreatment with a KOR antagonist can improve cognitive performance that is typically degraded under stress-like conditions, an effect that would be particularly desirable in humans when stress is accompanied by danger or the potential for harm. Considering prior work showing that pretreatment with this same class of agents has anxiolytic effects and attenuates the development of conditioned fear (Knoll et al, 2007) in a rodent version of a method often used to study PTSD in people (Mahan and Ressler, 2012), these new findings provide further evidence that KOR antagonists can prevent stress-induced processes that may render individuals vulnerable to acute injury and contribute to the development of psychiatric illness.
Acknowledgments
This study was supported by a National Defense Science and Engineering Graduate Fellowship (to AVV), DA009045 (to FIC), the Frazier Medical Research Institute (to BMC), and MH063266 (to WAC).
Dr Carlezon has a US patent covering the use of κ-antagonists in the treatment of depression (Assignee: McLean Hospital). In the past 3 years, Dr Carlezon has received compensation for professional services from The American College of Neuropsychopharmacology and Myneurolab.com. Dr Carroll discloses that he and the Research Triangle Institute are co-owners of US patents claiming the composition of JDTic. Dr Cohen has pending patents on pyrimidines to treat bipolar disorders, κ-opioid agonists in bipolar mania, and mitochondrial replacement. AVV and JMY declare no conflict of interest.
References
- American Psychiatric Association 2000Diagnostic and Statistical Manual of Mental Disorders4th edn, text rev.American Psychiatric Association: Washington, DC [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32:709–723. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunez C, Robbins TW. Effects of dopamine depletion of the dorsal striatum and further interaction with subthalamic nucleus lesions in an attentional task in the rat. Neuroscience. 1999;92:1343–1356. doi: 10.1016/s0306-4522(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology. 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Galloway MP. Facilitation of dopamine release in vivo by serotonin agonists: studies with microdialysis. Eur J Pharmacol. 1991;200:1–8. doi: 10.1016/0014-2999(91)90658-d. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Keegan MJ, Galloway MP. Serotonin-facilitated dopamine release in vivo: pharmacological characterization. J Pharmacol Exp Ther. 1993;265:373–377. [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology. 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Lemos JC, Chavkin C. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One. 2009;4:e8528. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, et al. Neuron. 2011. pp. 498–511. [DOI] [PMC free article] [PubMed]
- Campeau S, Liberzon I, Morilak D, Ressler K. Stress modulation of cognitive and affective processes. Stress. 2011;14:503–519. doi: 10.3109/10253890.2011.596864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Béguin C, Knoll AT, Cohen BM. Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol Ther. 2009;123:334–343. doi: 10.1016/j.pharmthera.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas M. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56 (Suppl 1:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll I, Thomas JB, Dykstra LA, Granger AL, Allen RM, Howard JL, et al. Pharmacological properties of JDTic: a novel kappa-opioid receptor antagonist. Eur J Pharmacol. 2004;501:111–119. doi: 10.1016/j.ejphar.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chilcoat HD, Breslau N. Investigations of causal pathways between PTSD and drug use disorders. Addictive Behav. 1998;23:827–840. doi: 10.1016/s0306-4603(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Lentjes EG, Heijnen CJ, Stalla GK, et al. Elevated plasma corticotrophin-releasing hormone levels in veterans with posttraumatic stress disorder. Prog Brain Res. 2008;167:287–291. doi: 10.1016/S0079-6123(07)67025-3. [DOI] [PubMed] [Google Scholar]
- Donzanti BA, Althaus JS, Payson MM, Von Voigtlander PF. Kappa agonist-induced reduction in dopamine release: site of action and tolerance. Res Commun Chem Pathol Pharmacol. 1992;78:193–210. [PubMed] [Google Scholar]
- Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology. 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann NY Acad Sci. 2009;1179:120–143. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Marshall AD, Taft CT. Posttraumatic stress disorder: etiology, epidemiology, and treatment outcome. Ann Rev Clin Psychol. 2006;2:161–197. doi: 10.1146/annurev.clinpsy.2.022305.095305. [DOI] [PubMed] [Google Scholar]
- Keller MC, Neale MC, Kendler KS. Association of different adverse life events with distinct patterns of depressive symptoms. Am J Psychiatry. 2007;164:1521–1529. doi: 10.1176/appi.ajp.2007.06091564. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Ruscio AM, Shear K, Wittchen HU. Epidemiology of anxiety disorders. Curr Top Behav Neurosci. 2010;2:21–35. [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress and depression. Brain Res. 2010;1314C:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in behavioral models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz D, Meloni EG, et al. Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry. 2011;70:425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott VJ, Millar AM, McIntosh JF, Shah DK, Fisher DJ, Blais CM, et al. Separate and combined effects of low dose ketamine and nicotine on behavioral and neural correlates of sustained attention. Biol Psychiatry. 2011;88:83–93. doi: 10.1016/j.biopsycho.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, et al. Antidepressant-like effects of k-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Mahan AJ, Ressler KL. Fear conditioning, synaptic plasticity, and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C. Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci USA. 2010;107:11608–11613. doi: 10.1073/pnas.1000751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Carroll FI, Béguin C, Carlezon WA, Jr, Cohen BM, et al. Duration of action of a broad range of selective kappa opioid receptor antagonists is positively correlated with c-Jun N-terminal Kinase-1 activation. Mol Pharmacol. 2011;80:920–929. doi: 10.1124/mol.111.074195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro TA, Berry LM, Van't Veer A, Béguin C, Carroll FI, Zhao Z, et al. Long-acting kappa opioid antagonists nor-BNI, GNTI, and JDTic: pharmacokinetics in mice and lipophilicity. BMC Pharmacol. 2012;12:5. doi: 10.1186/1471-2210-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Van't Veer AV, Carlezon WA., Jr Tracking down the molecular substrates of stress: new roles for p38a MAPK and kappa-opioid receptors. Neuron. 2011a;71:383–384. doi: 10.1016/j.neuron.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Van't Veer AV, Parsegian A, Gallo MS, Chen M, Neve RL, et al. Activation of CREB in the nucleus accumbens shell produces anhedonia and resistance to extinction of fear in rats. J Neurosci. 2011b;31:3095–3103. doi: 10.1523/JNEUROSCI.5973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth C, Paine TA, Rittiner J, Béguin C, Carroll FI, Roth BL, et al. Role of kappa-opioid receptors in the effects of salvinorin A and ketamine on attention behavior in rats. Psychopharmacology. 2010;210:263–274. doi: 10.1007/s00213-010-1834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, et al. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolarakis KE, Almeida OF, Herz A. Stimulation of hypothalamic beta-endorphin and dynorphin release by corticotropin-releasing factor (in vitro) Brain Res. 1986;399:152–155. doi: 10.1016/0006-8993(86)90610-4. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Yamaguchia Y, Futamia Y, Togashia H, Izumia T, Matsumotoa M, et al. Corticotropin releasing factor enhances attentional function as assessed by the five-choice serial reaction time task in rats. Behav Brain Res. 2009;198:429–433. doi: 10.1016/j.bbr.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA., Jr Sensitivity of the 5-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biol Psychiatry. 2007;62:687–693. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson R, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Robbins T. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Baunez C, Everitt BJ, Robbins TW. Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behav Neurosci. 2001;115:799–811. doi: 10.1037//0735-7044.115.4.799. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Saranson I, Bransome EB, Beck LH. A continuous performance test of brain damage. J Consult Clin Psychol. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, et al. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003;54:1382–1388. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;60:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, French AM. Age-related differences in sensitivity to the antinociceptive effects of kappa opioids in adult male rats. Psychopharmacology. 2002;162:255–264. doi: 10.1007/s00213-002-1102-6. [DOI] [PubMed] [Google Scholar]
- Song ZH, Takemori AE. Stimulation by corticotropin-releasing factor of the release of immunoreactive dynorphin A from mouse spinal cords in vitro. Eur J Pharmacol. 1992;222:27–32. doi: 10.1016/0014-2999(92)90458-g. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Colago EE, Pickel VM. Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J Neurosci. 1999;19:1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology. 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Van't Veer A, Carroll FI, Carlezon WA., Jr2011Antagonism of kappa-opioid receptors reduces corticotropin-releasing factor induced effectsProgram No. 791.10. 2011 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2011. Online.
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;15:371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]