Abstract
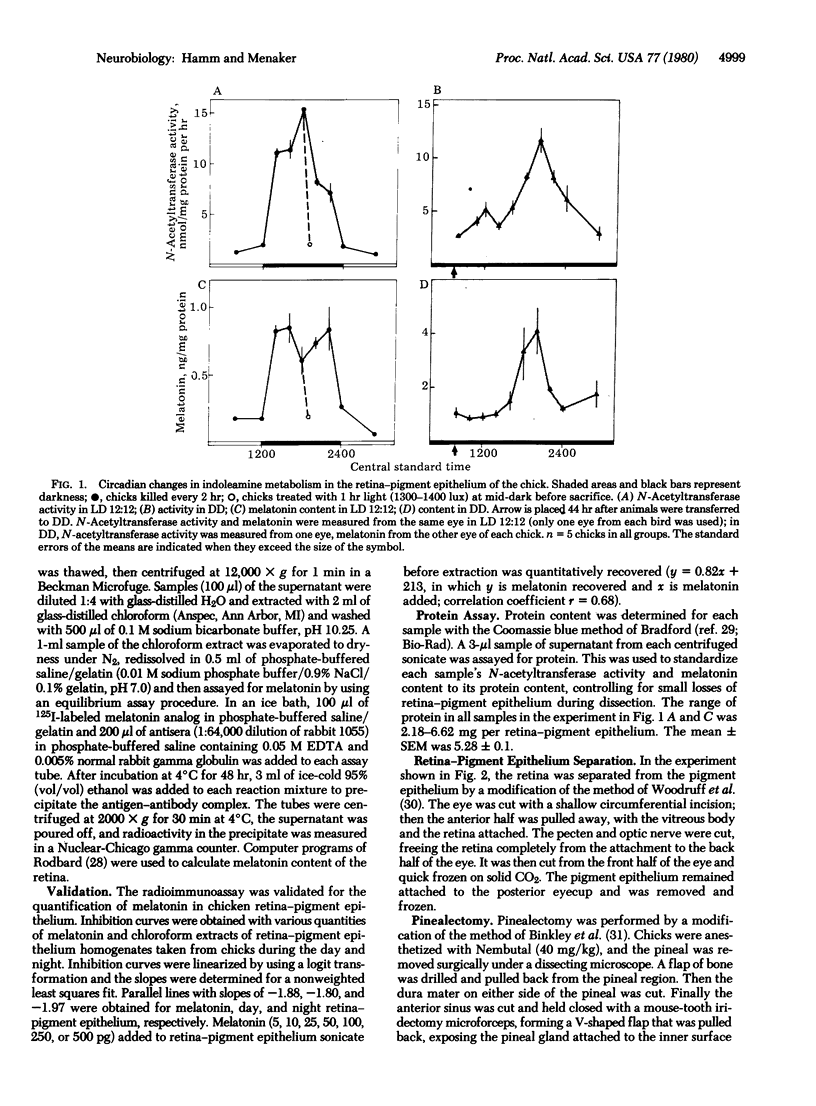
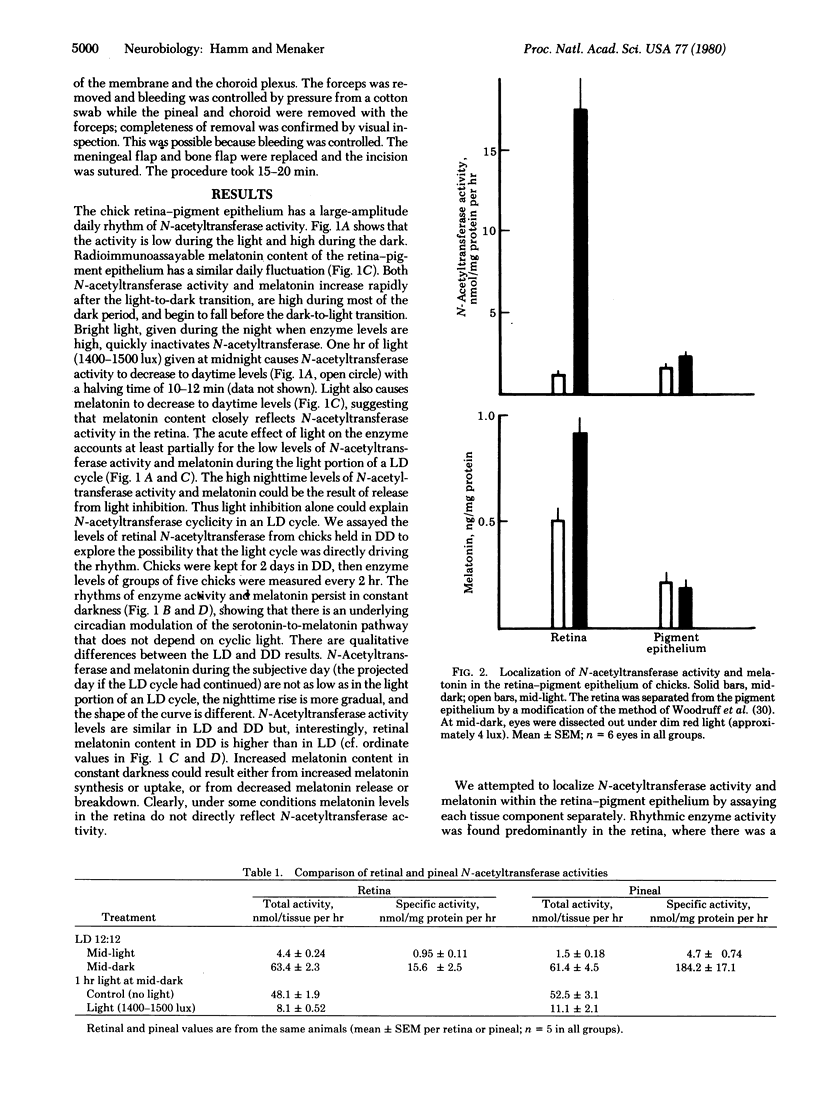
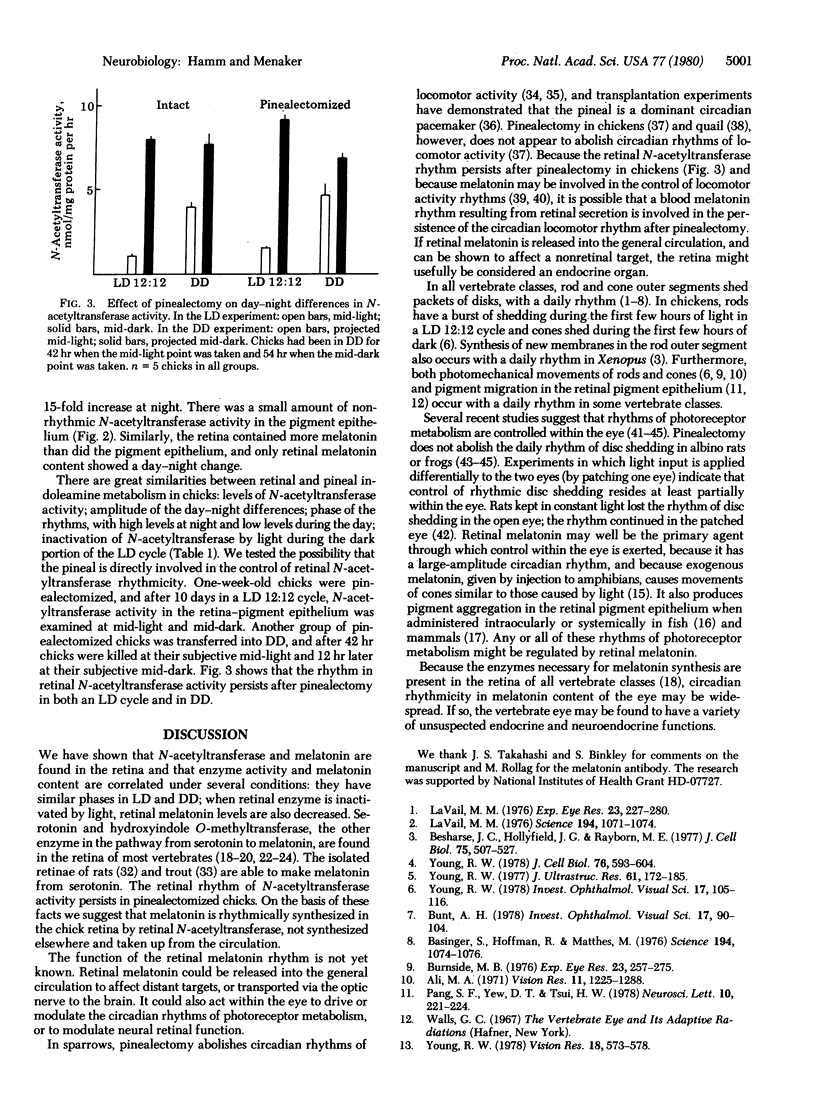
There is a large-amplitude circadian rhythm of indoleamine metabolism in the retina-pigment epithelium of the chicken. N-Acetyltransferase activity (arylamine acetyltransferase; acetyl-CoA:arylamine N-acetyltransferase, EC 2.3.1.5) and melatonin content are 15-fold higher at night than during the day in a cycle of a 4-fold increase during the subjective night. Light at midnight inactivates N-acetyltransferase and lowers melatonin. N-Acetyltransferase activity is found predominantly in the retina. The circadian rhythm of this enzyme activity persists in pinealectomized chicks. Thus the pineal is not responsible for retinal indoleamine rhythms. Retinal and pineal levels of N-acetyltransferase activity behave similarly under several conditions. In the chicken, the eye is a major site of rhythmic indoleamine metabolic activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M. A. Les réponses rétinomotrices: caractères et mécanismes. Vision Res. 1971 Nov;11(11):1225–1288. doi: 10.1016/0042-6989(71)90010-1. [DOI] [PubMed] [Google Scholar]
- Basinger S., Hoffman R., Matthes M. Photoreceptor shedding is initiated by light in the frog retina. Science. 1976 Dec 3;194(4269):1074–1076. doi: 10.1126/science.1086510. [DOI] [PubMed] [Google Scholar]
- Besharse J. C., Hollyfield J. G., Rayborn M. E. Turnover of rod photoreceptor outer segments. II. Membrane addition and loss in relationship to light. J Cell Biol. 1977 Nov;75(2 Pt 1):507–527. doi: 10.1083/jcb.75.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkley S., Hryshchyshyn M., Reilly K. N-acetyltransferase activity responds to environmental lighting in the eye as well as in the pineal gland. Nature. 1979 Oct 11;281(5731):479–481. doi: 10.1038/281479a0. [DOI] [PubMed] [Google Scholar]
- Binkley S., Kluth E., Menaker M. Pineal function in sparrows: circadian rhythms and body temperature. Science. 1971 Oct 15;174(4006):311–314. doi: 10.1126/science.174.4006.311. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bunt A. H. Fine structure and radioautography of rabbit photoreceptor cells. Invest Ophthalmol Vis Sci. 1978 Feb;17(2):90–104. [PubMed] [Google Scholar]
- Burnside M. B. Possible roles of microtubules and actin filaments in retinal pigmented epithelium. Exp Eye Res. 1976 Aug;23(2):257–275. doi: 10.1016/0014-4835(76)90208-6. [DOI] [PubMed] [Google Scholar]
- Cardinali D. P., Rosner J. M. Metabolism of serotonin by the rat retina in vitro. J Neurochem. 1971 Sep;18(9):1769–1770. doi: 10.1111/j.1471-4159.1971.tb03752.x. [DOI] [PubMed] [Google Scholar]
- Cardinali D. P., Rosner J. M. Retinal localization of the hydroxyindole-O-methyl transferase (HIOMT) in the rat. Endocrinology. 1971 Jul;89(1):301–303. doi: 10.1210/endo-89-1-301. [DOI] [PubMed] [Google Scholar]
- Chèze G., Ali M. A. Rôle de l'épiphyse dans la migration du pigment épithélial rétinien chez quelques Téléostéens. Can J Zool. 1976 Apr;54(4):475–481. [PubMed] [Google Scholar]
- Currie J. R., Hollyfield J. G., Rayborn M. E. Rod outer segments elongate in constant light: darkness is required for normal shedding. Vision Res. 1978;18(8):995–1003. doi: 10.1016/0042-6989(78)90027-5. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Sensitive assay for serotonin N-acetyltransferase activity in rat pineal. Anal Biochem. 1972 Nov;50(1):174–179. doi: 10.1016/0003-2697(72)90496-4. [DOI] [PubMed] [Google Scholar]
- Easter S. S., Jr, Macy A. Local control of retinomotor activity in the fish retina. Vision Res. 1978;18(8):937–942. doi: 10.1016/0042-6989(78)90021-4. [DOI] [PubMed] [Google Scholar]
- Gaston S., Menaker M. Pineal function: the biological clock in the sparrow? Science. 1968 Jun 7;160(3832):1125–1127. doi: 10.1126/science.160.3832.1125. [DOI] [PubMed] [Google Scholar]
- Gern W. A., Ralph C. L. Melatonin synthesis by the retina. Science. 1979 Apr 13;204(4389):183–184. doi: 10.1126/science.432640. [DOI] [PubMed] [Google Scholar]
- Hauschild D. C., Laties A. M. An indoleamine-containing cell in chick retina. Invest Ophthalmol. 1973 Jul;12(7):537–540. [PubMed] [Google Scholar]
- Hollyfield J. G., Basinger S. F. Cyclic metabolism of photoreceptor cells. Invest Ophthalmol Vis Sci. 1978 Feb;17(2):87–89. [PubMed] [Google Scholar]
- LaVail M. M., Mullen R. J. Role of the pigment epithelium in inherited retinal degeneration analyzed with experimental mouse chimeras. Exp Eye Res. 1976 Aug;23(2):227–245. doi: 10.1016/0014-4835(76)90206-2. [DOI] [PubMed] [Google Scholar]
- LaVail M. M. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976 Dec 3;194(4269):1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- LaVail M. M., Ward P. A. Studies on the hormonal control of circadian outer segment disc shedding in the rat retina. Invest Ophthalmol Vis Sci. 1978 Dec;17(12):1189–1183. [PubMed] [Google Scholar]
- O'Day W. T., Young R. W. Rhythmic daily shedding of outer-segment membranes by visual cells in the goldfish. J Cell Biol. 1978 Mar;76(3):593–604. doi: 10.1083/jcb.76.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S. F., Yew D. T. Pigment aggregation by melatonin in the retinal pigment epithelium and choroid of guinea-pigs, Caviaporcellus. Experientia. 1979 Feb 15;35(2):231–233. doi: 10.1007/BF01920634. [DOI] [PubMed] [Google Scholar]
- Quay W. B. Retinal and pineal hydroxyindole-o-methyl transferase activity in vertebrates. Life Sci. 1965 May;4(9):983–991. doi: 10.1016/0024-3205(65)90202-x. [DOI] [PubMed] [Google Scholar]
- Rollag M. D., Niswender G. D. Radioimmunoassay of serum concentrations of melatonin in sheep exposed to different lighting regimens. Endocrinology. 1976 Feb;98(2):482–489. doi: 10.1210/endo-98-2-482. [DOI] [PubMed] [Google Scholar]
- Suzuki O., Noguchi E., Miyake S., Yagi K. Occurrence of 5-hydroxytryptamine in chick retina. Experientia. 1977 Jul 15;33(7):927–928. doi: 10.1007/BF01951286. [DOI] [PubMed] [Google Scholar]
- Takahashi J. S., Menaker M. Physiology of avian circadian pacemakers. Fed Proc. 1979 Nov;38(12):2583–2588. [PubMed] [Google Scholar]
- Tamai M., Teirstein P., Goldman A., O'Brien P., Chader G. The pineal gland does not control rod outer segment shedding and phagocytosis in the rat retina and pigment epithelium. Invest Ophthalmol Vis Sci. 1978 Jun;17(6):558–562. [PubMed] [Google Scholar]
- Turek F. W., McMillan J. P., Menaker M. Melatonin: effects on the circadian locomotor rhythm of sparrows. Science. 1976 Dec 24;194(4272):1441–1443. doi: 10.1126/science.1006311. [DOI] [PubMed] [Google Scholar]
- Wainwright S. D. Development of hydroxyindole-O-methyltransferase activity in the retina of the chick embryo and young chick. J Neurochem. 1979 Mar;32(3):1099–1101. doi: 10.1111/j.1471-4159.1979.tb04600.x. [DOI] [PubMed] [Google Scholar]
- Woodruff M. L., Bownds D., Green S. H., Morrisey J. L., Shedlovsky A. Guanosine 3',5'-cyclic monophosphate and the in vitro physiology of frog photoreceptor membranes. J Gen Physiol. 1977 May;69(5):667–679. doi: 10.1085/jgp.69.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W. The daily rhythm of shedding and degradation of cone outer segment membranes in the lizard retina. J Ultrastruct Res. 1977 Nov;61(2):172–185. doi: 10.1016/s0022-5320(77)80084-1. [DOI] [PubMed] [Google Scholar]
- Young R. W. The daily rhythm of shedding and degradation of rod and cone outer segment membranes in the chick retina. Invest Ophthalmol Vis Sci. 1978 Feb;17(2):105–116. [PubMed] [Google Scholar]
- Young R. W. Visual cells, daily rhythms, and vision research. Vision Res. 1978;18(5):573–578. doi: 10.1016/0042-6989(78)90205-5. [DOI] [PubMed] [Google Scholar]
- Zimmerman N. H., Menaker M. The pineal gland: a pacemaker within the circadian system of the house sparrow. Proc Natl Acad Sci U S A. 1979 Feb;76(2):999–1003. doi: 10.1073/pnas.76.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]