Abstract
Background
In order to maximize the benefits of HIV care and treatment investments in sub-Saharan Africa, programs can broaden to target other diseases amenable to screening and efficient management. We nested cervical cancer screening into family planning clinics at select sites also receiving PEPFAR support for antiretroviral therapy (ART) rollout. This was done using visual inspection with acetic acid (VIA) by maternal child health nurses. We report on achievements and obstacles in the first year of the program in rural Mozambique.
Methods
VIA was taught to clinic nurses and hospital physicians, with a regular clinical feedback loop for quality evaluation and retraining. Cryotherapy using carbon dioxide as the refrigerant was provided at clinics; loop electrosurgical excision procedure (LEEP) and surgery were provided at the provincial hospital for serious cases. No pathology services were available.
Results
Nurses screened 4651 women using VIA in Zambézia Province in year one of the program, more than double the Ministry of Health service target. VIA was judged positive for squamous intraepithelial lesions in 8% (n=380) of the women (9% if age ≥30 years (n=3154) and 7% if age <30 years (n=1497); p=0.02). Of the 380 VIA-positive women, 4% (n=16) had lesions (0.3% of 4651 total screened) requiring referral to Quelimane Provincial Hospital. Fourteen (88%) of these 16 women were seen at the hospital, but records were inadequate to judge outcomes. Of women screened, 2714 (58%) either had knowledge of their HIV status prior to VIA or were subsequently sent for HIV testing, of which 583 (21%) were HIV positive.
Conclusions
Screening and clinical services were successfully provided on a large scale for the first time ever in these rural clinics. However, health manpower shortages, equipment problems, poor paper record systems and a limited ability to follow-up patients inhibited the quality of the cervical cancer screening services. Using prior HIV investments, chronic disease screening and management for cervical cancer is feasible even in severely resource-constrained rural Africa.
Keywords: cervical cancer, VIA, PEPFAR, HIV, resource limited setting
Background
The World Health Organization estimated that, in Mozambique, cervical cancer was the most common cancer among women aged 15 to 44 years as of 2010, accounting for an estimated 3700 cases and 2400 deaths per year [1]. Globally, cervical cancer is the second most common cancer in women with approximately 500,000 new cases and 275,000 deaths per year, of which roughly 85% of cases occur in low-income countries [2]. Compared to global case averages in a population of approximately 6.8 billion people, the number of cervical cancer cases and deaths are 2.4 times and 2.7 times higher, respectively, in Mozambique. The nation is hampered in confronting cervical cancer by poor logistics and scarce health personnel; Mozambique ranks 165 of 169 nations on the World Development Index, reflecting its extremely low-economic resources [3].
In higher-income countries, the use of Papanikolaou (Pap) smear screening has correlated strongly with a reduction in both cervical cancer incidence and mortality [4,5]. Due to a lack of technical skills, human resources and the financing required to maintain effective cytology-based follow-up services, sustainable Pap smear-based screening programs have not been mounted in the lowest-income countries [6–10]. In late 2009, the Mozambican Ministry of Health (Ministério da Saúde or MISAU) initiated a rollout of cervical cancer screening using visual inspection with acetic acid (VIA) in four of its eleven provinces; Maputo, Sofala, Nampula and Zambézia (Figure 1). VIA has previously been established as an effective, low cost alternative to cytology-based screening in low-income countries [11–18].
Figure 1.
Map of Mozambique, Zambézia Province with districts highlighted that provide VIA screening services. VIA, visual inspection with acetic acid.
International funding specifically targeting cancer prevention is typically limited to hepatitis B vaccine provision through the Global Alliance for Vaccines and Immunization (GAVI), the World Health Organization Expanded Program on Immunization (WHO/EPI) and bilateral vaccine programs. In order to provide technical assistance for the introduction of this new service in Mozambique and due to the strong connection between human papillomavirus (HPV) infection and HIV, MISAU has partnered with international organizations funded through the United States Agency for International Development (USAID) and the US President's Emergency Plan for AIDS Relief (PEPFAR) in order to support program rollout.
In February 2010, Friends in Global Health, LLC (FGH) partnered with MISAU for the rollout of VIA screening and cryotherapy treatment. FGH is a PEPFAR-funded HIV care and treatment partner supported by the Centers for Disease Control and Prevention and affiliated with the Vanderbilt Institute for Global Health. Four health facilities and one reference centre in which support is also provided towards the rollout of antiretroviral therapy (ART) were selected for VIA capacitation in Zambézia Province, Mozambique's second most populated province with approximately 4 million people and <14% who live in an urban area. In 2009, Zambézia Province had an HIV prevalence estimate of 12.6% amongst adults aged 15 to 49 years and 15.3% among women [19–21]. We describe key challenges of rollingout a VIA-based cervical cancer screening program in a rural, extremely resource-limited setting.
Methods
FGH began its support of the VIA cervical cancer screening program as part of its PEPFAR-funded package of HIV care and treatment services in three of Zambézia's three rural districts selected in accordance with the MISAU national plan (Figure 1) for scale up. VIA and cryotherapy services were established in the main clinic of each of the districts of Inhassunge and Namacurra and in two health facilities (September 17th and Coalane clinics) within Quelimane city. All four health facilities provide ART. The Provincial Hospital of Quelimane also performs a small number of VIA screening examinations (approximately 18/month) and serves as the referral site for women with advanced disease needing loop electrosurgical excision procedure (LEEP) or surgery.
According to national protocols, all women between 30 and 55 years of age are eligible for screening [22]. However, due to existing perceptions that cervical cancer in Mozambique may be occurring at earlier ages, MISAU gave permission to expand screening to all sexually active women in Zambézia Province. At the clinic level, cervical cancer screening is performed by midlevel maternal child health (MCH) nurses and is embedded within the family planning clinics. In many of these clinics, family planning is performed within the same physical space and by the same nurses in which FGH is supporting the prevention of mother to child transmission services. Women are offered a screening examination by the MCH nurse. During this examination, women are evaluated for clinical signs of sexually transmitted infections (STI) and other gynaecologic pathologies (e.g., fistulas and uterine prolapse). A cotton swab is then applied and excess mucus is removed, prior to application of 5% acetic acid. One minute later, findings are reported as positive or negative for likely squamous intraepithelial lesions (SIL) by the MCH nurse. A positive VIA test is characterized by an opaque, dense, acetowhite lesion on the cervix in close proximity to the squamocolumnar junction or external os. Immediate treatment with cryotherapy (carbon dioxide (CO2) as it is the only available refrigerant) is offered in the same session as the screening, if the lesion involved is <75% of the transformational zone, does not involve the endocervix or there is no evidence of invasive cancer. Women with lesions involving >75% of the transformation zone, endocervical involvement or suspicious of invasive cancer are referred to the Quelimane Provincial Hospital for LEEP, biopsy or surgery [22].
Data are collected using the MISAU paper-based monitoring system, that is, an individual patient medical record form and a register book for the cervical cancer screening programme. Data may also be recorded in the family planning register and/or STI register, depending on findings and actions taken at the clinical visit.
Utilizing the technical assistance infrastructure already established through the roll-out of the PEPFAR-funded HIV care and treatment programme, FGH provided logistical and training support to screening, including equipment purchase and distribution, minor facility renovations, in-service training of national health system nurses and doctors, weekly clinical mentoring and on-the-job training of nurses tasked to perform VIA and cryotherapy and assistance for program data collection and analysis. The National Committee for Bioethics for Health in Mozambique and the Institutional Review Board of Vanderbilt University approved this analysis, including data collection from patient records.
Results
Start-up process
At start-up, we assembled a technical assistance team, performed a needs assessment of physical space and equipment needs, purchased/imported equipment and consumables, trained staff and assisted in deployment of the data management system. Preparations for the initiation of services took less than three months with importation of equipment requiring the greatest amount of time.
Prior to initiation of services, MCH nurses and physicians of the MISAU national health system in each of the screening health facilities were provided one week of cervical cancer training, notably to build skills in VIA and cryotherapy performance. Facilitators of the training included a mix of specialists from MISAU, FGH and Jhpiego™ (a national level USAID-funded technical partner to MISAU on cervical cancer). Trainings for nurses included a daily mixture of didactic classes, practical training with patients in which a minimum of six VIAs and two cryotherapy procedures had to be performed in the presence of the trainers and a review of VIA images. At the end of the week, nurses were determined to be competent if they scored greater than 80% on all of the activities throughout the week. Two gynaecologists in the Provincial Hospital (one Mozambican and one Cuban) also received a separate training on LEEP and colposcopy. During this training, each gynaecologist received a mixture of didactic classes and practical patient-based training and had to perform a minimum of three LEEP procedures in the presence of the trainers.
Enrolment/screening
Between 1 February 2010 and 31 January 2011; 4651 women were screened for cervical cancer using VIA in Zambézia Province (Table 1), 68% of whom were ≥30 years old. This more than doubled the annual screening goal established by MISAU for the first year of programme implementation, which was 1920 women (two women/day/site). VIA was judged positive in 8% (n=380) of women. For women ≥30 years of age, 9% (n=3154) were VIA positive and for women <30 years of age, 7% (n=1497) were VIA positive (p=0.02). Only 61% (n=221) of the 364 VIA-positive women received treatment on the day of the screening. Reasons for not receiving needed cryotherapy include equipment problems from either malfunction or theft of parts, as well as unavailability of trained personnel to perform cryotherapy due to other workload demands. By the last quarter of the year, 96% of cryotherapy eligible women received treatment on the day of the VIA screen compared to only 53% in the first quarter of the year (Table 2). There were no bleeding episodes or severe adverse effects reported in any women receiving cryotherapy. However, follow-up for these women is not yet systematic.
Table 1.
Number of women undergoing cervical cancer screening with visual inspection with acetic acid, by Zambézia Province Health Facility, Mozambique, February 2010 to January 2011
| VIA negative | VIA positive | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Health facility | n | <30 years old | ≥30 years old | <30 years old | ≥30 years old | Cryotherapy performed | Suspected ICC |
| Provincial Hospital | 208 | 10 | 176 | 2 | 20 | 9 | 8 |
| Inhassunge | 1721 | 291 | 1296 | 27 | 107 | 97 | 4 |
| Namacurra | 1060 | 321 | 593 | 40 | 106 | 53 | 1 |
| September 17th | 1265 | 581 | 635 | 20 | 29 | 39 | 2 |
| Coalane | 397 | 193 | 175 | 12 | 17 | 23 | 1 |
| Total | 4651 | 1396 | 2875 | 101 | 279 | 221 | 16 |
ICC, invasive cervical cancer; VIA, visual inspection with acetic acid.
Table 2.
Percentage of women meeting the criteria for cryotherapy who received treatment on the same day as their screening test, per three-month period, Zambézia Province, Mozambique, 2010 to 2011
| Months | VIA positive | Cryotherapy performed | % |
|---|---|---|---|
| February to April | 136 | 72 | 53 |
| May to July | 134 | 75 | 56 |
| August to October | 46 | 30 | 65 |
| November to January | 46 | 44 | 96 |
| Total | 362 | 221 | 61 |
VIA, visual inspection with acetic acid.
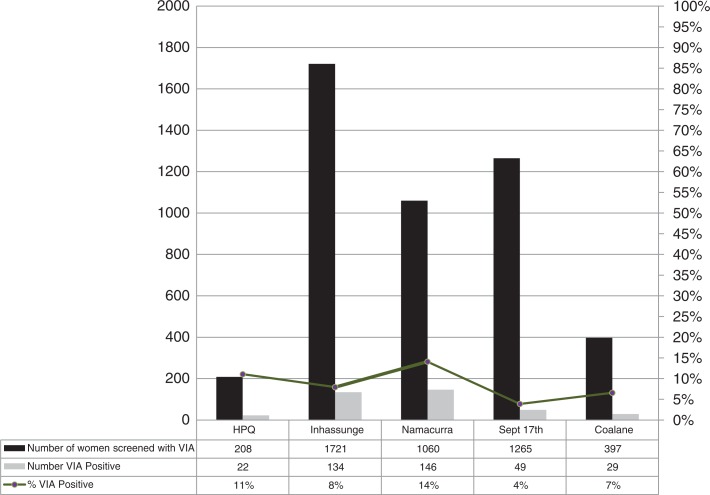
Over the course of the year, VIA positivity rates decreased from 9% at the beginning of the reporting period to 5% at the end of the reporting period, with Namacurra district showing the highest overall positivity rate at 14% and the Quelimane city clinic September 17th the lowest at 4% (Figure 2). Of the 380 women positive by VIA, 4% (n=16) had lesions (0.3% of 4651 total screened) requiring referral for further evaluation. Fourteen (88%) of these 16 women were seen for follow-up at the Quelimane Provincial Hospital where register data only documented the patient's name, from whence they were referred, and the reason for referral. We do not know their outcomes or the services provided. No pathology services were available and neither Pap smears nor HPV tests were performed for SIL screening, though they have been used in Maputo, the national capital [23–26].
Figure 2.
Proportion of women screened with VIA with a positive screen result by clinic, Zambézia Province. VIA, visual inspection with acetic acid.
Fifty-two percent (n=2445) of women screened knew their HIV status prior to being screened. Of these, 21% (n=521) were HIV positive by self-report. Of the 2206 women who did not know their HIV status, only 12% (n=269) were referred for HIV counselling and testing. Of these, 23% (n=62) were HIV positive. Of the 583 women finally known to be HIV positive, 37 (6%) were also VIA positive on screening (OR, 0.82, 95% CI: 0.56 to 1.21).
As a secondary benefit of the VIA screening examination, woman are also evaluated for clinical symptoms of STIs and for other gynaecologic pathology. Over the course of the study period, 5% (n=218) of women were clinically diagnosed with symptoms of STIs that would not have likely been diagnosed if cervical cancer screening had not been offered. Of these, 98% (n=214) were documented as treated, again as judged by syndromic management and chart review. Two women were diagnosed with rectovaginal fistulas and eight were diagnosed with uterine prolapse; nine of ten were referred for further treatment.
Discussion
With PEPFAR support, MISAU staff offered VIA screening to all women of reproductive age attending five family planning clinics in Zambézia Province. Nurses screened 4651 women (more than double the target number) over the first year of program functioning, in partnership with FGH for program technical assistance and access of HIV programmatic resources. Prior to initiation of this service, there were no available cervical screening or colposcopy services and no cryotherapy or LEEP treatment for cervical precancerous lesions in Zambézia Province. For the vast majority of women screened, the VIA screening consultation provided them with their first gynaecologic examination outside of pregnancy. VIA positivity in women <30 years of age was comparable to women between 30 and 55 years. This may be due to initiation of sexual activity at an earlier age in our population or possibly to the high burden of HIV resulting in more susceptible woman to the development of precancerous lesions. Overall, VIA positivity was 8% in our first year. This is comparable to other similar programmes where percent positivity ranged from 3.8% to 12.7% [6,7,9,10].
Results from the first year of screening in Zambézia province show that it is possible to implement a successful VIA screening program in severely resource-limited rural settings. We feel that there are three factors that contributed favourably to the successes experienced in the first year of roll-out in Zambézia: (1) strong MISAU leadership at a national level with clear guidelines and realistically attainable goals; (2) a strong collaborative relationship between clinic personnel and FGH; and (3) a hands-on supportive approach from FGH using both local and expatriate expertise fostered a strong mentoring relationship for the local nurses.
Challenges
Though screening activities exceeding MISAU expectations, we nonetheless identified five problems common to all the clinics:
Physical infrastructure
The physical conditions of clinics in Zambézia Province have suffered from neglect following a costly 16-year civil war (1976 to 1992); only recently have repairs and new construction been sponsored, largely from PEPFAR fiscal infusions. Hence, as a solution to this problem, space for VIA services required family planning clinics to have minor renovations that were financed with funds provided through the PEPFAR HIV care and treatment program, in all four clinics along with provision of all equipment such as gynaecology examination tables, examination lights, specula, cryosurgery equipment and consumables such as latex gloves and CO2.
Human resources/equipment
Mozambique's health sector human resources are among the world's most limited. Typically, clinics in Mozambique are run by nurses, sometimes with the aid of physician assistants (Técnicos de Medicina). Task shifting to MCH nurses is accompanied by an excessive workload and turn-over rates are high, particularly in the rural areas. Our year one implementation of VIA screening had a high turnover in clinic nurses and hospital physicians who had been trained in the performance of VIA and cryotherapy. Staff turnover is high in general within the Mozambican national health system and not necessarily reflective of just the family planning clinic and cervical cancer screening program. Due to a generalized staffing shortage, those who stayed were often pulled from the VIA service to cover other areas within the clinic. At start-up, 10 MCH nurses and six physicians were trained; however, by year end, no physicians and only five of these MCH nurses continued providing VIA services. As a consequence, service delivery was often delayed or unavailable for substantial periods of time.
The introduction of VIA and cryotherapy services brought new equipment that was unfamiliar to the health staff. Cryotherapy equipment was imported either from South Africa or the US. During year one, two other reasons for services at multiple sites being delayed or halted included: (1) theft of equipment parts of the cryotherapy apparatus, requiring weeks to purchase replacement material; and (2) unavailability of technicians trained on the maintenance and repairs of equipment with problems. We had not anticipated the theft problem, as it is rarely a problem for other equipment in the clinics. In order to overcome this problem, initiatives were implemented to better secure equipment from theft and intensive training to increase the number of technicians for equipment maintenance.
While poor uptake of cryotherapy on the same day as the positive VIA screen was common in the beginning of the program (only 53%), the last quarter's data showed 96% of women receiving cryotherapy on the same day that the VIA was judged suggestive for SIL. MISAU staff did not believe that patient reluctance to get care was a factor in failure to obtain same-day cryotherapy, but we have no data on this issue.
Monitoring and evaluation
Prior to the introduction of PEPFAR-supported HIV care and treatment services, all health records were maintained on paper registers and aggregate reporting was submitted monthly. The scale-up of HIV care and treatment has introduced electronic patient tracking systems for HIV services, but chronic disease services such as “see and treat” cervical cancer screening have not yet benefitted from electronic record systems. As a result, collection of program data requires that numerous forms to be completed with excessive repetition of information.
Four percent (n=16) of VIA positive women were referred for colposcopy due to the size of the lesion identified on VIA screening. The current monitoring and evaluation system does not allow for ease in follow-up of these 16 women between the clinic in which the VIA was performed and outcomes of their subsequent visit to the referral centre, though we know that 14 of 16 women did present to the Provincial Hospital in Quelimane. Due to poor hospital record-keeping, we could not analyze program outcomes for women with advanced disease and judge whether appropriate therapy had been instituted. Conversations with MISAU have already begun and are under review to explore the introduction of an electronic database for the cervical cancer screening programme, which would evolve the monitoring and evaluation of the program from aggregate data collection alone to patient level data in which the patient could be followed across multiple services and between the main clinic and the referral centre.
Quality assurance of screening
FGH currently provides weekly intensive on-the-job mentoring by nurse specialists trained in VIA with the nurses of the national health system who provide VIA screening examinations. This includes oversight of VIA technique and performance and provision of a second opinion on cases in which the MCH nurse has questions about the screening result. While these basic on-the-job mentoring activities were provided to evaluate the quality of VIA screening and nurse decision making, we believe that digital cervicography (a permanent photographic record) and back-up cytology and pathology testing would improve our ability to evaluate screening quality and judge true positivity rates.
Failure to screen for HIV
Only 12% of women with HIV status unknown at the time of the VIA screen were referred for subsequent HIV counselling and testing. This represents a huge missed opportunity and as a solution, we are now working intensively to ensure full availability of provider initiated counselling and testing (PICT) services within the family planning clinics to obviate the need for referrals elsewhere for HIV testing.
Besides the potential health benefits in preventing morbidity and mortality associated with HPV infection, other secondary benefits of implementing VIA cervical cancer screening are worth highlighting: such as improved knowledge of women screened as to their own gynaecological health, diagnosis and treatment of concurrent STIs/HIV, and the identification and referral for treatment of other gynaecological pathologies such as rectovaginal fistulas and uterine prolapse. Basic knowledge of our patients as to their own gynaecologic health was low. Qualitative studies as to the acceptability of VIA screening as well as basic gynaecologic health knowledge are currently underway in Zambézia Province to quantitate this gap. In countries such as Zambia, relatively low cost digital cervicography has been introduced with success into VIA screening programs [27]. This technology projects an image of the cervix to a bedside monitor or television screen and allows for immediate education of patients about examination results. As well, the capturing of the cervical examination image allows for greater quality assurance as to the treatment decisions made and provides an electronic platform for program data capture. We feel that addition of this type of technology could counter many of the weaknesses of our current programme, improving continuing nursing education and QA, and we are seeking the support of MISAU to introduce this simple technology in Zambézia Province. Better computing infrastructure would also enable a roll-out of electronic medical records beyond the HIV services. Paper records and forms proved to be an inefficient use of nursing time for an already overstretched health workforce and also did not permit prompt access to patient data to guide care decisions and program policies.
Conclusions
Our experiences reflect the realities of severe resource constraints in the world's poorest nations. Both health manpower shortages and logistical limitations constrained more complete success. Inability to “see and treat” the patient on the same visit was noted in almost half of patients at the programme start, but this was nearly resolved within the year. Lack of a proper patient follow-up system lead to an inability to document whether suspected cancer patients actually got needed care. In addition, our data may be interpreted to suggest that screening of women at all ages is indicated; we caution that we do not have cytopathology to document whether or not our younger patients might not have had more false positives than our older patients. Despite challenges, cervical cancer screening using VIA is feasible in resource-constrained rural Africa, and we hope that our experiences, frankly presented here, can be useful to others.
Acknowledgements
Funding sources
This study was financially supported by the Centers for Disease Control and Prevention, PEPFAR grant U2GPS000631.
Competing interests
The authors declare they have no competing interests.
Authors' contributions
TDM prepared the study proposal, collected and analyzed the data, interpreted the findings and wrote the manuscript. CSM was involved in developing the study proposal, supervising the data collection and reviewing the manuscript. AC was involved in collecting and analyzing data and reviewing the manuscript. AJB was involved in supervising the data collection and reviewing the manuscript. MS was involved in analyzing data and reviewing the manuscript. SHV was involved in the study proposal and writing of the manuscript. All authors have read and approved the final manuscript.
Abbreviations
ART, antiretroviral therapy; FGH, Friends in Global Health, LLC; LEEP, loop electrosurgical excision procedure; MCH, maternal child health; MISAU, Ministério da Saúde; Pap, Papanikolaou; PEPFAR, US President's Emergency Plan for AIDS Relief; STI, sexually transmitted infections; SIL, squamous intraepithelial lesions; VIA, visual inspection with acetic acid; USAID, United States Agency for International Development.
References
- 1.Human Papillomavirus and Related Cancers. Mozambique [Internet]. [cited 2011 Aug 22]. Available from http://apps.who.int/hpvcentre/statistics/dynamic/ico/country_pdf/MOZ.pdf?CFID=4005139&CFTOKEN=50211239.
- 2.World Health Organization [Internet] Sexual and reproductive health [cited 2011 Aug 22]. Available from: http://www.who.int/reproductivehealth/topics/cancers/en/
- 3.Indices & Data. Human Development Reports (HDR), [Internet] United Nations Development Programme (UNDP) [cited 2011 Aug 23]. Available from: http://hdr.undp.org/en/statistics/
- 4.Miller Report on consensus conference on cervical cancer screening and management. Int J Cancer [Internet] 2000 doi: 10.1002/(sici)1097-0215(20000501)86:3<440::aid-ijc22>3.0.co;2-a. Wiley Online Library [cited 2011 Aug 22]. Available from: http://onlinelibrary.wiley.com.proxy.library.vanderbilt.edu/doi/10.1002/(SICI)1097-0215(20000501)86:3%3C440::AID-IJC22%3E3.0.CO;2-A/pdf. [DOI] [PubMed] [Google Scholar]
- 5.Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004;364(9430):249–56. doi: 10.1016/S0140-6736(04)16674-9. [DOI] [PubMed] [Google Scholar]
- 6.Muwonge R, Manuel M, da G, Filipe AP, Dumas JB, Frank MR, Sankaranarayanan R. Visual screening for early detection of cervical neoplasia in Angola. Int J Gynaecol Obstet. 2010;111(1):68–72. doi: 10.1016/j.ijgo.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Moodley J, Kawonga M, Bradley J, Hoffman M. Challenges in implementing a cervical screening program in South Africa. Cancer Detect Prev. 2006;30(4):361–8. doi: 10.1016/j.cdp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Chumworathayi B, Blumenthal PD, Limpaphayom KK, Kamsa-Ard S, Wongsena M, Supaatakorn P. Effect of single-visit VIA and cryotherapy cervical cancer prevention program in Roi Et, Thailand: a preliminary report. J Obstet Gynaecol Res. 2010;36(1):79–85. doi: 10.1111/j.1447-0756.2009.01089.x. [DOI] [PubMed] [Google Scholar]
- 9.Vedantham H, Silver MI, Kalpana B, Rekha C, Karuna BP, Vidyadhari K, et al. Determinants of VIA (visual inspection of the cervix after acetic acid application) positivity in cervical cancer screening of women in a peri-urban area in Andhra Pradesh, India. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1373–80. doi: 10.1158/1055-9965.EPI-09-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngoma T, Muwonge R, Mwaiselage J, Kawegere J, Bukori P, Sankaranarayanan R. Evaluation of cervical visual inspection screening in Dar es Salaam, Tanzania. Int J Gynaecol Obstet. 2010;109(2):100–4. doi: 10.1016/j.ijgo.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal P, Batra S, Gandhi G, Zutshi V. Comparison of papanicolaou test with visual detection tests in screening for cervical cancer and developing the optimal strategy for low resource settings. Int J Gynecol Cancer. 2010;20(5):862–8. doi: 10.1111/IGC.0b013e3181e02f77. [DOI] [PubMed] [Google Scholar]
- 12.Quentin W, Adu-Sarkodie Y, Terris-Prestholt F, Legood R, Opoku BK, Mayaud P. Costs of cervical cancer screening and treatment using visual inspection with acetic acid (VIA) and cryotherapy in Ghana: the importance of scale. Trop Med Int Health. 2011;16(3):379–89. doi: 10.1111/j.1365-3156.2010.02722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nessa A, Hussain MA, Rahman JN, Rashid MH, Muwonge R, Sankaranarayanan R. Screening for cervical neoplasia in Bangladesh using visual inspection with acetic acid. Int J Gynaecol Obstet. 2010;111(2):115–8. doi: 10.1016/j.ijgo.2010.06.004. Epub 2010 Aug 2. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y-Z, Ma J-F, Zhao F-H, Xiang XE, Ma ZH, Shi YT, et al. Three-year follow-up results of visual inspection with acetic acid/Lugol's iodine (VIA/VILI) used as an alternative screening method for cervical cancer in rural areas. Chin J Cancer. 2010;29(1):4–8. doi: 10.5732/cjc.009.10687. [DOI] [PubMed] [Google Scholar]
- 15.Qiao Y-L. Perspective of cervical cancer prevention and control in developing countries and areas. Chin J Cancer. 2010;29(1):1–3. doi: 10.5732/cjc.009.10570. [DOI] [PubMed] [Google Scholar]
- 16.Mwanahamuntu MH, Sahasrabuddhe VV, Kapambwe S, Pfaendler KS, Chibwesha C, Mkumba G, et al. Advancing cervical cancer prevention initiatives in resource-constrained settings: insights from the cervical cancer prevention program in Zambia. PLoS Med. 2011;8(5):e1001032. doi: 10.1371/journal.pmed.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mwanahamuntu MH, Sahasrabuddhe VV, Stringer JS, Parham GP. Integrating cervical cancer prevention in HIV/AIDS treatment and care programmes. Bull World Health Organ. 2008;86(8):D–E. doi: 10.2471/BLT.08.056275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahasrabuddhe VV, Bhosale RA, Kavatkar AN, Nagwanshi CA, Joshi SN, Jenkins CA, et al. Comparison of visual inspection with acetic acid (VIA) and cervical cytology to detect high grade cervical neoplasia among HIV-infected women in India. Int J Cancer [Internet] 2012. [cited 2011 Sep 23];130:234–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21387289. [DOI] [PMC free article] [PubMed]
- 19.Moon T, Burlison J, Sidat M, Pires P, Silva W, Solis M, et al. Lessons learned while implementing an HIV/AIDs care and treatment program in rural Mozambique. Retrovirol Res Treat. 2010;3:1–14. doi: 10.4137/RRT.S4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.População por distritos da Zambézia [Internet] 2007. [cited 2011 Sep 23]. Available from: http://www.ine.gov.mz/censo2007/rdcenso09/zambezia/popZamb.
- 21. INSIDA/HIV/SIDA/Home – Ministério da Saúde [Internet] [cited 22 Aug 2011]. Available at: http://www.misau.gov.mz/pt/hiv_sida/insida.
- 22.Cunha MM [Internet] Normas Nacionais para Prevenção do Cancro do Colo Uterino, 2a Edição: Ministerio da Saúde [cited 23 Sep 2011] Available from: http://www.misau.gov.mz. Accesses September 23, 2011.
- 23.Carrilho C, Cirnes L, Alberto M, Buane L, Mendes N, David L. Distribution of HPV infection and tumour markers in cervical intraepithelial neoplasia from cone biopsies of Mozambican women. J Clin Pathol. 2005;58(1):61–8. doi: 10.1136/jcp.2004.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrilho C, Gouveia P, Cantel M, Alberto M, Buane L, David L. Characterization of human papillomavirus infection, P53 and Ki-67 expression in cervix cancer of Mozambican women. Pathol Res Pract. 2003;199(5):303–11. doi: 10.1078/0344-0338-00422. [DOI] [PubMed] [Google Scholar]
- 25.Carrilho C, Alberto M, Buane L, David L. Keratins 8, 10, 13, and 17 are useful markers in the diagnosis of human cervix carcinomas. Hum Pathol. 2004;35(5):546–51. doi: 10.1016/j.humpath.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Carrilho C, Cantel M, Gouveia P, David L. Simple mucin-type carbohydrate antigens (Tn, sialosyl-Tn, T and sialosyl-T) and gp 230 mucin-like glycoprotein are candidate markers for neoplastic transformation of the human cervix. Virchows Arch. 2000;437(2):173–9. doi: 10.1007/s004280000218. [DOI] [PubMed] [Google Scholar]
- 27.Mwanahamuntu MH, Sahasrabuddhe VV, Pfaendler KS, Mudenda V, Hicks ML, Vermund SH, et al. Implementation of “see-and-treat” cervical cancer prevention services linked to HIV care in Zambia. AIDS. 2009;23(6):N1–5. doi: 10.1097/QAD.0b013e3283236e11. [DOI] [PMC free article] [PubMed] [Google Scholar]