Abstract
Background
Tuberculosis (TB) continues to be the most frequent cause of illness and death from an infectious agent globally, and its interaction with HIV is having devastating effects. To investigate how HIV alters the immune response to Mycobacterium tuberculosis (Mtb), we assessed basal and Mtb-induced proliferation, cytokine production, and expression of signalling lymphocytic activation molecule (SLAM), inducible costimulator (ICOS) and programmed death-1 (PD-1) on T lymphocytes from HIV-positive individuals coinfected with TB, HIV-positive subjects, TB patients and healthy donors (HD).
Findings
HIV-TB patients showed increased ICOS, SLAM and PD-1 basal levels on T lymphocytes, whereas HIV-positive individuals displayed elevated levels of SLAM and PD-1, TB patients high levels of SLAM, and HD low levels of the three proteins. Mtb-stimulation enhanced ICOS expression in the four groups, but only TB and HD increased SLAM and PD-1 levels.
Conclusions
These data show the immune deregulation that takes place during the immune response against TB in different study populations.
Keywords: human, AIDS, tuberculosis, T cells, ICOS, SLAM, PD-1, cytokines
Introduction
Tuberculosis (TB) continues to be the most frequent cause of illness and death from an infectious agent, and its interaction with HIV infection presents devastating effects. Globally, 9.2 million new cases and 1.7 million deaths occur annually due to TB, of which 0.7 million cases and 0.2 million deaths correspond to HIV-positive individuals. HIV infection has emerged by far, as the most important of all the predisposing factors for the development of TB, and TB is often the sentinel illness of HIV infection. TB causes worsening of HIV disease status, increases viral replication, decreases immunity and hastens the onset of AIDS [1]. IFN-γ production by Th1 lymphocytes and macrophage activation are crucial in the defence against mycobacteria [2]. On the other hand, HIV infection is associated with a profound dysregulation of the immune system and alterations in the cytokine profile [3].
Several T cell molecules such as the signalling lymphocytic activation molecule (SLAM), inducible costimulator (ICOS) and programmed death-1 (PD-1) have been shown to modulate the level and pattern of cytokines produced by T cells [4–6]. These costimulatory receptors fine tune the expansion and properties of activated T cells. ICOS signalling might promote expansion of effector cells that would traffic into inflamed tissues, interact with APC there and be regulated by PD-1 and its ligands [7]. Also, it has been shown that PD-1:PD-Ls interactions can regulate T cell proliferation, cytokine production [5], and the balance among tolerance, autoimmunity, infection and immunopathology [8]. In addition, PD-1 has a relevant role in HIV infection, since it has been reported that HIV-specific T cells express elevated levels of PD-1 and that this expression correlates with the viral load and inversely with CD4+ T cell counts. More importantly, antibody blockade of the PD-1/PD-L1 pathway was sufficient to both increase and stimulate virus-specific T cell proliferation and cytokine production. Moreover, it has been proposed that PD-1 is the major factor of immune exhaustion and activation in HIV infection [9]. Previously, we reported that both ICOS and SLAM activation promoted the induction of protective Th1 cytokine responses against Mycobacterium tuberculosis (Mtb) [4,6] and the PD-1:PDLs pathway negatively modulates T cell effector functions during human TB [5]. Overall, a balance between activating and inhibitory signals ensures the development of an effective immune response. Therefore, to test the hypothesis that HIV infection could favour an alteration in the host immune response to Mtb, we investigated the regulation of the expression of the signalling molecules SLAM, ICOS and PD-1 during HIV, TB and HIV-TB coinfection.
Materials and methods
Patients
Four groups of individuals were enrolled: (1) asymptomatic HIV-1 infected patients (HIV-positive), as determined by ELISA and confirmatory Western Blot and no history of being exposed to TB; (2) individuals with active TB; (3) HIV-1 infected patients with active TB (HIV-TB), without other opportunistic infections; and (4) Bacillus Calmette-Guerin-vaccinated healthy donors (HD) with no history of TB contact. Patients with active TB were evaluated at Hospital F.J. Muñiz. HIV-positive patients and HIV-TB individuals were enrolled at Hospital J.A. Fernández. Diagnosis of TB was based on clinical and radiological data together with the identification of acid-fast bacilli in sputum or a positive culture of TB bacilli. HIV-TB individuals showed lower CD4 counts than HIV-positive individuals (median±SD: HIV-positive group: 353±233 cells/µl; HIV-TB group: 106±195 cells/µl, p<0.01, n=15 individuals per group, Mann Whitney test), but there were no differences in CD4 percentages between groups (median±SD: HIV-positive group: 20±8.8; HIV-TB group: 13±8.8, p=0.1726, n=15 individuals per group, Mann Whitney test). CD4 counts were not determined in TB patients. However, several reports indicate that TB patients display higher CD4 counts when comparing TB patients with HIV-TB patients [10,11] and lower when contrasting with HD values [11]. We did not observe statistical differences in HIV viral load between HIV-positive and HIV-TB individuals (median±SD: HIV-positive group: 39.619±74.745 HIV copies/ml; HIV-TB group: 68.554±2.4915 HIV copies/ml, p=n.s.). All participating patients had received less than one-week anti-TB therapy and no ARV treatment. Informed consent was obtained, according to the local ethics committee.
Antigen
In vitro stimulation of cells was performed with previously titrated M. tuberculosis H37Rv (Mycobacteria Research Laboratories, Colorado State University, Fort Collins, CO, USA), prepared by probe sonication.
Culture conditions
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation on Ficoll-Paque and cultured with M. tuberculosis (10 µg/ml) in 24- or 96-well plates with RPMI supplemented with L-glutamine, gentamincin and 10% human serum. After five days, cells were pulsed with [3H]TdR (1 µCi/well) and harvested 16 hours later, and [3H]TdR incorporation was measured in a liquid scintillation counter. IFN-γ and IL-10 production was measured by ELISA (eBioscience, USA).
Phenotyping
PBMC were stained with mAbs specific for CD3, ICOS, SLAM and PD-1 (eBioscience) before and after culture with sonicated M. tuberculosis. Negative control samples were incubated with irrelevant, isotype-matched mAbs in parallel with experimental samples. Samples were analyzed on a FACSCanto flow cytometer (BD Biosciences, USA).
Statistical analysis
Statistical analysis was performed using the non-parametric Wilcoxon Rank Sum test for paired samples and the Mann Whitney test for between-groups comparisons. Values of p<0.05 were considered significant.
Results and discussion
Immunity to TB requires the induction of IFN-γ, a key cytokine mainly produced by T cells that enhances macrophage function, crucial to control the pathogen. To investigate the alterations that HIV infection induces in the immune response during TB, we evaluated T cell proliferation, cytokine production and the expression of the costimulatory molecules ICOS, SLAM and PD-1 in individuals infected with HIV, dually infected with HIV-TB, patients with active TB and HD after in vitro stimulation with M. tuberculosis.
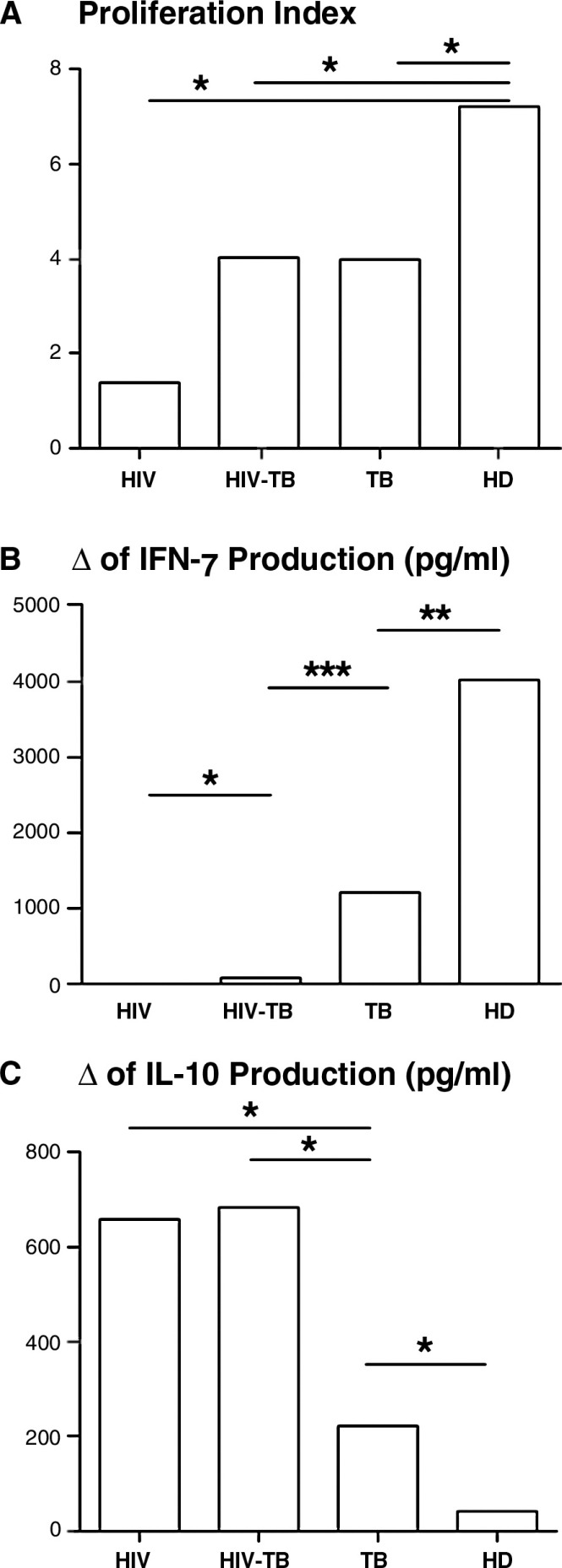
Since protection against M. tuberculosis requires T cell activation and IFN-γ production [12], and IL-10 inhibits pro-inflammatory responses to the pathogen [13], we first studied the cytokine and proliferating responses of HIV-positive, HIV-TB, TB and HD groups to M. tuberculosis. Antigen stimulation induced a significant proliferation in HIV-TB, TB and HD groups, whereas HIV-positive individuals showed impaired proliferation in response to the antigen compared to media (data not shown). Of note, basal proliferation was similar among groups (data not shown). Moreover, we observed a significantly higher proliferation index (PI) in HD compared with HIV-positive, HIV-TB and TB patients, showing the latter three groups a gradual increment in their PI from HIV-positive → HIV-TB → TB individuals (Figure 1A). Importantly, cells from HD volunteers responded to Mtb stimulation because of their BCG-vaccinated condition. The fact that these individuals display a significant response to M. tuberculosis is because of the previously described cross-reactivity between M. tuberculosis and BCG antigens [14].
Figure 1.
In vitro responses to M. tuberculosis of HIV, HIV-TB, TB and HD individuals. (A) PBMC from individuals belonging to each group were cultured in the presence or absence of sonicated M. tuberculosis for five days and proliferation was determined by [3H-tymidine] incorporation. PI for each individual was calculated as: cpm after M. tuberculosis-stimulation/cpm after culturing with media. Each bar represents the median of the PI in each group (10 individuals per group). (B and C) PBMC from individuals belonging to each group were cultured in the presence or absence of antigen for 48 hours and (B) IFN-γ and (C) IL-10 production was measured by ELISA. Each bar shows the median of the IFN-γ or IL-10 production increment from media levels in each group (10 individuals per group). The values of delta (Δ) were calculated as follows: pg/ml of cytokine produced after M. tuberculosis stimulation − pg/ml of cytokine produced after culture with medium. *p<0.05, *p<0.01, ***p<0.005. HD, healthy donors; PBMC, peripheral blood mononuclear cells; PI, proliferation index.
Additionally, M. tuberculosis stimulation of PBMC from HIV-positive, HIV-TB, TB patients and HD induced a similar profile of differences in IFN-γ production (Figure 1B). Interestingly, IL-10 production was significantly increased in HIV-positive and HIV-TB patients after M. tuberculosis stimulation, compared to TB patients and HD (Figure 1C). These data suggest that chronic HIV infection not only reduces CMI to M. tuberculosis but also redirects the pattern of cytokines towards an anti-inflammatory phenotype. Other authors explored the cytokine responses during HIV infection or coinfection HIV-TB. Zhang et al. studied the proliferative and cytokine responses to Mtb (Erdman strain) in HIV-positive and coinfected patients and observed a similar pattern in proliferation and IFN-γ production. In contrast, they observed similar IL-10 responses between both groups, perhaps because of the different stimuli they used [15]. Also, Bal et al. also described the alterations in the cytokine profile induced by mitogens during TB, HIV, HIV-TB coinfection and in HD and observed that the secretion of IFN-γ was significantly lower in coinfected individuals than in TB patients and HD, but IL-10 production was lower in coinfected compared to HD and TB patients and did not differ from HIV-TB and HIV-positive individuals [16]. We can speculate from other works that M. tuberculosis as a hole bacteria will induce IL-10 by both monocytes, mediated by innate signals [17] and M. tuberculosis-specific T lymphocytes, arbitrated by specific recognition [17]. Moreover, we have previously described that the major source of IL-10 during TB infection are T lymphocytes [6], therefore we can hypothesize that, in coinfected individuals, T lymphocytes will be producers of this cytokine. Overall, published data on cytokine responses to Mtb antigens is extremely diverse, suggesting thus the need to further investigate the cytokine profile of our study populations in the context of Mtb-specific stimulation.
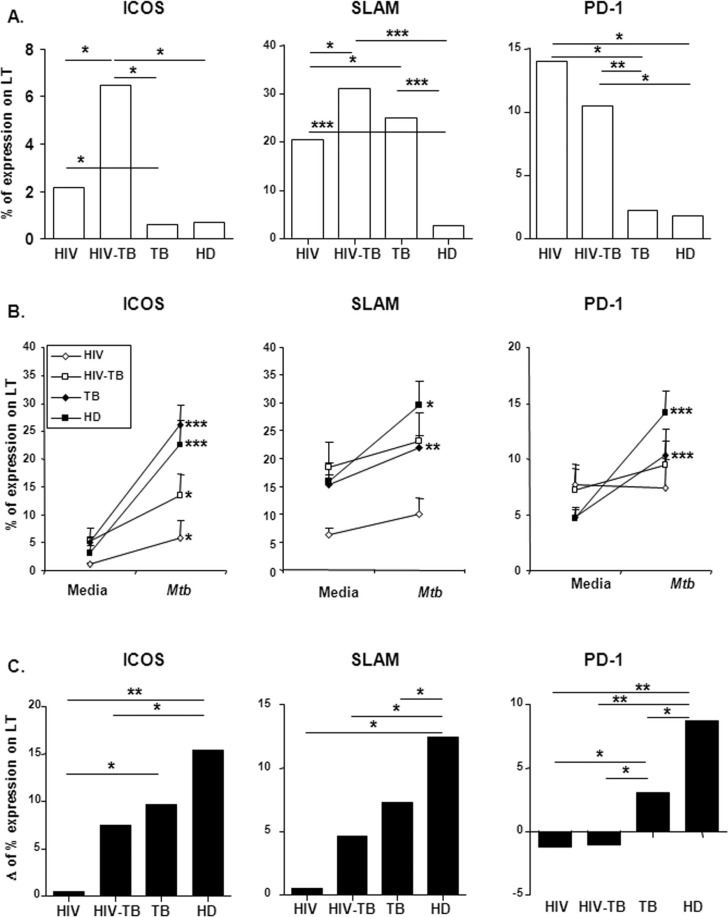
Continuing our study, and in order to characterize the immune dysregulation induced by HIV infection in M. tuberculosis immune responses, we analyzed basal expression of molecules involved in T cell regulation of cytokine responses against mycobacteria: ICOS, an activation-dependent T cell molecule; SLAM, a promoter of T cell proliferation and IFN-γ production; and PD-1, also crucial for effective immune responses to HIV [4–6][18]. Only coinfected patients showed increased ICOS levels compared to other groups (Figure 2A), indicating that the T cell activation necessary for a high ICOS expression occurs when both infections are present, regardless of the IFN-γ response to the bacteria. Moreover, basal expression of SLAM was significantly higher on T cells from HIV, HIV-TB and TB patients compared to HD (Figure 2A). To note, we believe that the differences in CD4 counts between HIV-positive and HIV-TB patients is not a cause for the observed differences in signalling molecule's expression between both groups, since we observed a similar percentage of CD4+ T cells. Therefore, the observed differences in ICOS expression would be due to a discrepancy in T cell activation induced by the presence of both pathogens.
Figure 2.
Expression and regulation of costimulatory molecules on T lymphocytes before and after M. tuberculosis stimulation from HIV, HIV-TB, TB and HD individuals. (A) PBMC from individuals belonging to each group were stained for ICOS, SLAM or PD-1 and the expression of each molecule was evaluated using two-color flow cytometry. ICOS, SLAM or PD-1 expression on T cells was determined by first gating on total CD3+ cells, then evaluating each molecule expression. Bars represent the median of ICOS, SLAM or PD-1 basal expression for each group. (B) PBMC from patients and HD were cultured in the presence or absence of sonicated M. tuberculosis. ICOS, SLAM or PD-1 expression was determined on T cells after five-day stimulation as in (A). The mean±SEM for each molecule expression is shown. (C) Increment in ICOS, SLAM and PD-1 expression from media levels was evaluated on T cells from individuals belonging to each group. The values of delta (Δ) were calculated as follows: percentage of CD3+ molecule+ T cells after M. tuberculosis stimulation − percentage of CD3+ molecule+ T cells after culture with medium. The median of molecule's delta is shown for each group. Ten individuals per group were evaluated. *p<0.05, ***p<0.005. HD, healthy donors; PBMC, peripheral blood mononuclear cells; ICOS, inducible costimulator; SLAM, signalling lymphocytic activation molecule; PD-1, programmed death-1.
Finally, we observed that basal expression of PD-1 on T lymphocytes from HIV-positive and HIV-TB individuals was similar and significantly higher than TB patients and HD (Figure 2A).
When studying the Ag-induced regulation of the receptors, we observed that all subject groups had an increased ICOS expression in response to M. tuberculosis stimulation compared to media levels (Figure 2B). Nevertheless, such relative increment reached the highest values in HD, followed by TB and HIV-TB individuals in the same extent, with HIV-positive patients displaying the lowest increment (Figure 2C). ICOS promotes protective Th1 cytokine responses to M. tuberculosis [4] and inhibits the early steps of HIV-1 replication [19]. The significant differences observed in ICOS increment between HD and HIV or HIV/TB patients (Figure 2C) indicate that HIV impairs the upregulation of the receptor. Moreover, although there are no differences between HD and TB patients regarding ICOS increment in Figure 2C, it should be kept in mind that TB patients have also certain degree of impairment on their immune response to M. tuberculosis, therefore the fact that there are no significant differences in Figure 2C is not surprising. Hence, our results support that HIV infection impairs the M. tuberculosis-induced upregulation of ICOS (Figure 2B and C). These observations suggest a vital role for ICOS in coinfected individuals, since although both HIV-positive and HIV-TB patients produce high levels of IL-10 after Ag-stimulation, only HIV-TB individuals, displaying the highest ICOS expression, secrete more IFN-γ than HIV-positive patients.
The analysis of Ag-induced regulation of SLAM expression indicated that HIV infection impairs upregulation of the receptor, since both HIV-positive and HIV-TB individuals did not augment their SLAM levels on T cells compared to unstimulated cells (Figure 2B). On the contrary, TB patients and HD increased their SLAM percentages on T lymphocytes in response to M. tuberculosis stimulation as previously reported [6]. Comparisons on the relative increment between groups revealed that only HD increased their SLAM expression from media levels (Figure 2C). The impairment in SLAM upregulation observed in Figure 2C has to be analyzed comparing with HD's increment, since TB patients have a reduced (yet significant from media levels) increment in SLAM levels after antigen challenge. These data suggest that T cells from infected individuals are activated at basal state, and HIV infection negatively modulate the upregulation of SLAM. Moreover, although HIV-TB patients show high expression of the protein, this expression is not enough to induce a Th1 cytokine profile in response to M. tuberculosis challenge in terms of IFN-γ production. Although, in TB patients, the SLAM increment was lower than HD, we previously demonstrated that SLAM engagement could enhance M. tuberculosis-specific Th1 responses in TB patients [6]. We previously described that ICOS correlates with Mtb-induced IFN-γ production in TB patients [4], and the correlation analysis of the data from our work also revealed that ICOS correlates with IFN-γ production in HIV-infected individuals (data not shown, Spearman coefficient 0.6199, p<0.05). Thus, our results formally demonstrate a direct correlation between the levels of ICOS and cytokine secretion in coinfected HIV-TB patients. Furthermore, we also observed a direct correlation between the expression of SLAM and ICOS (data not shown, Spearman coefficient 0.8403, p<0.0001), suggesting that the regulation of costimulatory molecule's levels in coinfected patients is primarily dependent on the specific response of their T cells to M. tuberculosis.
Of note, SLAM and ICOS, both inducible T cell molecules, belong to two different families of receptors (SLAM to the IgG superfamily and ICOS to the TNFR family). Therefore, they will have different transcription factor requirements for protein expression. But more importantly, they work differently in terms of T cell costimulation: SLAM signalling promotes Th1 patterns [20] whereas ICOS signalling induces a Th1 or Th2 cytokine pattern, depending on the surrounding environment [21]. Therefore, and taking into account that T cell costimulation is a hierarchical process with elements of mutual interdependence between individual costimulators [21], it is not surprising that both molecules show different expression patterns during the infection with HIV and coinfection with TB.
Finally, we observed that M. tuberculosis-stimulation failed to induce any significant increase in PD-1 expression on T cells from HIV-positive and HIV-TB individuals, indicating an exhaustion of T cells in these patients. In contrast, TB patients and HD showed a significant increment in PD-1 on their T cells in response to Ag stimulation (Figure 2B). The evaluation of the relative increment in PD-1 after Ag stimulation among groups showed a gradual increment from HIV-positive → HIV-TB → TB → HD individuals (Figure 2C). Hence, although HIV-positive and HIV-TB patients presented higher amounts of PD-1 at basal conditions, infection with HIV impaired the functional response of T lymphocytes in terms of upregulation of signalling molecules, necessary for a fully functional T cell response to M. tuberculosis. Expression of PD-1 is regulated not only by T cell activation status [5] but also by HIV peptides [22]. Besides, PD-1 signalling inhibits T cell effector functions during TB [5] and HIV [22]. The finding of a higher expression of PD-1 on T cells from HIV-TB and HIV-positive individuals support the idea of upregulation of PD-1 by viral proteins, in addition to the enhanced cell activation described for both infections [23,24]. Therefore, higher basal levels of PD-1 on T cells from HIV-TB and HIV-positive subjects may induce dysfunctional M. tuberculosis-specific T cells, reflected in lower levels of IFN-γ production and proliferation in vitro against the antigen. After Ag-stimulation, neither HIV-TB nor HIV-positive patients could increase their PD-1 levels, further supporting the fact that HIV infection impedes a successful immune response to the pathogen.
TB, HIV-positive and specially coinfected patients show a proinflammatory state, which means their peripheral blood cells are in contact with a milieu where cytokines, chemokines and other factors are present [25,26]. That situation provoked by the infection(s) induces non-specific activation of T lymphocytes, therefore increasing the expression of activation molecules. That could be the case for ICOS, SLAM and PD-1 molecules. We therefore propose that five days of culture in media alone will induce downregulation of those molecules because of the absence of stimuli, as proposed for FoxP3 expression on T cells [27].
HIV infection induces dysregulation of several T cell processes, including cytokine secretion, cytotoxic capacity and T cell cooperation [28]. Expression of surface molecules reflects these changes, and therefore activation markers such as CD38, CD70, HLA-DR (also modulating antigen presentation) and CD45R0 among others will be dysregulated [29]. In turn, advanced TB infection provokes a diminished capacity on T cell responses to M. tuberculosis antigens [30]. The overall result is a diminished T cell response capacity, reflected on a smaller amount of IFN-γ production by T cells [30]. Our results are in concordance with the above-mentioned observations, since at baseline, HIV patients preferentially express low levels of ICOS, intermediate levels of SLAM and high levels of PD-1; HIV-TB individuals exhibit high levels of the three molecules, while TB patients show high levels only of SLAM on their T cells. The mechanisms involved need to be further elucidated and may lead to better understand the pathogenesis of both diseases.
Acknowledgements
The authors would like to thank Dr. Oscar Bottasso for his helpful comment on the manuscript and Dr. Gabriela Turk and Mr. Sergio Mazzini for continuous support.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JOJ, VP, IBA and GJM carried out the immunological studies. NL, OS, PC, RMM, EA and HS participated in its design and coordination and helped to draft the manuscript. MFQ conceived of the study, participated in the design of the study, performed the statistical analysis and wrote the manuscript. All authors read and approved the final manuscript.
Abbreviations
ELISA, enzyme-linked immunosorbent assay; HD, healthy donors; ICOS, inducible costimulator; IFN-γ, interferon γ; Mtb, Mycobacterium tuberculosis; PBMC, peripheral blood mononuclear cells; PD-1, programmed death-1; PI, proliferation index; SLAM, signalling lymphocytic activation molecule; TB, tuberculosis.
Funding sources
This investigation received financial support from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (PICT 34271 MFQ and PICT 2010-0656 MFQ), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP 0010 MFQ) and the University of Buenos Aires (UBACyT X631 MFQ).
References
- 1.WHO. Geneva, Switzerland: WHO; 2008. Global tuberculosis control – surveillance, planning, financing. WHO Report 2008 WHO/HTM/TB/2008393. [Google Scholar]
- 2.Lienhardt C, Azzurri A, Amedei A, Fielding K, Sillah J, Sow OY, et al. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur J Immunol. 2002;32:1605–13. doi: 10.1002/1521-4141(200206)32:6<1605::AID-IMMU1605>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Hertoghe T, Wajja A, Ntambi L, Okwera A, Aziz MA, Hirsch C, et al. T cell activation, apoptosis and cytokine dysregulation in the (co)pathogenesis of HIV and pulmonary tuberculosis (TB) Clin Exp Immunol. 2000;122:350–7. doi: 10.1046/j.1365-2249.2000.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quiroga MF, Pasquinelli V, Martinez GJ, Jurado JO, Zorrilla LC, Musella RM, et al. Inducible costimulator: a modulator of IFN-gamma production in human tuberculosis. J Immunol. 2006;176:5965–74. doi: 10.4049/jimmunol.176.10.5965. [DOI] [PubMed] [Google Scholar]
- 5.Jurado JO, Alvarez IB, Pasquinelli V, Martinez GJ, Quiroga MF, Abbate E, et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol. 2008;181:116–25. doi: 10.4049/jimmunol.181.1.116. [DOI] [PubMed] [Google Scholar]
- 6.Pasquinelli V, Quiroga MF, Martinez GJ, Zorrilla LC, Musella RM, Bracco MM, et al. Expression of signaling lymphocytic activation molecule-associated protein interrupts IFN-gamma production in human tuberculosis. J Immunol. 2004;172:1177–85. doi: 10.4049/jimmunol.172.2.1177. [DOI] [PubMed] [Google Scholar]
- 7.Loke P, Allison JP. Emerging mechanisms of immune regulation: the extended B7 family and regulatory T cells. Arthritis Res Ther. 2004;6:208–14. doi: 10.1186/ar1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–45. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann DE, Walker BD. Programmed death-1 as a factor in immune exhaustion and activation in HIV infection. Curr Opin HIV AIDS. 2008;3:362–7. doi: 10.1097/COH.0b013e3282f9ae8b. [DOI] [PubMed] [Google Scholar]
- 10.Diagbouga S, Fumoux F, Ledru E, Sanou PT, Barro D, Marchal G. Lack of direct correlation between CD4 T-lymphocyte counts and induration sizes of the tuberculin skin test in human immunodeficiency virus type 1 seropositive patients. Int J Tuberc Lung Dis. 1998;2:317–23. [PubMed] [Google Scholar]
- 11.Uppal SS, Tewari SC, Verma S, Dhot PS. Comparison of CD4 and CD8 lymphocyte counts in HIV-negative pulmonary TB patients with those in normal blood donors and the effect of antitubercular treatment: hospital-based flow cytometric study. Cytometry B Clin Cytom. 2004;61:20–6. doi: 10.1002/cyto.b.20018. [DOI] [PubMed] [Google Scholar]
- 12.Spellberg B, Edwards JE., Jr Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 13.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–7. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 14.Pai M, Dheda K, Cunningham J, Scano F, O'Brien R. T-cell assays for the diagnosis of latent tuberculosis infection: moving the research agenda forward. Lancet Infect Dis. 2007;7:428–38. doi: 10.1016/S1473-3099(07)70086-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Gong J, Iyer DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94:2435–42. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bal AM, Lakhashe SK, Thakar MR, Tripathy SP, Paranjape RS. Dysregulation of proinflammatory and regulatory cytokines in HIV infected persons with active tuberculosis. Cytokine. 2005;30:275–81. doi: 10.1016/j.cyto.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 17.de la Barrera S, Aleman M, Musella R, Schierloh P, Pasquinelli V, Garcia V, et al. IL-10 down-regulates costimulatory molecules on Mycobacterium tuberculosis-pulsed macrophages and impairs the lytic activity of CD4 and CD8 CTL in tuberculosis patients. Clin Exp Immunol. 2004;138:128–38. doi: 10.1111/j.1365-2249.2004.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A, et al. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179:1979–87. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 19.Zhou X, Kubo M, Nishitsuji H, Kurihara K, Ikeda T, Ohashi T, et al. Inducible-costimulator-mediated suppression of human immunodeficiency virus type 1 replication in CD4(+) T lymphocytes. Virology. 2004;325:252–63. doi: 10.1016/j.virol.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 20.Veillette A. SLAM-family receptors: immune regulators with or without SAP-family adaptors. Cold Spring Harb Perspect Biol. 2010;2:a002469. doi: 10.1101/cshperspect.a002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroczek RA, Mages HW, Hutloff A. Emerging paradigms of T-cell co-stimulation. Curr Opin Immunol. 2004;16:321–7. doi: 10.1016/j.coi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Muthumani K, Choo AY, Shedlock DJ, Laddy DJ, Sundaram SG, Hirao L, et al. Human immunodeficiency virus type 1 Nef induces programmed death 1 expression through a p38 mitogen-activated protein kinase-dependent mechanism. J Virol. 2008;82:11536–44. doi: 10.1128/JVI.00485-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catalfamo M, Di Mascio M, Hu Z, Srinivasula S, Thaker V, Adelsberger J, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci USA. 2008;105:19851–6. doi: 10.1073/pnas.0810032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues DS, Medeiros EA, Weckx LY, Bonnez W, Salomao R, Kallas EG. Immunophenotypic characterization of peripheral T lymphocytes in Mycobacterium tuberculosis infection and disease. Clin Exp Immunol. 2002;128:149–54. doi: 10.1046/j.1365-2249.2002.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shebl FM, Yu K, Landgren O, Goedert JJ, Rabkin CS. Increased levels of circulating cytokines with HIV-related immunosuppression. AIDS Res Hum Retroviruses. 2011 doi: 10.1089/aid.2011.0144. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nixon DE, Landay AL. Biomarkers of immune dysfunction in HIV. Curr Opin HIV AIDS. 2010;5:498–503. doi: 10.1097/COH.0b013e32833ed6f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang HX, Zhang W, Zhao LD, Li Y, Zhang FC, Tang FL, et al. Are CD4 + CD25-Foxp3+ cells in untreated new-onset lupus patients regulatory T cells? Arthritis Res Ther. 2009;11:R153. doi: 10.1186/ar2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imami N, Hardy G, Pires A, Burton C, Pido-Lopez J, Mela C, et al. Immune reconstitution in HIV-1-infected patients. Curr Opin Investig Drugs. 2002;3:1138–45. [PubMed] [Google Scholar]
- 29.Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–8. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 30.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]