Abstract
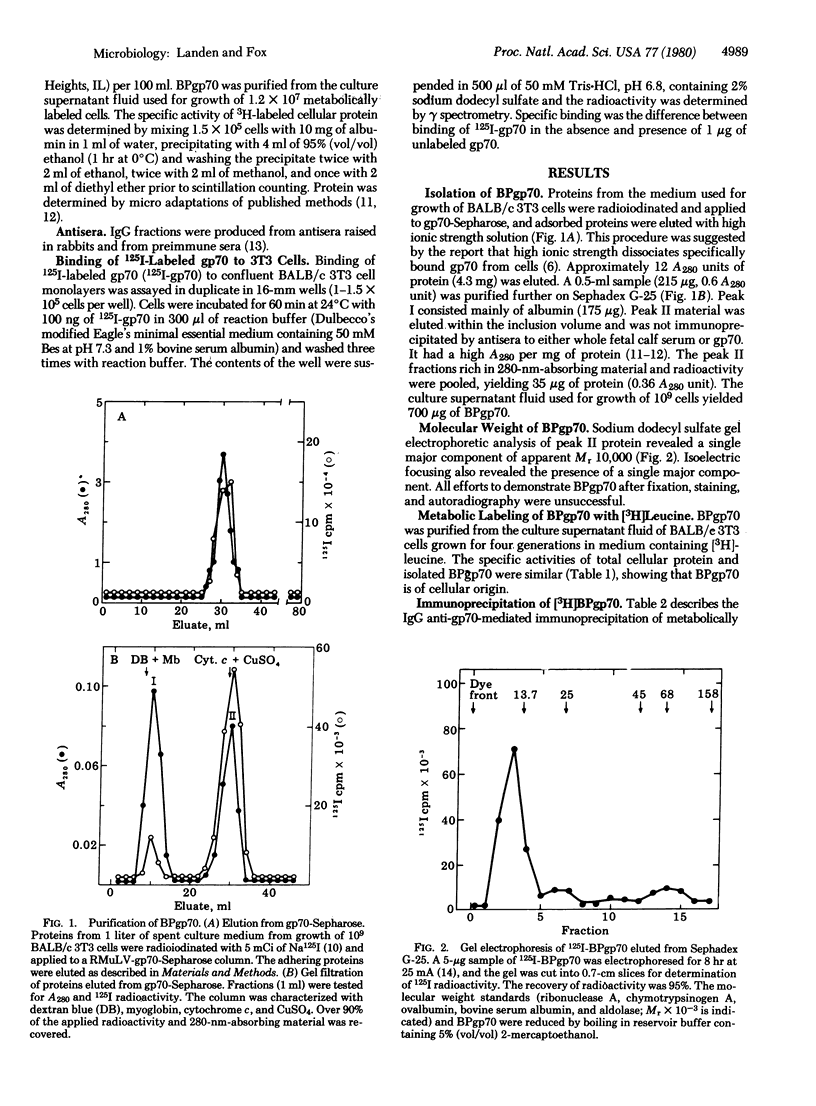
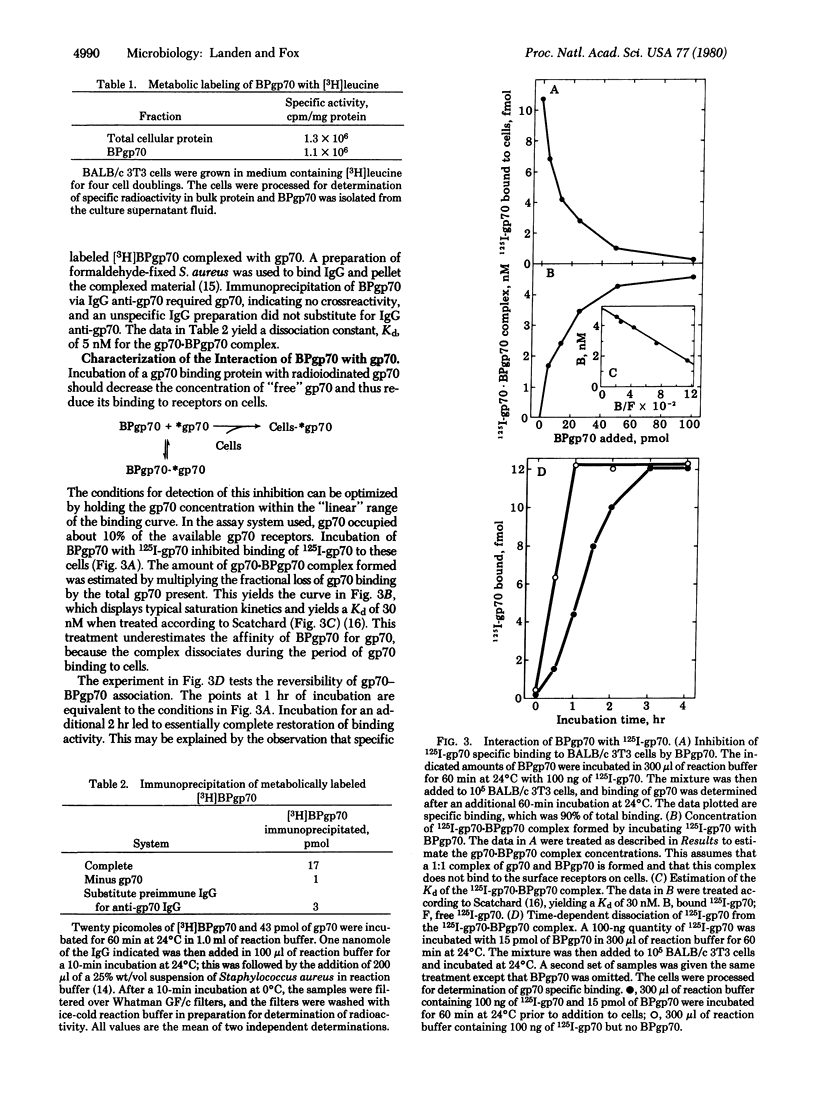
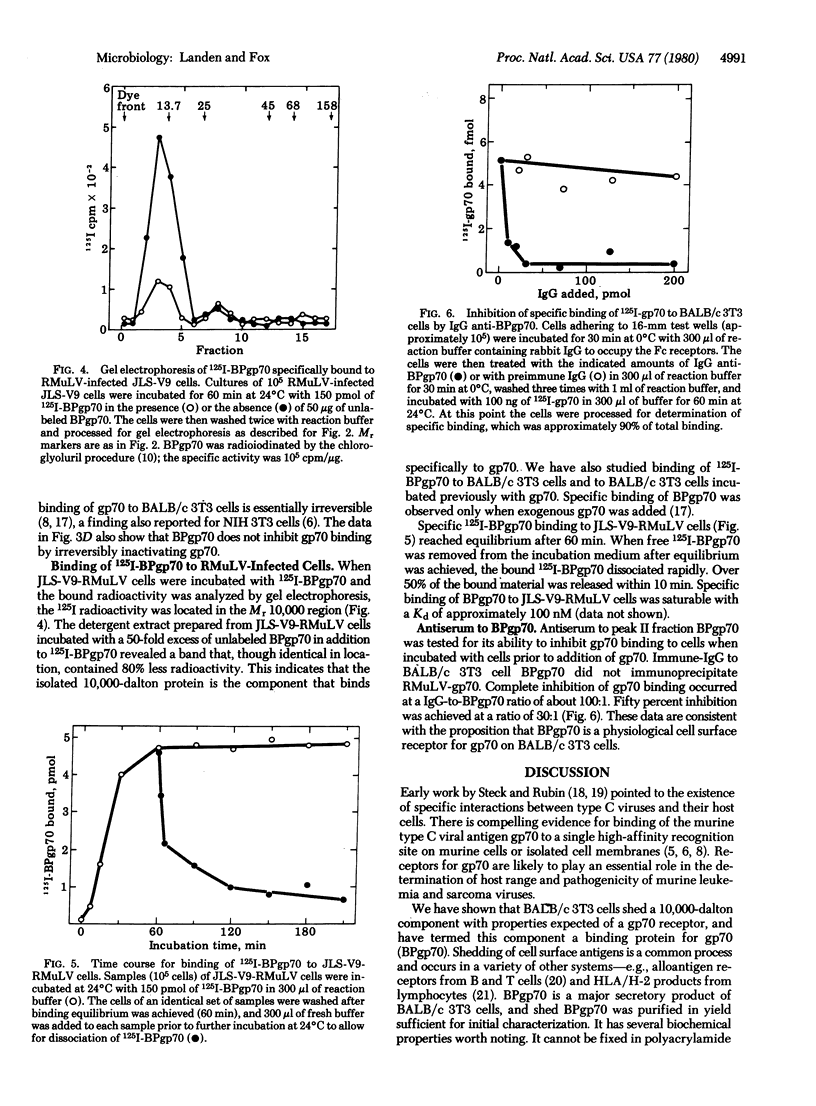
A protein that avidly binds gp70, the envelope antigen of Rauscher murine leukemia virus (RMuLV), has been purified from the culture medium used for growth of BALB/c 3T3 mouse cells. Gel filtration chromatrography revealed the apparent Mr 10,000 BPgp70 was efficiently labeled when BALB/c 3T3 cells were grown in medium containing [3H]leucine, indicating a cellular origin for BPpg70. Metabolically labeled [3H]BPgp70 was not immunoprecipitated by IgG-anti RMuLV-gp&) alone, but was immunoprecipitated when gp70 was added, an indicaton of BPgp70 x gp70 complex formation. The dissociation constant estimated by immunoprecitipation agreed with the apparent Kd for binding of gp70 to BALB/c 3T3 cells. BPgp70 reversibly inhibited specific binding of 125I-labeled BMuLV-gp70 to BALB/c 3T3 cells when it was incubated with the 125I-labeled gp70 first. These data yielded a dissociation constant similar to that calculated from the immunoprecipitation data. 125I-Labeled BPgp70 also bound specifically to cells infected with RMuLV, but not to uninfected cells. Incubation of BALB/c 3T3 cells with the IgG fraction of an antiserum to BPgp70 inhibited the specific binding of 125I-labeled gp70 to these cells, but preimmune IgG did not. Complete inhibition was achieved at a less than 100:1 ratio of IgG anti-BPgp70 to gp70 binding sites.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishayee S., Strand M., August J. T. Cellular membrane receptors for oncovirus envelope glycoprotein: properties of the binding reaction and influence of different reagents on the substrate and the receptors. Arch Biochem Biophys. 1978 Jul;189(1):161–171. doi: 10.1016/0003-9861(78)90129-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Callahan G. N., Ferrone S., Poulik M. D., Reisfeld R. A., Klein J. Characterization of Ia antigens in mouse serum. J Immunol. 1976 Oct;117(4):1351–1355. [PubMed] [Google Scholar]
- Dales S. Early events in cell-animal virus interactions. Bacteriol Rev. 1973 Jun;37(2):103–135. doi: 10.1128/br.37.2.103-135.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLarco J., Todaro G. J. Membrane receptors for murine leukemia viruses: characterization using the purified viral envelope glycoprotein, gp71. Cell. 1976 Jul;8(3):365–371. doi: 10.1016/0092-8674(76)90148-3. [DOI] [PubMed] [Google Scholar]
- Fowler A. K., Twardzik D. R., Reed C. D., Weislow O. S., Hellman A. Binding characteristics of Rauscher leukemia virus envelope glycoprotein gp71 to murine lymphoid cells. J Virol. 1977 Dec;24(3):729–735. doi: 10.1128/jvi.24.3.729-735.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAFUSA H. ANALYSIS OF THE DEFECTIVENESS OF ROUS SARCOMA VIRUS. 3. DETERMINING INFLUENCE OF A NEW HELPER VIRUS ON THE HOST RANGE AND SUSCEPTIBILITY TO INTERFERENCE OF RSV. Virology. 1965 Feb;25:248–255. doi: 10.1016/0042-6822(65)90203-5. [DOI] [PubMed] [Google Scholar]
- Jakoi E. R., Marchase R. B. Ligatin from embryonic chick neural retina. J Cell Biol. 1979 Mar;80(3):642–650. doi: 10.1083/jcb.80.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Gallo R. C. Characterization of Rauscher murine leukemia virus envelope glycoprotein receptor in membranes from murine fibroblasts. J Virol. 1978 Dec;28(3):686–696. doi: 10.1128/jvi.28.3.686-696.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Rifkin D., Compans R. W. Identification of the spike proteins of Rous sarcoma virus. Virology. 1971 Nov;46(2):485–489. doi: 10.1016/0042-6822(71)90049-3. [DOI] [PubMed] [Google Scholar]
- Steck F. T., Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. I. Establishment of interference. Virology. 1966 Aug;29(4):628–641. doi: 10.1016/0042-6822(66)90287-x. [DOI] [PubMed] [Google Scholar]
- Steck F. T., Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. II. Early steps of infection by RSV of cells under conditions of interference. Virology. 1966 Aug;29(4):642–653. doi: 10.1016/0042-6822(66)90288-1. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of ribonucleic acid tumor viruses. Purification of envelope, core, and internal components. J Biol Chem. 1976 Jan 25;251(2):559–564. [PubMed] [Google Scholar]
- Takemoto L. J., Fox C. F., Jensen F. C., Elder J. H., Lerner R. A. Nearest-neighbor interactions of the major RNA tumor virus glycoprotein on murine cell surfaces. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3644–3648. doi: 10.1073/pnas.75.8.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Giuli C., Kawai S., Dales S., Hanafusa H. Absence of surface projections of some noninfectious forms of RSV. Virology. 1975 Jul;66(1):253–260. doi: 10.1016/0042-6822(75)90195-6. [DOI] [PubMed] [Google Scholar]



