Abstract
Context
In the electrically induced cramp model, the tibial nerve is stimulated at an initial frequency of 4 Hz with increases in 2-Hz increments until the flexor hallucis brevis cramps. The frequency at which cramping occurs (ie, threshold frequency [TF]) can vary considerably. A potential limitation is that multiple subthreshold stimulations before TF might induce fatigue, which is operationally defined as a decrease in maximal voluntary isometric contraction (MVIC) force, thereby biasing TF.
Objective
To determine if TF is similar when initially stimulated at 4 Hz or 14 Hz and if MVIC force is different among stimulation frequencies or over time (precramp, 1 minute postcramp, and 5 minutes postcramp).
Design
Crossover study.
Setting
Laboratory.
Patients or Other Participants
Twenty participants (13 males: age = 20.6 ± 2.9 years, height = 184.4 ± 5.7 cm, mass = 76.3 ± 7.1 kg; 7 females: age = 20.4 ± 3.5 years, height = 166.6 ± 6.0 cm, mass = 62.4 ± 10.0 kg) who were prone to cramps.
Intervention(s)
Participants performed 20 practice MVICs. After a 5-minute rest, three 2-second MVICs were recorded and averaged for the precramp measurement. Participants were stimulated at either 4 Hz or 14 Hz, and the frequency was increased in 2-Hz increments from each initial frequency until cramp. The MVIC force was reevaluated at 1 minute and 5 minutes postcramp.
Main Outcome Measure(s)
The TF and MVIC force.
Results
Initial stimulation frequency did not affect TF (4 Hz = 16.2 ± 3.8 Hz, 14 Hz = 17.1 ± 5.0 Hz; t19=1.2, P = .24). Two participants had inaccurate TFs when initially stimulated at 14 Hz; they cramped at 10 and 12 Hz in the 4-Hz condition. The MVIC force did not differ between initial frequencies (F1,19 = 0.9, P = .36) but did differ over time (F2,38 = 5.1, P = .01). Force was lower at 1 minute postcramp (25.1 ± 10.1 N) than at precramp (28.7 ± 7.8 N; P < .05) but returned to baseline at 5 minutes postcramp (26.7 ± 8.9 N; P > .05).
Conclusions
The preferred initial stimulation frequency might be 4 Hz because it did not alter or overestimate TF. The MVIC force was lower at 1 minute postcramp, suggesting the induced cramp rather than the varying electrical frequencies affected force. A 1- to 5-minute rest should be provided postcramp induction if multiple cramps are induced.
Key Words: electromyography, fatigue, neuromuscular stimulation, tetany
Key Points
An initial stimulation frequency of 4 Hz or 14 Hz did not affect cramp threshold frequency.
Muscle cramping rather than varying electrical stimulation frequency affected maximal voluntary isometric contraction force.
When testing, scientists should wait at least 5 minutes between cramp episodes to ensure the muscle has returned to baseline.
Percutaneous electrical stimulation is a reliable and generally well-tolerated model for inducing muscle cramps and might be helpful for studying the pathogenesis and treatment of skeletal muscle cramps.1,2 Most researchers studying electrically induced muscle cramps1–4 have used a modification of the model described by Bertolasi et al,5 who used trains of electrical stimuli consisting of 40 stimuli beginning at an initial electrical stimulation frequency of 1 Hz to induce cramps in the flexor hallucis brevis. Modifications to this technique include using an initial stimulation frequency of 4 Hz, increasing stimulation frequency in 2-Hz increments, and separating stimulations by 60 seconds of rest.1–3
The minimum stimulation frequency at which a cramp occurs is termed threshold frequency (TF). In a previous investigation, we3 reported that individuals with a history of muscle cramping have a lower TF than those who report no history of cramping. This suggests that lower TF might be associated with the occurrence of cramp symptoms and adds validity to the assumption that lower TFs represent a greater propensity to cramp. This observation was important because several scientists4,6 have used changes in TF to indicate a decreased or increased susceptibility to cramp after interventions (ie, lower TFs after intervention indicate an increased susceptibility and vice versa).
Discrepancy exists in the literature regarding the methods and initial frequency used to induce cramps with percutaneous electrical stimulation. Scientists have used initial frequencies of 1 Hz,5,7 4 Hz,1–3,6 5 Hz,7 and 10 Hz4 to study muscle cramping. Starting at a low frequency ensures a reliable measurement of TF by preventing overshooting cramp TF because some patients develop cramps at low stimulation frequencies (eg, 5 Hz).7 However, other researchers1,2,4 have indicated that TF is much higher (ie, 16–32 Hz) when no intervention is applied to healthy, cramp-prone individuals. Thus, a higher initial stimulation frequency might be used without overshooting TF.
Whereas beginning at lower initial frequencies prevents overshooting the TF of an individual, applying many subthreshold electrical stimuli to a small muscle, such as the flexor hallucis brevis, might induce fatigue. Low-frequency electrical stimulation can cause fatigue,8 and we have observed that inducing 2 cramps within 1 minute of each other resulted in a second cramp that was weaker and lasted a shorter duration.1 Moreover, fatigue has been observed to cause delays in rates of relaxation, thereby increasing the likelihood of fused tetani,9 and might increase the excitability of the α motor-neuron pool by increasing muscle spindle activity.10 Some researchers have suggested that this mechanism causes exercise-associated muscle cramps.11 If increased excitatory afferent drive occurs with the electrically induced cramp model, then many subcramp threshold stimuli might condition the muscle to cramp prematurely, leading to inaccurate measurements of cramp TF and inaccurate conclusions about an individual's susceptibility to cramp based on some other intervention (eg, exercise).
Therefore, the purpose of our study was to determine if TF is similar when initially stimulated at 4 Hz or 14 Hz and if MVIC force is different between stimulation frequencies or over time (precramp, 1 minute, and 5 minutes postcramp). We sought to answer 3 questions. First, does a difference exist in TF if initially stimulated at 4 Hz or 14 Hz? Second, if a difference exists in TF between the varying initial frequencies, is this difference caused by fatigue, which was operationally defined by lower maximal voluntary isometric contraction (MVIC) force? Third, if the subthreshold stimuli decrease MVIC force, is MVIC force lower at 1 minute and 5 minutes postcramp induction? We hypothesized that cramp TF and MVIC force would not be different between initial frequencies.
METHODS1
Experimental Design
A crossover design guided data collection. The order of treatments was randomized and counterbalanced with a balanced Latin square. Our dependent variables were flexor hallucis brevis cramp TF in hertz and MVIC force in newtons. Initial electrical stimulation frequency (4 Hz, 14 Hz) and time (precramp, 1 minute postcramp, 5 minutes postcramp) were our independent variables. We used 14 Hz as our other initial stimulation frequency because we wanted to provide at least 1 electrical stimulation trial before cramping, and previous data on participants with a history of muscle cramping indicate they have a TF around 16 Hz.2
Participants
A convenience sample of male and female participants with a self-reported history of muscle cramping within the 6 months before data collection were recruited from various exercise science classes and word of mouth. Twenty individuals (13 males: age = 20.6 ± 2.9 years, height = 184.4 ± 5.7 cm, mass = 76.3 ± 7.1 kg; 7 females: age = 20.4 ± 3.5 years, height = 166.6 ± 6.0 cm, mass = 62.4 ± 10.0 kg) completed the study. Exclusion criteria consisted of (1) pregnancy; (2) injury or surgery to the dominant limb within the 12 months before testing; or (3) any self-reported neurologic, cardiovascular, or neuromuscular disease. The dominant limb was defined as the limb participants used to kick an imaginary ball and was the limb tested. Participants provided written informed consent, and the study was approved by the Institutional Review Board of Brigham Young University.
Procedures
Participants reported to a laboratory on 2 days separated by 72 hours. They were instructed to maintain their current diets, drink water consistently before testing, and abstain from exercise or strenuous activity for 24 hours before testing. Participants self-reported compliance with instructions before testing each day.
They lay supine with their ankles hanging off a table and were instructed to relax during testing. Standard electromyography (EMG) preparatory procedures2 were performed to the medial plantar aspect of the foot, area around the medial malleolus, and ipsilateral tibial tuberosity. An 8-mm Ag-AgCl stimulating electrode (Biopac Systems, Inc, Santa Barbara, CA) was placed slightly inferior to the medial malleolus. The tibial nerve was stimulated submaximally 2 to 4 times with a 1-ms electrical stimulus at 80 V to determine the site around the medial malleolus that caused the greatest hallux flexion. An 8-cm2 dispersive electrode (Biopac Systems, Inc) was placed over the lateral malleolus. Electrodes were secured with medical tape and an elastic wrap at these locations. Two EMG measurement electrodes (Biopac Systems, Inc) were placed 2 cm apart over the midbelly of the flexor hallucis brevis, and 1 ground measurement electrode (Biopac Systems, Inc) was placed over the ipsilateral tibial tuberosity. Electrode sites were labeled with a permanent marker for subsequent testing.
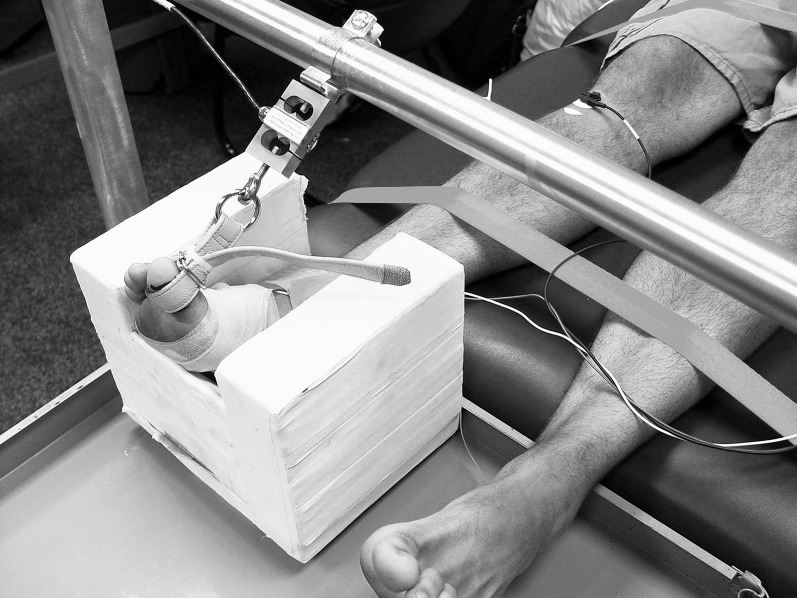
The big toe was placed in a toe harness that was attached to a strain gauge. Nylon straps were tightened over the participant's midthigh and shin to prevent movement of the hip and knee. The ankle was placed in a foam block with a foot pad at 120° to keep the ankle in slight plantar flexion and to prevent the ankle from extreme inversion and eversion (Figure). Participants were instructed to keep the plantar aspect of the foot against this foam block when performing MVICs. Next, they performed 20 practice 2-second flexor hallucis brevis MVICs with a 1-minute rest between MVICs. During pilot testing, this quantity of practice trials was determined to be an appropriate number. To prevent use of the gastrocnemius, we monitored its activity with a biofeedback unit (Pathway TR-10C; Prometheus Group, Dover, NH). Gastrocnemius EMG activity was sampled from the midbelly of the gastrocnemius. Activity exceeding 8 mV constituted an unsuccessful MVIC attempt. If participants performed an MVIC incorrectly, they rested for 1 minute and repeated the attempt. No EMG data were collected from the gastrocnemius; rather, we used the biofeedback unit to help participants perform flexor hallucis brevis contractions more successfully. These settings have been used successfully in previous experiments with high intratester reliability (intraclass correlation coefficient [ICC] [3,3] = 0.92).12 After 20 successful MVICs, participants rested 5 minutes and performed three 2-second MVICs. The force of these MVICs was recorded and averaged.
Figure.
Great toe harness and strain gauge attachment.
After 30 minutes of rest, we attempted to induce cramps via electrical stimulation. Electrical stimulation consisted of 80-V trains of 2-second polyphasic bursts repeated each minute. Initial bursts were either 4 Hz or 14 Hz, and each subsequent burst was 2 Hz greater than the previous burst. Trains continued until the muscle cramped. For example, in the 4-Hz trial, participants were stimulated at 4 Hz for 2 seconds, so they received a total of 8 bursts over the course of the 2-second stimulation period. If they did not cramp after the end of stimulation, they rested for 1 minute, the investigator increased train frequency to 6 Hz, and the participants were stimulated again. This continued until cramp occurred.
A muscle cramp was defined by 3 criteria: (1) involuntary contraction of the flexor hallucis brevis immediately after stimulation that resulted in sustained great toe flexion, (2) verification by the participant that a cramp had occurred, and (3) an average EMG root mean square amplitude greater than 2 standard deviations above the 1-second baseline EMG average root mean square amplitude.2 Muscle cramps induced with this model and using similar criteria have both high intrasession (ICC[3,1] = 0.844)2 and intersession (ICC[3,1] > 0.963) reliability.1,2
If a cramp did not resolve spontaneously after 12 seconds, the primary investigator applied a stretch to the flexor hallucis brevis by hyperextending the great toe until the cramp was alleviated. Cramp TF was recorded, and flexor hallucis brevis MVIC force was reassessed at 1 and 5 minutes postcramp induction. Using the other initial frequency, we repeated the procedures 72 hours after the first testing session.
Instrumentation
The muscle action potentials of the flexor hallucis brevis were collected using the MP150 analog-to-digital data acquisition system with AcqKnowledge software (version 3.7.3; Biopac Systems, Inc). Signals were amplified using the TEL 100C (Biopac Systems, Inc) with a gain set to 5000 from disposable, long-term recording electrodes (model EL502-10; Biopac Systems, Inc) with a center-to-center interelectrode distance of 2 cm. Amplifier impedance was 2 MΩ with a common-mode rejection ratio of 11 dB and a signal-to-noise ratio of 0.75 dB. The EMG signals were sampled at 2000 Hz and band-pass filtered with the low- and high-frequency filters set at 10 and 500 Hz, respectively. The total EMG recording consisted of 1 second of resting activity, 2 seconds of stimulation, and 12 seconds of poststimulus activity.
The train of electrical stimuli was delivered to the tibial nerve by a Grass S88 stimulator and SIU5 Stimulus Isolation Unit (Astro-Med, Inc, West Warwick, RI). An 8-mm Ag-AgCl shielded active electrode (model EL258S; Biopac Systems, Inc) and an 8-cm2 dispersive electrode was connected to the foot of each participant. The intensity and duration of the stimulus were set at 80 V and 2 seconds, respectively, because this intensity and duration have been shown to induce muscle cramps in healthy participants.1,3
To determine flexor hallucis brevis force, the big toe was placed in a toe harness that was attached to a strain gauge rated for loads less than 11 kg and calibrated with a 4-kg weight. The muscle action potentials of the flexor hallucis brevis during MVIC were sampled using the same EMG variables. No electrical stimulus was applied during MVIC.
Statistical Analysis
Differences between initial electrical stimulation TFs were analyzed with a dependent t test because sex does not influence TF (K. C. Miller, unpublished data, August 2011). A 3-way repeated-measures analysis of variance (ANOVA) was used to determine if sex affected MVIC force. Given that no main effect of sex (F1,18 = 2.5, P = .13) and no interactions among sex, initial frequency used, or time were observed (F2,36 = 1.4, P = .25), sex was removed as an independent variable for MVIC force. A 2-way repeated-measures ANOVA with initial stimulation frequency and time as the independent variables then was calculated. Tukey-Kramer multiple comparison tests were conducted when we identified interactions or main effects. The α level was set at .05. We used Number Cruncher Statistical Software (NCSS 2001; Kaysville, UT) for all computations and analyses. Data are reported as means ± standard deviations.
RESULTS
Cramp TF was unaffected by the initial stimulation frequency (4 Hz = 16.2 ± 3.8 Hz; 14 Hz = 17.1 ± 5.0 Hz; t19 = 1.2, P = .24). Cramp TF was not observed in 2 participants when 14 Hz was used as the initial starting frequency. The TFs in the 4-Hz trial for these participants were 10 Hz and 12 Hz; both of them cramped at 14 Hz in the 14-Hz trial. No other electrical stimulations were applied to these participants in the 14-Hz trial.
We did not find an interaction between initial stimulation frequency and time for flexor hallucis brevis force (F2,38 = 2.3, P = .11) and did not find that force was different between initial stimulation frequencies (F1,19 = 0.9, P = .36). However, force changed over time (F2,38 = 5.1, P = .01). It was higher at precramp (28.7 ± 7.8 N) than at 1 minute after cramp induction (25.1 ± 10.1 N; P < .05) but returned to baseline levels at 5 minutes after cramp induction (26.7 ± 8.9 N; P > .05). Force measures were reliable over days (ICC[3,3] = 0.81).
DISCUSSION
Our main observation was that cramp TF was similar regardless of whether the initial electrical stimulation frequency was 4 Hz or 14 Hz (TF for 4 Hz = approximately 16 Hz, TF for 14 Hz = approximately 17 Hz). Scientists have suggested that cramps occur because fatigue alters α motor- neuron excitability13 based on the observation that electrically induced fatigue increases muscle spindle activity10 and decreases Golgi tendon organ activity.14 Based on the lack of changes in TF, it appears the greater number of subthreshold stimuli in the 4-Hz trial was not enough to alter afferent drive or the excitability of the α motor-neuron pool. The authors observing changes in afferent drive (eg, latency) used supramaximal stimuli (100 Hz) to induce fatigue.10,14 When low-frequency electrical stimulations (eg, 30 Hz) induce fatigue, muscle afferent latency often is unaffected.15 These low-frequency stimulations more closely resemble the motor-unit firing rates of humans during MVICs (ie, <32 Hz).16 An alternative possibility for the lack of differences in TF was that our 60-second rest intervals between stimulations were long enough to allow muscle afferents to return to normal resting activity levels. Authors10,11 showing changes in muscle afferent discharge have noted that muscle recovery required 60 to 120 seconds. Therefore, scientists can be confident in their TF measures regardless of the initial electrical stimulation as long as a low-frequency electrical stimulation is used and sufficient rest is provided between electrical stimulations.
A second observation in our study was that MVIC force between initial stimulation frequencies did not differ. Other8 researchers have observed that fatigue can be induced after low-frequency electrical stimulation. Papaiordanidou et al8 noted decreases in MVIC force and maximal M-wave amplitude of the abductor pollicis after 17 trains of 30-Hz stimulation with a pulse duration of 450 μs and a duty cycle of 40% (4 seconds on, 6 seconds off). These observations suggest that muscle excitability was lowered after stimulation, and the authors inferred the presence of peripheral fatigue. The discrepancy between our data and those of Papaiordanidou et al8 are likely due to the different electrical stimulation variables used and the amount of rest given between stimuli. We provided fewer trains (2 trains in 2 seconds) with 60 seconds of rest between trains. Thus, our model likely did not induce fatigue. Our variables were selected because they are typical of those used in this cramp protocol.1–3,6
Whereas MVIC did not differ between electrical frequencies, MVIC force decreased over time. We conclude that cramping rather than varying the number of electrical stimulations needed to induce subsequent cramping induces muscular fatigue. Previously, we1 observed that when 2 successive cramps were induced within 1 minute of each other, the second cramp was often less intense and weaker. The practical implication of these observations is that when testing, scientists should wait between 1 minute and 5 minutes after cramp induction if several cramps are induced in the same session. Because TF often is used as a quantitative measure of an individual's susceptibility to cramp and 4 Hz often is used as the initial stimulation frequency,2,3,6,17 the confidence of the reliability of TF measures in these studies is improved because substantial fatigue does not occur if 4 Hz is used as the initial stimulation frequency.
We must mention 2 considerations if scientists want to use a higher initial electrical stimulation frequency when inducing cramps with percutaneous electrical stimulation. First, TF possibly will be less than the initial frequency. This occurred in 10% (2/20) of our participants. Other scientists have not observed cramping when low-frequency electrical stimulation was used. Benatar et al7 noted no cramps when healthy participants were stimulated at 1 Hz or 5 Hz. Even when 10 Hz was used, cramps occurred in only 1 participant.7 Thus, a higher starting frequency, such as 14 Hz, might be used without fear of an inaccurate TF in most participants. Second, starting participants at low-stimulation frequencies might serve the important function of familiarizing them with the cramp protocol; that is, the lower initial stimulation frequencies might decrease muscle guarding and apprehension so they can relax more effectively during the higher stimulation frequencies experienced later in the protocol. Because TF was not higher in the 14-Hz trial, increased guarding and apprehension did not seem to bias the TF measure in the 14-Hz trial. Thus, scientists should not fear starting at a higher initial frequency because of a lack of familiarization of participants. However, we recommend using 4 Hz as the initial frequency because reliable measures of TF are obtained with minimal to no risk of obtaining an inaccurate measure of TF, and decisions about the effectiveness of treatments often are based on TF.4,6,17
CONCLUSIONS
The TF appears to be reliable regardless of whether 4 Hz or 14 Hz is used as the initial stimulation frequency in the electrical-stimulation cramp-induction model. These data increase the confidence in the reliability of other investigations2,3,6,17 in which the researchers used low initial stimulation frequencies and measured TF. However, the induced cramps alter MVIC force, which possibly is due to fatigue, but MVIC force returns to normal within 5 minutes of cramp induction.
ACKNOWLEDGMENTS
This study was presented at the National Athletic Trainers' Association 2009 Annual Meeting, and an abstract was published as Miller KC, Knight KL. The relationship between the beginning electrical stimulation frequency and a person's “true” cramp threshold frequency. J Athl Train. 2009;44(suppl 1):S89.
We thank Steven Wilding for his assistance with data collection and the Mary Lou Fulton Endowment for generously funding this research.
Footnotes
Portions of the Methods section were adapted from Miller KC, Knight KL, Wilding SR, Stone MB. Duration of electrically induced muscle cramp increased by increasing stimulation frequency. J Sport Rehabil. 2012;21(2):182–185. Adapted with permission from Human Kinetics, Inc.
REFERENCES
- 1.Miller KC, Knight KL. Pain and soreness associated with a percutaneous electrical stimulation muscle cramping protocol. Muscle Nerve. 2007;36(5):711–714. doi: 10.1002/mus.20857. [DOI] [PubMed] [Google Scholar]
- 2.Stone MB, Edwards JE, Babington JP, Ingersoll CD, Palmieri RM. Reliability of an electrical method to induce muscle cramp. Muscle Nerve. 2003;27(1):122–123. doi: 10.1002/mus.10296. [DOI] [PubMed] [Google Scholar]
- 3.Miller KC, Knight KL. Electrical stimulation cramp threshold frequency correlates well with the occurrence of skeletal muscle cramps. Muscle Nerve. 2009;39(3):364–368. doi: 10.1002/mus.21170. [DOI] [PubMed] [Google Scholar]
- 4.Serrao M, Arendt-Nielsen L, Ge HY, Pierelli F, Sandrini G, Farina D. Experimental muscle pain decreases the frequency threshold of electrically elicited muscle cramps. Exp Brain Res. 2007;182(3):301–308. doi: 10.1007/s00221-007-0985-1. [DOI] [PubMed] [Google Scholar]
- 5.Bertolasi L, De Grandis D, Bongiovanni LG, Zanette GP, Gasperini M. The influence of muscular lengthening on cramps. Ann Neurol. 1993;33(2):176–180. doi: 10.1002/ana.410330207. [DOI] [PubMed] [Google Scholar]
- 6.Stone MB, Edwards JE, Huxel KC, Cordova ML, Ingersoll CD, Babington JP. Threshold frequency of an electrically induced cramp increases following a repeated, localized fatiguing exercise. J Sports Sci. 2010;28(4):399–405. doi: 10.1080/02640410903508854. [DOI] [PubMed] [Google Scholar]
- 7.Benatar M, Chapman KM, Rutkove SB. Repetitive nerve stimulation for the evaluation of peripheral nerve hyperexcitability. J Neurol Sci. 2004;221(1–2):47–52. doi: 10.1016/j.jns.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Papaiordanidou M, Guiraud D, Varray A. Does central fatigue exist under low-frequency stimulation of a low fatigue-resistant muscle? Eur J Appl Physiol. 2010;110(4):815–823. doi: 10.1007/s00421-010-1565-9. [DOI] [PubMed] [Google Scholar]
- 9.Bigland-Ritchie B, Johansson R, Lippold OC, Woods JJ. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. J Neurophysiol. 1983;50(1):313–324. doi: 10.1152/jn.1983.50.1.313. [DOI] [PubMed] [Google Scholar]
- 10.Nelson DL, Hutton RS. Dynamic and static stretch responses in muscle spindle receptors in fatigued muscle. Med Sci Sports Exerc. 1985;17(4):445–450. doi: 10.1249/00005768-198508000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Schwellnus MP. Cause of exercise associated muscle cramps (EAMC)-altered neuromuscular control, dehydration, or electrolyte depletion? Br J Sports Med. 2009;43(6):401–408. doi: 10.1136/bjsm.2008.050401. [DOI] [PubMed] [Google Scholar]
- 12.Miller KC, Knight KL, Wilding SR, Stone MB. Duration of electrically induced muscle cramp increased by increasing stimulation frequency. J Sport Rehabil. 2012;21(2):182–185. doi: 10.1123/jsr.21.2.182. [DOI] [PubMed] [Google Scholar]
- 13.Schwellnus MP, Derman EW, Noakes TD. Aetiology of skeletal muscle “cramps” during exercise: a novel hypothesis. J Sports Sci. 1997;15(3):277–285. doi: 10.1080/026404197367281. [DOI] [PubMed] [Google Scholar]
- 14.Hutton RS, Nelson DL. Stretch sensitivity of Golgi tendon organs in fatigued gastrocnemius muscle. Med Sci Sports Exerc. 1986;18(1):69–74. [PubMed] [Google Scholar]
- 15.Balestra C, Duchateau J, Hainaut K. Effects of fatigue on the stretch reflex in a human muscle. Electroencephalogr Clin Neurophysiol. 1992;85(1):46–52. doi: 10.1016/0168-5597(92)90101-g. [DOI] [PubMed] [Google Scholar]
- 16.Bellemare F, Woods JJ, Johansson R, Bigland-Ritchie B. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. J Neurophysiol. 1983;50(6):1380–1392. doi: 10.1152/jn.1983.50.6.1380. [DOI] [PubMed] [Google Scholar]
- 17.Miller KC, Mack GW, Knight KL, et al. Three percent hypohydration does not affect the threshold frequency of electrically induced cramps. Med Sci Sports Exerc. 2010;42(1):2056–2063. doi: 10.1249/MSS.0b013e3181dd5e3a. [DOI] [PubMed] [Google Scholar]