Abstract
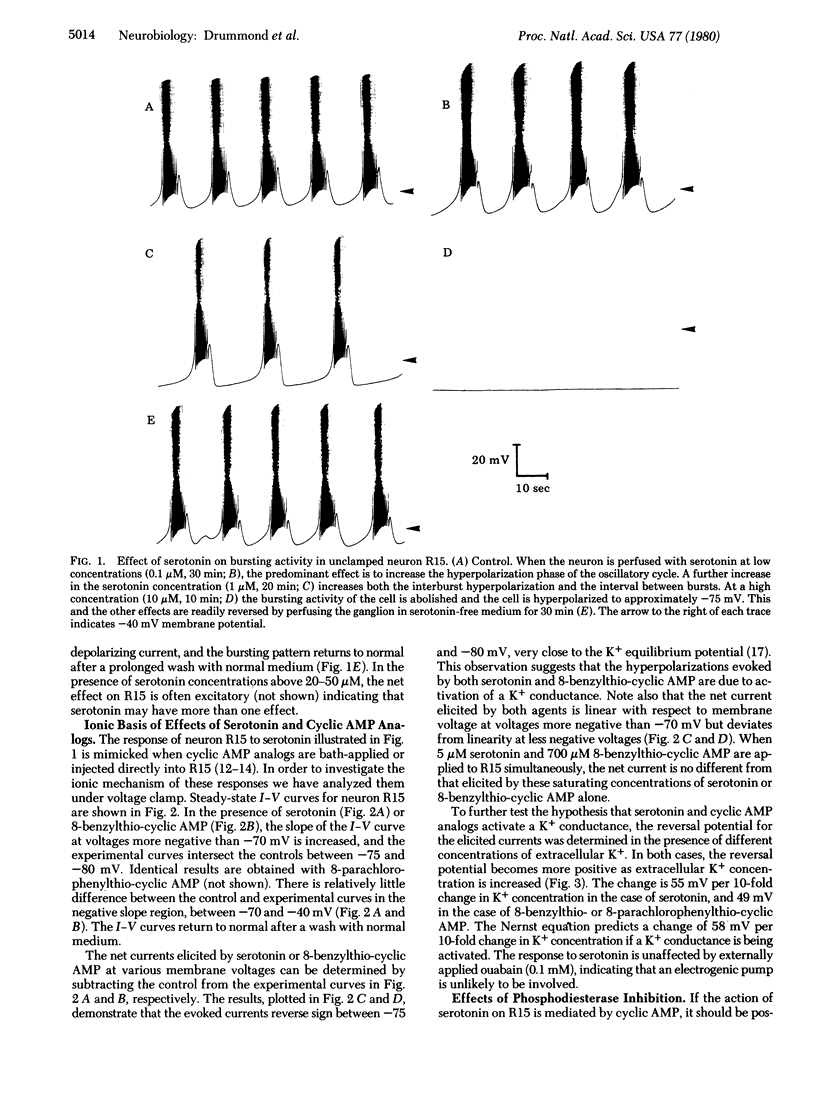
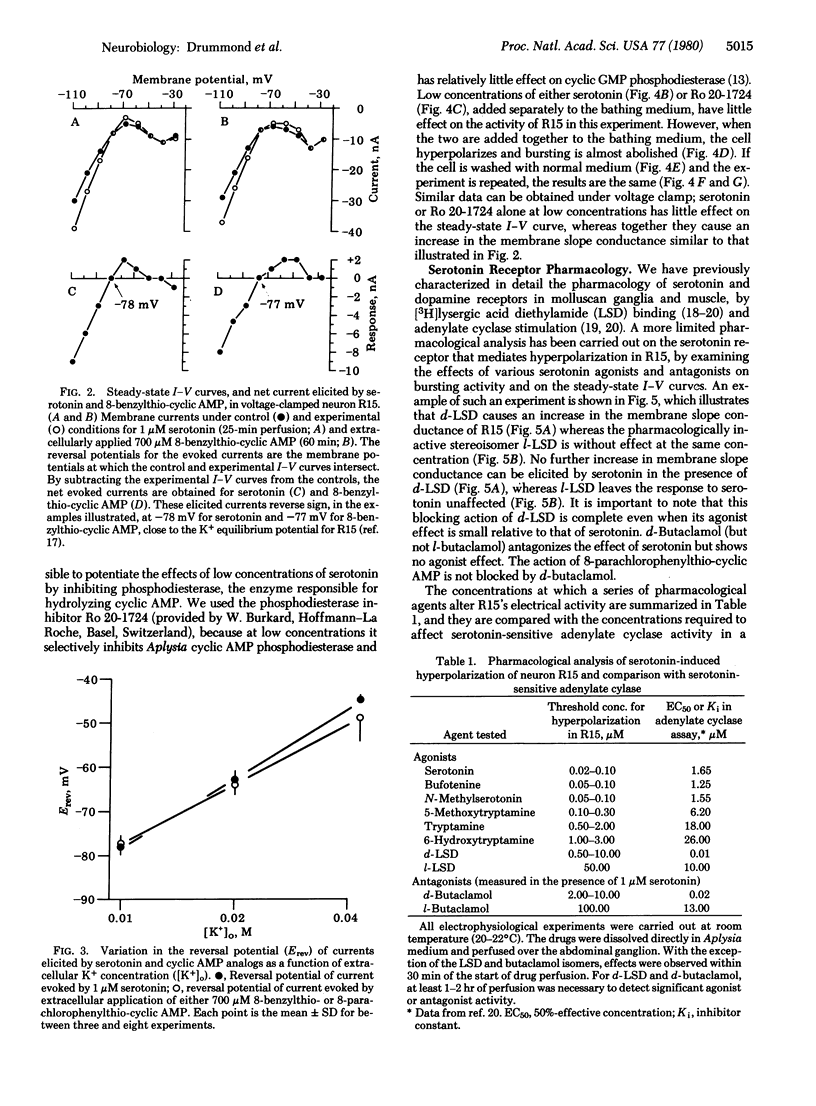
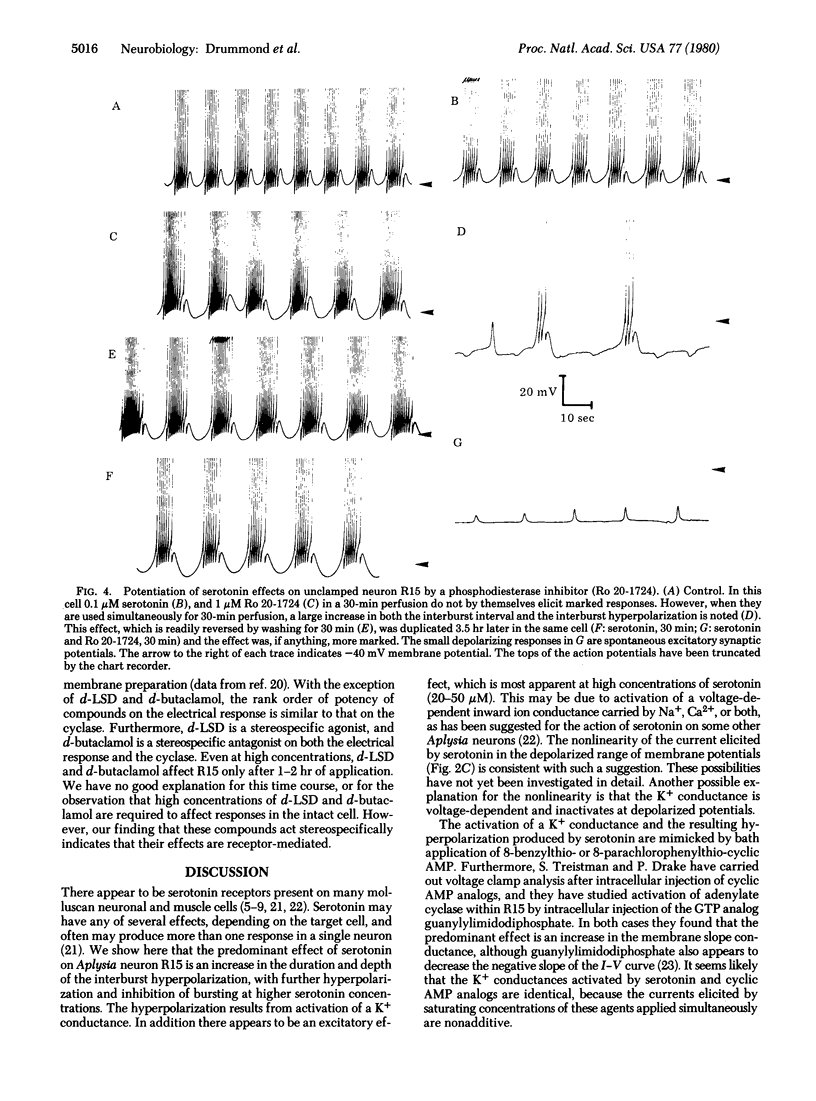
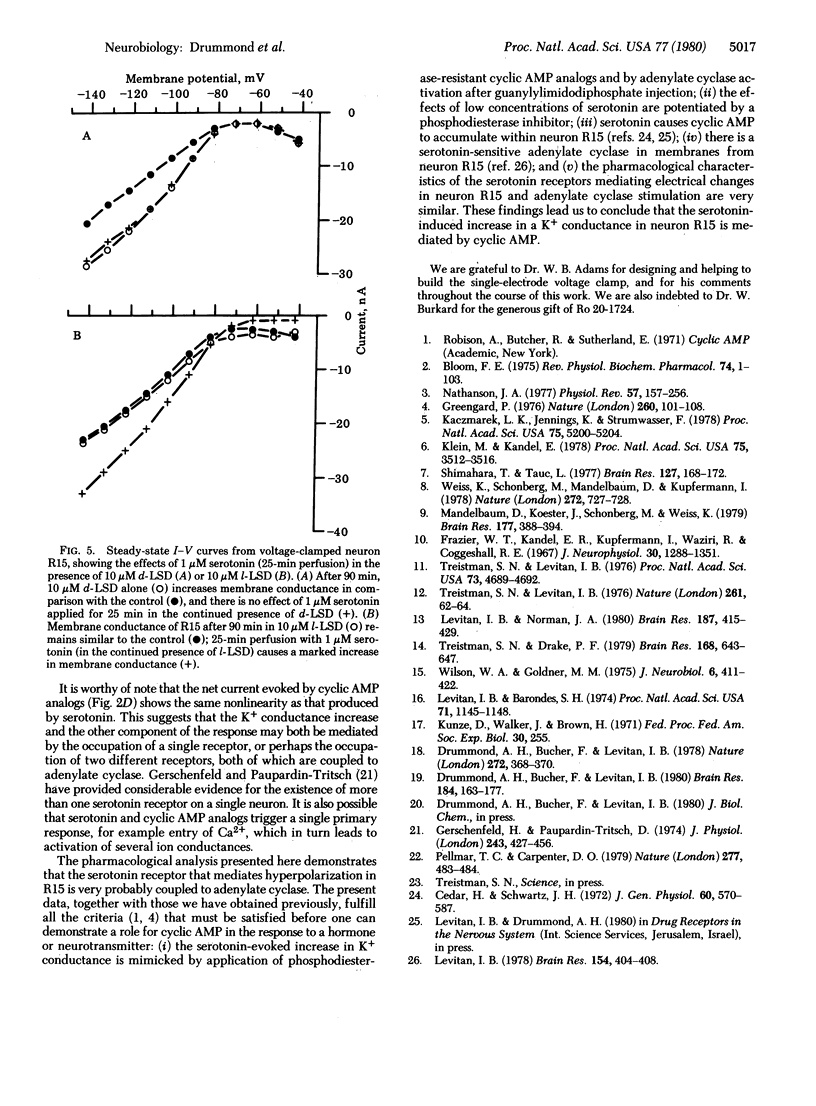
Addition of serotonin to the medium bathing an Aplysia abdominal ganglion causes a change in the endogenous bursting activity of the identified neuron R15. At serotonin concentrations in the micromolar range, the predominant effect is an increase in depth and duration of the interburst hyperpolarization and consequent decrease in burst rate. At higher concentrations (10 microM) serototin can inhibit bursting completely. We have shown previously that these changes can be mimicked by bath application or intracellular injection of several cyclic AMP analogs substituted at the 8 position. Voltage clamp analysis indicates that serotonin and cyclic AMP analogs both cause an increase in membrane slope conductance in R15, with reversal potentials for the responses between -75 and -80 mV, close to the K+ equilibrium potential. When the K+ concentration in the bathing medium is changed, the reversal potentials change in a manner suggesting that serotonin and cyclic AMP analogs on K+ conductance are not additive. Furthermore, the effects of low concentrations of serotonin can be potentiated by the phosphodiesterase inhibitor Ro 20-1724. A pharmacological analysis indicates that the serotonin receptor that mediates hyperpolarization in R15 is similar to the serotonin receptor that we have shown to be coupled to adenylate cyclase. The present electrophysiological and pharmacological observations, together with our previous biochemical and pharmacological results, demonstrate that the serotonin-induced hyperpolarization of neuron R15 is mediated by cyclic AMP.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom F. E. The role of cyclic nucleotides in central synaptic function. Rev Physiol Biochem Pharmacol. 1975;74:1–103. doi: 10.1007/3-540-07483-x_19. [DOI] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. Cyclic adenosine monophosphate in the nervous system of Aplysia californica. II. Effect of serotonin and dopamine. J Gen Physiol. 1972 Nov;60(5):570–587. doi: 10.1085/jgp.60.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. H., Bucher F., Levitan I. B. Distribution of serotonin and dopamine receptors in Aplysia tissues: analysis by [3H]LSD binding and adenylate cyclase stimulation. Brain Res. 1980 Feb 17;184(1):163–177. doi: 10.1016/0006-8993(80)90595-8. [DOI] [PubMed] [Google Scholar]
- Drummond A. H., Bucher F., Levitan I. B. LSD labels a novel dopamine receptor in molluscan nervous system. Nature. 1978 Mar 23;272(5651):368–370. doi: 10.1038/272368a0. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Paupardin-Tritsch D. Ionic mechanisms and receptor properties underlying the responses of molluscan neurones to 5-hydroxytryptamine. J Physiol. 1974 Dec;243(2):427–456. doi: 10.1113/jphysiol.1974.sp010761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Possible role for cyclic nucleotides and phosphorylated membrane proteins in postsynaptic actions of neurotransmitters. Nature. 1976 Mar 11;260(5547):101–108. doi: 10.1038/260101a0. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. K., Jennings K., Strumwasser F. Neurotransmitter modulation, phosphodiesterase inhibitor effects, and cyclic AMP correlates of afterdischarge in peptidergic neurites. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5200–5204. doi: 10.1073/pnas.75.10.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I. B. Adenylate cyclase in isolated Helix and Aplysia neuronal cell bodies: stimulation by serotonin and peptide-containing extract. Brain Res. 1978 Oct 13;154(2):404–408. doi: 10.1016/0006-8993(78)90714-x. [DOI] [PubMed] [Google Scholar]
- Levitan I. B., Barondes S. H. Octopamine- and serotonin-stimulated phosphorylation of specific protein in the abdominal ganglion of Aplysia californica. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1145–1148. doi: 10.1073/pnas.71.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I. B., Norman J. Different effects of cAMP and cGMP derivatives on the activity of an identified neuron: biochemical and electrophysiological analysis. Brain Res. 1980 Apr 14;187(2):415–429. doi: 10.1016/0006-8993(80)90212-7. [DOI] [PubMed] [Google Scholar]
- Mandelbaum D. E., Koester J., Schonberg M., Weiss K. R. Cyclic AMP mediation of the excitatory effect of serotonin in the heart of Aplysia. Brain Res. 1979 Nov 16;177(2):388–394. doi: 10.1016/0006-8993(79)90792-3. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A. Cyclic nucleotides and nervous system function. Physiol Rev. 1977 Apr;57(2):157–256. doi: 10.1152/physrev.1977.57.2.157. [DOI] [PubMed] [Google Scholar]
- Pellmar T. C., Carpenter D. O. Voltage-dependent calcium current induced by serotonin. Nature. 1979 Feb 8;277(5696):483–484. doi: 10.1038/277483a0. [DOI] [PubMed] [Google Scholar]
- Shimahara T., Tauc L. Cyclic AMP induced by serotonin modulates the activity of an identified synapse in Aplysia by facilitating the active permeability to calcium. Brain Res. 1977 May 20;127(1):168–172. doi: 10.1016/0006-8993(77)90389-4. [DOI] [PubMed] [Google Scholar]
- Treistman S. N., Drake P. F. The effects of cyclic nucleotide agents on neurons in Aplysia. Brain Res. 1979 Jun 8;168(3):643–647. doi: 10.1016/0006-8993(79)90321-4. [DOI] [PubMed] [Google Scholar]
- Treistman S. N., Levitan I. B. Alteration of electrical activity in molluscan neurones by cyclic nucleotides and peptide factors. Nature. 1976 May 6;261(5555):62–64. doi: 10.1038/261062a0. [DOI] [PubMed] [Google Scholar]
- Treistman S. N., Levitan I. B. Intraneuronal guanylyl-imidodiphosphate injection mimics long-term synaptic hyperpolarization in Aplysia. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4689–4692. doi: 10.1073/pnas.73.12.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss K. R., Schonberg M., Mandelbaum D. E., Kupfermann I. Activity of an individual serotonergic neurone in Aplysia enhances synthesis of cyclic adenosine monophosphate. Nature. 1978 Apr 20;272(5655):727–728. doi: 10.1038/272727a0. [DOI] [PubMed] [Google Scholar]
- Wilson W. A., Goldner M. M. Voltage clamping with a single microelectrode. J Neurobiol. 1975 Jul;6(4):411–422. doi: 10.1002/neu.480060406. [DOI] [PubMed] [Google Scholar]




